OUscoil Atha Cliath The University of Dublin
Terms and Conditions of Use of Digitised Theses from Trinity College Library Dublin Copyright statement
All material supplied by Trinity College Library is protected by copyright (under the Copyright and Related Rights Act, 2000 as amended) and other relevant Intellectual Property Rights. By accessing and using a Digitised Thesis from Trinity College Library you acknowledge that all Intellectual Property Rights in any Works supplied are the sole and exclusive property of the copyright and/or other I PR holder. Specific copyright holders may not be explicitly identified. Use of materials from other sources within a thesis should not be construed as a claim over them.
A non-exclusive, non-transferable licence is hereby granted to those using or reproducing, in whole or in part, the material for valid purposes, providing the copyright owners are acknowledged using the normal conventions. Where specific permission to use material is required, this is identified and such permission must be sought from the copyright holder or agency cited.
Liability statement
By using a Digitised Thesis, I accept that Trinity College Dublin bears no legal responsibility for the accuracy, legality or comprehensiveness of materials contained within the thesis, and that Trinity College Dublin accepts no liability for indirect, consequential, or incidental, damages or losses arising from use of the thesis for whatever reason. Information located in a thesis may be subject to specific use constraints, details of which may not be explicitly described. It is the responsibility of potential and actual users to be aware of such constraints and to abide by them. By making use of material from a digitised thesis, you accept these copyright and disclaimer provisions. Where it is brought to the attention of Trinity College Library that there may be a breach of copyright or other restraint, it is the policy to withdraw or take down access to a thesis while the issue is being resolved.
Access Agreement
By using a Digitised Thesis from Trinity College Library you are bound by the following Terms & Conditions. Please read them carefully.
Investigation o f cerebral perfusion changes following
MDMA “Ecstasy” administration in an animal model
using bolus-tracking arterial spin labelling MRI
by
Jennifer Rouine
Thesis subm itted for the degree o f Doctor o f Philosophy at the
University o f Dublin, Trinity College
Subm itted October 2011
2 2 OCT 2 iir /
I Declaration
T h is thesis is su b m itte d by th e u n d e r s ig n e d fo r the d e g re e o f D o c to r o f P h ilo so p h y at the U niversity o f D ublin, T rinity C o lle g e and has not been s u b m itte d as an e x e rc ise for a d eg ree at this o r an y o th e r u n iv e rsity and it is entirely m y o w n w o rk . 1 ag re e to d e p o s it this thesis in the U n iv e r s ity ’s o p en a c c e s s institutional re p o sito ry o r allo w th e library to do so on m y behalf, su b ject to Irish C o p y rig h t L egislation and T rin ity C o lle g e Library c o n d itio n s o f use and a c k n o w le d g e m e n t.
T h e recreation al drug o f ab u se 3 ,4 -m e th y le n e d io x y m e th a m p h e ta m in e (M D M A ; E c sta sy ) carries a risk o f cereb ro v a scu la r a c c id e n ts (C V A ) that m ay relate to the role o f seroton in (5 -H T ) and/or d o p a m in e in the reg u la tio n o f c ereb ro v a scu la r ton e. R e cen t a d v a n c e s in m a g n e tic reso n a n c e im a g in g (M R I) h a v e en a b led m easu rem en t o f cerebral b lo o d p erfu sion u sin g con trast a g en t-free ap p ro a ch es su ch a s b o lu s-tra ck in g arterial spin la b e lin g (b tA S L ). T h is in v estig a tio n a sse sse d c h a n g e s in cerebral p erfu sion f o llo w in g sy ste m ic M D M A ad m in istration to rats u sin g b tA S L M R I. A d u lt m a le W istar rats w e r e a d m in istered M D M A (5 or 2 0 m g/k g; i.p .) or sa lin e , a n a e sth etise d 1, 3 or 2 4 hours later and a h igh reso lu tio n an a to m ica l scan fo llo w e d by a c o n tin u o u s A S L (c A S L ) se q u e n c e w a s c o n d u cted u sin g a 7 T esla M RI scan n er. P e r fu sio n -w e ig h te d im a g e s w ere gen erated by su b traction o f la b elled from con trol im a g e s and ex p e rim e n ta l data w a s fitted to a q u an titative m o d el o f cerebral p erfu sio n to gen erate m ean transit tim e (M T T ), cap illary transit tim e (C T T ) and sign al am p litu d e. M T T and C T T are in v e r se ly proportional to cerebral b lo o d flo w (C B F ) and C B F squared r e sp e c tiv e ly , and sig n a l a m p litu d e is proportional to cerebral b lo o d v o lu m e (C B V ). M D M A in d u ced a red u ction in M T T and C T T and an in crea se in sig n a l a m p litu d e in prim ary m otor, se co n d a ry m otor and so m a to se n so r y co rte x I and 3 hours fo llo w in g ad m in istration . S u ch e ffe c ts w ere n o t o b ta in ed in su b -co rtica l re g io n s. T h e acu te e ffe c ts o f M D M A on cerebral p erfu sion m ay g o so m e w a y to w a rd s p r o v id in g a m e ch a n ism to ex p la in the o c c u r ren ce o f C V A in v u ln era b le recreation al e c sta sy users.
e ffec t o f the d o p a m in e D] re c e p to r a n ta g o n is t S C H 2 3 3 9 0 (1 m g /k g ) w a s also d e te rm in e d . W h ile Di re cep to r a n ta g o n ism p ro v o k e d a d ec rea se in cerebral p erfusion in th e visual and parietal asso c ia tio n cortex, it failed to influence the c h a n g e s in cortical p e rfu sio n o b ta in e d w ith M D M A indicating th a t d o p a m in e Di re cep to rs play a role in re g u la tin g b lo o d flow in s o m e brain regions but no t M D M A - r e la te d p erfu sio n c h a n g e s in the frontal cortex. In c o n c lu sio n a lthough 5 - H T depletio n m a y play a role in m e d ia tin g c h a n g e s in cortical p erfu sio n asso c ia te d w ith M D M A ad m in istratio n , m e c h a n is m s in d e p e n d e n t o f 5 - H T such as d irec t d ru g action on, o r 5 -H T and d o p a m in e Di re cep to r in d e p e n d e n t re g u la tio n o f the c e rebral m ic ro v a sc u la tu re unit sh o u ld also be c o n sid ered .
Finally as re peated M D M A e x p o s u r e leads to lo ng-term 5 -H T depletio n , lo ng-term c h a n g e s in C B F and C B V w ere also a sse sse d 8 w e e k s fo llo w in g a re p eated re g im e o f M D M A (5 and 10 m g/k g ; i.p., tw ic e daily for 4 days). P rior e x p o s u r e to M D M A , h a v in g no effect alo n e , atten u a te d p erfusion c h a n g e s asso c ia te d w ith ac u te M D M A (2 0 m g /k g ) c h a lle n g e . In a d d itio n , prior M D M A e x p o s u r e w a s asso c ia te d with a long-term re d u ctio n in cortical 5- H T co n c en tratio n . T h e results sug g est that a functional deficit d e v e lo p s w ith prio r e x p o s u r e in relation to c e re b r o v a s c u la r to n e an d /o r n e u r o v a s c u la r c o u p lin g in r e s p o n se to acute ch a lle n g e. T h e results h av e im plica tions in relation to long-term d eficits in the re g u la tio n o f cerebral perfusion a sso c ia te d w ith prio r M D M A ex p o su re .
Firstly, 1 wish to thank my supervisor Dr. A ndrew Harkin. Your constant guidance, support
and insight were invaluable and 1 am so grateful for all o f your help over the last four years.
I would also like to say a big thanks to OH and Rustam who helped me so much with
learning about the MRl and how to conduct my scans to the best o f my ability. A big thanks
is also due to Ann in Pharmacy who gave up so many o f her m ornings to help me
photograph my Evans blue infused brains.
A very special thanks goes to Valentina Gigliucci for all o f her dedication to m aking me the
best “master apprentice” in HPLC that she possibly could. I never would have gotten all o f
it done without your help ;-).
In addition a big thank you must go to all o f the m em bers o f the TC/A H lab, both past and
present. In particular I would like to thank Lorna and Natacha for teaching me both lab and
in vivo
techniques that 1 have used over the course o f my research. 1 would also like to say a
big thanks to Aine, Jen, Katie, Eimear, Martina, Barry, Shane, Eadaoin, Raasay and Sinead
for making my years here in TC IN fun and full o f good m emories ©.
IV Table of Contents
I D e clara tio n i
II S u m m a r y ii
III A c k n o w le d g e m e n ts iv
IV T a b le o f C o n te n ts v
V List o f F igures ix
VI List o f T a b le s xi
VII A b b re v ia tio n s xii
1. I n tr o d u c tio n
1.1 3 ,4 - m e th y le n e d io x y m e th a m p h e ta m in e 3
1.1.1 In troduc tion 3
1.1.2 R ecreational use o f M D M A “ E c s ta s y ” 5
1.1.3 M D M A to xicity 7
1.1.4 M D M A n e u ro to x ic ity 9
1.2 M a g n e tic R e s o n a n c e Im ag in g 11
1.3 A rterial Spin L ab e llin g 13
1.4 C ere b ral B lood F lo w 15
1.4.1 In troduc tion 15
1.4.2 N e u r o v a s c u la r C o u p lin g 16
1.4.3 R eg u latio n o f C ere b ral B lo o d F lo w 17
(a) E ndothelial C ells 17
(b) P ericy te s 18
(c) N e u ro tra n s m itte rs 20
1.5 S u m m a r y 27
[image:8.525.38.515.60.713.2]2.1 M ateria ls 33
2.1.1 A n im a ls 33
2.1.2 E x p erim e n ta l T re a tm e n ts 33 2.1.3 H igh P e rf o rm a n c e Liquid C h ro m a to g ra p h y
R ea g e n ts 33
2.1.4 M R I re a g e n ts 34
2.1.5 E v a n s blue assa y re a g e n ts 34 2.1.6 G eneral L a b o r a to ry C h e m ic a ls 34 2.1.7 G eneral L a b o r a to ry Plastics 34
2.1.8 A n a e sth e tic s 35
2.2 M e th o d s 36
2.2.1 A n im a ls 36
2.2.2 D rug P repa ration and A d m in istra tio n 36 2.2.3 M o n ito rin g B o d y T e m p e r a tu r e 36 2.2.4 A n a e sth e sia an d A n im a l P repa ration 37 2.2.5 M ag n e tic R e s o n a n c e Im ag in g 38 2.2.6 Test for E x tra v a sa tio n o f E v an s B lue 41 2.2.7 High P e rf o rm a n c e Liquid C h ro m a to g ra p h y 42
2.2.8 Statistical A n a ly s is 43
3. R e g io n a l, tim e an d d o se d e p e n d e n t e ffe c ts o f M D M A “ E c s ta s y ” on ce reb ra l p erfu sio n d e te r m in e d by b o lu s-tr a c k in g a r te r ia l sp in la b e llin g (b tA S L ) M R I
3.1 In troduc tion 47
3.2 E x p erim e n ta l P ro c e d u re 54
3.3 R esu lts 55
3.3.1 M D M A p r o v o k e s a tim e d e p e n d e n t d e c re a se in M T T and C T T an d an increase in signal a m p litu d e
M T T a n d C T T w ith a c o r r e s p o n d i n g i n c r e a s e in
s ig n a l a m p l i t u d e in t h e p r i m a r y m o t o r c o r t e x 65 3 .3 .3 C o r tic a l a n d stria ta l 5 - H T a n d 5 - H I A A
c o n c e n t r a t i o n f o l l o w i n g M D M A a d m i n i s t r a t i o n 72
3 .4 D i s c u s s i o n 7 6
4. Investigation o f the role o f 5-H T and d opam ine in m ediating increased cortical
perfusion follow ing M D M A “ E cstasy”
4.1 I n t r o d u c t i o n 85
4 .2 E x p e r i m e n t a l P r o c e d u r e 89 S t u d y I : C a n t h e 5 - H T r e l e a s i n g a g e n t f e n f l u r a m i n e o r t h e 5- H T2 r e c e p t o r a g o n i s t D O l m i m i c c h a n g e s in c o r t i c a l p e r f u s i o n
a s s o c i a t e d w i t h M D M A ? 89 S t u d y 2: C a n c e n tr a l 5 - H T d e p l e t i o n o r 5 - H T r e c e p t o r b l o c k a d e i n f l u e n c e M D M A - i n d u c e d c h a n g e s in c o r t i c a l p e r f u s i o n ? 8 9 S t u d y 3: C a n b l o c k a d e o f t h e 5 - H T t r a n s p o r t e r p r e v e n t M D M A - i n d u c e d c h a n g e s in c o rtic a l p e r f u s i o n ? 9 0 S t u d y 4: C a n p r i o r t r e a t m e n t w ith th e s e l e c t i v e d o p a m i n e
D|/5 r e c e p t o r a n t a g o n i s t , S C H 2 3 3 9 0 , i n f l u e n c e M D M A - i n d u c e d c h a n g e s in c o r tic a l p e r f u s i o n ? 9 0
4 .3 R e s u l t s 91
4.3.1 F e n f l u r a m i n e , b u t n o t D O I , m i m i c s M D M A - i n d u c e d c h a n g e s in c o r tic a l p e r f u s i o n 91 4 . 3 . 2 5 - H T d e p l e t i o n p r o v o k e s an i n c r e a s e in c o r tic a l
p e r f u s i o n a n d p o t e n t i a t e s M D M A r e la te d c h a n g e s 9 9 4 .3 .3 M D M A - i n d u c e d c h a n g e s in c o r t i c a l p e r f u s i o n
4.4.1 Increased cortical perfusion follow ing M D M A
is mimicked by 5-HT depletion
115
4.4.2 Increased cortical perfusion follow ing M D M A
is not mediated by 5-HT depletion
116
4.4.3 A role for dopamine?
117
4.4.4 A role for direct vascular actions o f M D M A ?
118
4.4.5 Concluding remarks
119
5. Investigation of the long-term effects of repeated M D M A “Ectsasy” exposure on
cerebral cortical perfusion with btASL M R I in rats
5.1 Introduction
123
5.2 Experimental Procedure
130
5.3 Results
131
5.3.1 Prior exposure to M D M A has no effect alone
but attenuates increased cerebral cortical perfusion
induced by acute M D M A challenge
131
5.3.2 Cortical 5-HT concentration in response to prior
M D M A exposure and acute M D M A challenge
139
5.4 Discussion
141
6. Discussion
6.1 Discussion
149
6.2 Future Directions
157
References
159
V III
Appendix
x v ii
V List o f Figures
F igure 1.1.1 S tru ctu re o f a m p h e ta m in e an d its d erivatives
F igure 1.2 Protons, o r h y d ro g e n ions, a lig n in g parallel and anti-parallel to a m a g n e tic field F igure 1.3 S c h e m a tic d ep ictin g th e b tA S L M R l te c h n iq u e
F igure 1.4.1 S c h e m a tic o f brain v asc u la tu re
Figure 1.4.3 S chem atic o f the neuro v ascu lar unit and the role it has to play in m odu latin g cerebral blood flow
F igure 3.3.1.1 M D M A p ro v o k e s a tim e d e p e n d e n t d ec rea se in M T T and CTT w ith a c o r re sp o n d in g increase in signal a m p litu d e in the p rim a ry m o to r corte x
F ig u re 3.3.1.2 M R im ag es re p re se n tin g the a p p e a ra n c e and cle a ra n c e o f co n tra st a g e n t o v e r tim e fo llo w in g in trav e n o u s adm in istratio n
F igure 3.3.2 D o s e - d e p e n d e n t d e c re a se s in M T T and C T T and increase in signal a m p litu d e in the prim ary m o to r cortex
F igure 3.3.3 M D M A p ro v o k e s a tim e d e p e n d e n t d e c re a se in 5 -H T and 5 -H I A A co n c en tratio n
F igure 4.3.1.1 F en flu ra m in e , like M D M A , p ro v o k e s a d e c re a se in M T T and C T T w ith a c o rre s p o n d in g increase in signal a m p litu d e in the p rim a ry m o to r cortex
F igure 4.3.1.2 C ortical 5 -H T c o n c e n tra tio n fo llo w in g fe n flu ra m in e a d m in istra tio n and asso c ia te d re p resen ta tiv e blood v o lu m e m ap s
representative blood volume maps
Figure 4.3.3 Cortical 5-HT concentration follow ing citalopram pre-treatment and associated
representative blood volume maps
Figure 5.3.1 Prior exposure to M D M A attenuates increased cerebral cortical perfusion
induced by acute M D M A challenge
VI List o f Tables
Table 3.3.1 M D M A provokes a time dependent decrease in M T T and C T T with a
corresponding increase in signal amplitude in cortex
Table 3.3.2 M D M A provokes a dose-dependent decrease in M TT and C T T with a
corresponding increase in amplitude in cortex
Table 4.3.1 Fenfluramine and M DM A provoke a decrease in M T T and C T T with a
corresponding increase in amplitude in cortex
Table 4.3.2 pCPA potentiates M DM A -induced decreases in M TT and C T T and
corresponding increase in amplitude in cortex
°C d e g r e e s C e lsiu s
5 -H IA A 5 -h y d r o x y in d o le -a c e tic acid
5 -H T ser o to n in /5 -h y d ro x y tr y p ta m in e
5 -H Tia type-1 a sero to n in receptor
5 -H Tid t y p e - 1 D seroton in recep tor 5 -H T 1 Du t y p e - 1 D a sero to n in recep tor 5 -H T 1 DP t y p e - 1 D p seroton in recep tor
5-H T2 ty p e -2 sero to n in receptor
5-H T2A ty p e -2 A seroton in recep tor 5-H T2B ty p e -2 B sero to n in receptor 5-H T2C ty p e -2 C sero to n in receptor
5-H T7 ty p e -7 sero to n in receptor
5 -H T -lR sero to n in im m u n o re a c tiv e
A D P a d e n o sin e d ip h osp h ate
A N O V A a n a ly sis o f varian ce
A S L arterial sp in la b e llin g
A T P a d e n o sin e trip h osp h ate
b tA S L b o lu s-tra c k in g arterial sp in la b e llin g
B B B b lo o d -b ra in barrier
B O L D b lo o d -o x y g e n le v e l d ep en d en t
Ca^"^ ca lc iu m
c r ch lo rid e
C B F cerebral b lo o d f l o w
C B V cerebral bloo d v o l u m e
C N S central n e r v o u s s y s t e m
C T T c apillary transit t im e
C V A cer e b r ov asc u lar a c c id e n t
Di t y p e- 1 d o p a m in e receptor
D2 t y p e- 2 d o p a m in e receptor
D3 ty p e -3 d o p a m in e receptor
D4 t y p e - 4 d o p a m in e receptor
D5 ty p e -5 d o p a m in e receptor
D A d o p a m in e
D A G d ia c y lg ly c e r o l
D A T d o p a m in e transporter
D O ! 1 -( 2 ,5 - d im e t h o x y - 4 - i o d o p h e n y l) - 2 - a m in o p r o p a n e E D T A e th y le n e d ia m in e te tr a -a c e tic acid
e N O S e n d o th e lial nitric o x i d e sy nthase
ET-1 e n d o t h e liu m - d e r iv e d c o n str ic tin g factor fM RI functional m a g n e tic r e so n a n c e im a g in g
g gram
G A B A y - a m in o butyric acid
H P L C high p e r for m an ce liquid c h r om atogr a p h y
H S P 9 0 heat s h o c k protein
L C B F local cerebral blood flow
M C A m id d le cerebral artery
M D A 3 ,4 -m e th y le n e d io x y a m p h e ta m in e
M D M A 3 ,4 -m e th y le n e d io x y m e th a m p h e ta m in e
M R l m a g n e tic re so n a n c e im ag in g
M T T m e a n transit tim e
m R N A m e s s e n g e r R N A
N A n o ra d re n a lin e
N a H 2 P 0 4 so d iu m d ih y d ro g e n p h o sp h a te
NaCI so d iu m chloride
N a O H s o d iu m h y d ro x id e
n g n a n o g ra m
N O nitric o x id e
p C P A /? « r a -c h lo ro p h e n y a la n in e
P K C protein kinase C
r C B F regional cerebral blood flow
rC B V regional cerebral blood v o lu m e
r f radio fr eq u en c y
rpm re v o lu tio n s p er m in u te
R T - P C R re v erse tra n sc rip tio n - p o ly m e r a se chain reaction
sec s e c o n d s
s.c. s u b c u ta n e o u s
S C H 2 3 3 9 0 7 c h lo r o 3 m e th y 11 p h e n y l 1,2,4 ,5 tetrah y d ro 3
S E M standard erro r o f th e m ean
S E R T sero to n in tra n sp o rte r
S S R l selec tive sero to n in re u p ta k e in h ibitor
T C A trich lo ro a cetic acid
V M A T v e sic u la r m o n o a m in e tra n sp o rte r
v/v v o lu m e p er v o lu m e
Chapter 1
Chapter I: Introduction
1.1 3,4-m ethylenedioxym ethamphetamine (MDMA)
I . l . l Introduction
3 ,4 -m e th y le n e d io x y m e th a m p h e ta m in e ( M D M A ) is a re ad ily a v a ila b le illicit p s y c h o a c tiv e drug. M D M A w a s first sy nthesised and p a te n te d by the G e rm a n p h a rm a c e u tic a l c o m p a n y M e r c k in 1914. M D M A is a sy n th etic d ru g an d m e m b e r o f th e a m p h e ta m in e fa m ily o f drugs. It is rin g -su b stitu te d and shares a sim ilar structure to o th e r a m p h e ta m in e d e riv a tiv e s in clu d in g m e th a m p h e ta m in e , 3 ,4 - m e th y le n e d io x y a m p h e ta m in e ( M D A ) an d the h allu c in o g e n m e sc a lin e (F ig u re 1.1.1).
..NH, CH, am pheiam iiic
NHCII
m etham phetam m e
NH,
3.4-m cthy)cnedk)xyam phetanw «e (MDA)
<
1
H
O il,
<:i
N n o i , CH ,
Cl
•NH, CH, 3.4-iiK ih>leiieJ«>xyclham phctam ine 3.4-m cth>leiiedK )xym elham phetam ine p<ifn-chk>roatnplKftaniitur
(VU)HA) (MDMA) (PCA)
F.C NHCH.CH,
fcn tliiram iiie
C H ,0
NH. C H ,0 C H ,0 CM,
NK,
O CH ,
p tiro-nicihoxyam phetam ine 3,4.5-inm elhoxyphcncthyianiin«
CPMA) (m escalincj
[image:21.538.70.502.62.242.2]Chapter 1: Introduction
The substituted am phetam ines differ from m etham phetam ine, and its parent com poundam phetam ine, by the presence o f a m eth ylen ed ioxy group attached to p osition s 3 and 4 o f the arom atic ring o f the am phetam ine m olecule. T his group o f com pounds also includes
3,4-m ethylen ed ioxyeth am p h etam in e (M D E A ), and m ethylenedioxyam phetam ine (M D A ) w hich are c lo se ly related to M D M A and share many o f its properties. N either am phetam ine
nor its derivatives are found in nature and are com p letely synthetic substances. T hey structurally resem ble adrenaline and dopam ine, with the substituted am phetam ines also resem bling serotonin (5-H T ), and act to enhance neurotransmitter release into the synaptic cleft. M D M A acts as a m onoam ine releaser, a direct and indirect m onoam inergic agonist
and a m onoam ine re-uptake inhibitor in the brain, it binds to all three o f the m onoam ine pre-synaptic transporters (Green et a l , 2 0 0 3 ), but has the highest affinity for the serotonin (5-H T ) transporter (SE R T ) and acts m ainly on the serotonergic system . It acts to a lesser extent on the dopam inergic and the noradrenergic system s resulting in increased release o f th ese neurotransmitters. Crespi and co llea g u es (1 9 9 7 ) have show n that the release o f 5-H T and dopam ine is both carrier-mediated and calcium -dependent (Ca^^-dependent) with M D M A acting to increase cyto so lic Ca^"^ lev els in neuronal term inals, thereby inducing ex o c y to sis. M D M A also binds to various receptors and its in vitro pharm acological profile
ranks its affin ities at th ese receptors as fo llo w s (B attaglia et a l , 1988; D e S ouza &
Battaglia, 1989): 5-H T uptake > a2 adrenergic = 5-H T i = Mi m uscarinic = a i adrenergic =
1.1.2 Recreational use o f MDMA “E c s ta sy ’
M DM A, when used recreationally, is usually taken orally in a tablet form referred to as
“ecstasy”, with tablets generally containing 50 - 150 mg o f the drug. The relative purity o f
the tablets varies and they have been shown to contain any amount o f extraneous
substances including caffeine, ephedrine, ketamine, paracetamol, LSD and other
amphetamine derivatives (Freese
et a i ,
2002; Parrott, 2004). Patterns o f ecstasy use vary
between countries with a high prevalence for binge use in the United Kingdom, 25% o f
subjects taking 4 or more tablets per session (W instock et a i , 2001).
Onset o f effects are typically observed between 20 and 60 min follow ing ingestion and
peak concentrations are observed at 1.5 - 3 hr with the primary effects o f the drug lasting
between 3 and 5 hr (Green
et al.. 2003). The half life (T
1/2) o f M DM A is approximately 8hr. MDMA undergoes metabolism by common metabolic pathways in the liver via several
cytochrome P450 enzym es including CYP2D6 and over a dozen metabolites o f M DM A
have been identified in animals and humans (Green
et a i ,
2003; Kreth
et a i , 2000).
Demethylation o f MDM A which produces reactive catechols is a major degradation step, as
is a parallel side chain pathway initiated by N-dem ethylation to form M DA (Chu
et a i ,
1996). An aromatic hydroxylation pathway also exists, and has been proposed to result in
the
production
o f
trihydroxymethamphetamine
via
6-hydroxymethyienedioxymethamphetamine. The main metabolites o f MDM A and MDA are
4-hydroxy-3-methoxymetamphetamine (HM M A) and 4-hydroxy-3-m ethoxyam phetamine
(HM A) (Green
et a i , 2003). An animal model o f CYP2D6/D1 deficiency, the female Dark
Agouti rat, is w idely used in MDMA research as these rats are poor metabolisers o f
Chapter 1: Introduction
Agouti rats when compared to Sprague Dawley rats. It has been estimated that between 5 -
10% o f Caucasians are deficient in this particular enzym e and are classified as poor
metabolisers (Gonzalez
et a i, 1988). It has been proposed that poor metabolism may
account for some apparently inexplicable or idiosyncratic toxic reactions to the drug
(Tucker
et a l, 1994). The kidneys are the main excretory organs and M DM A has a non
linear pharmacokinetic profile, probably due to either a saturable or inhibitable metabolic
pathway (de la Torre
et a l, 2004; Farre
et a i, 2004) increasing the chance o f accidental
overdose. There are 2 stereoisomers o f M D M A with S(+) being metabolised faster than R(-
) and demonstrating greater neurotoxicity in the rat (Kalant, 2001).
Increased synaptic 5-HT availability is believed to be responsible for the feelings o f
euphoria and enhanced confidence in addition to increased feelings o f serenity and
calm ness (Liechti
et a i, 2000a,b; Verheyden
et a i, 2003). Many studies have reported a
rapid increase in release o f 5-HT following M D M A administration using
in vivo
m icrodialysis (M eehan
et al., 2002; Shankaran and Gudelsky, 1999) and using
in vitro
approaches (Koch and Galloway, 1997; O ’ Loinsigh
et al., 2001). This is followed by a
pronounced decrease in brain levels o f 5-HT and its primary metabolite,
5-
hydroxyindoleacetic acid (5-H lA A ) and the activity o f the 5-HT synthesising enzym e
tryptophan hydroxylase. Within 24 hr brain 5-HT levels recover to normal baseline values
but 3 days following drug administration a sustained and regionally specific depletion o f 5-
HT and 5-HIAA is seen which has been shown to persist for up to 12 months in the rat
(Battaglia
et a l, 1987; Baumann
et a i, 2007; Harkin
et a l, 2001; M cK enna and Peroutka,
With respect to dopamine (DA) release, there is evidence that MDMA elicits this effect via
5-HT release (Koch and Galloway, 1997) via the
5-HT2A receptor (Nash, 1990) and via a
carrier-mediated mechanism independent o f 5-HT release (Nash and Brodkin, 1991). The
positive effects o f MDMA decrease while the negative effects increase with respondents
reporting an increasing tolerance to the drug with repeated use (Solow ij
et a l ,
1992;
W instock et a l ,
2001).
1.1.3 MDMA toxicity
The toxicity o f MDMA which is exhibited both peripherally and centrally has been
extensively reviewed elsewhere (Green
et a l ,
2003). It has been estimated that ingestion o f
the drug results in the deaths o f 15 persons per year in the UK. Nevertheless at the height o f
its popularity, when approximately 500,000 people consumed the drug in an uncontrolled
manner every week in the UK, it became evident that MDMA is actually not very
dangerous or toxic in the short-term. The major concern relating to MDM A is its putative
long-term neurotoxic effects that may not be apparent for many years after consumption.
Chapter I: Introduction
been reported to cause a marked hyperthermic response (Che
el al., 1995; Dafters, 1994;
O ’Shea
et al., 1998). However, at ambient temperatures below 20 - 22°C, a hypothermic
response has been observed following M DM A administration to rats (Marston
et al., 1999).
Dafters & Lynch (1998) observed that ambient temperatures o f 17°C resulted in a
hypothermic response in rats follow ing administration o f M DM A (10 - 15 mg/kg; s.c.).
These findings indicate that M DM A has a profound effect on thermoregulatory
mechanisms and that the substance is highly sensitive to external temperature changes. It
has been reported that acute 5-HT release is not directly responsible for hyperthermia, but
that 5-HT receptors modulate the response (Docherty & Green, 2010; Green
et a l ,
2004,
for review). In addition dopaminergic Di receptors and a p ,
a
2^- and p3- adrenoceptors havebeen implicated in the M DM A-induced hyperthermic response (Docherty & Green, 2010).
It is thought that MDMA may compromise thermoregulation or the body’s ability to
maintain a stable core body temperature despite changes in ambient temperature. This is o f
considerable relevance to human M DM A use as the vast majority o f MDMA consumption
occurs at “raves” where a high ambient temperature, overcrowding and excessive dancing
greatly influence the effects o f the drug (Parrott, 2011).
In addition to the thermoregulatory changes observed follow ing MDMA administration
further physiological changes have been reported. In rats “serotonin syndrome” is observed
follow ing
administration
o f
M DM A.
This
behavioural
syndrome
includes
hyperlocomotion, flattened body posture, head weaving, piloerection, hind limb retraction,
Straub tail, sweating and forepaw treading (Colado
et al., 1993; de Souza
et a l ,
1997;
Marston
et a l ,
1999; Shankaran & Gudelsky, 1999). MDMA administration to animals
stimulatory effects resulting in tachycardia and arrhythm ia in rats (Dum ont
et a l, 2009;
Gordon
et a l, 1991; O ’Cain
et a l,
2000; Vanattou-Saifoudine
et al., 2010b) and increased
blood pressure (Broadley, 2010). The num ber o f M D M A associated hospital admissions
presenting with cardiovascular toxicity suggests that M D M A profoundly affects parameters
such as heart rate and arterial pressure (Henry
et al., 1992) however, the cardiovascular
actions o f M D M A have not been well characterised. It is difficult to carry out studies in
hum ans m im icking the uncontrolled conditions the drug is normally taken under, such as
overcrowding, excessive dancing and loud music. From clinical studies that have been
conducted - albeit in a more controlled environm ent than that in which the drug is typically
consumed - M D M A has been shown to produce a m odest tachycardia and hypertension
(Downing, 1986; Mas
et al., 1999; Verheyden
et al., 2003; Vollenweider
et a l, 1998)
although these studies also reported severe responses in certain individuals. When
administered acutely in recreational doses to human volunteers (0.25 - 1.9 mg/kg; p.o.),
M D M A increased cardiovascular activity, which peaked between I and 2 hr following
administration (de la Torre
et a l,
2000a,b; Lester
et a l, 2000; Liechti & Vollenweider,
2000a,b). It could therefore be the case that M D M A exacerbates latent cardiovascular
problems and could pose serious threats in a dance club setting. The physiological changes
alluded to here are the most com m on indicators o f M D M A -induced toxicity.
1.1.4 MDMA neurotoxicity
With respect to long-term effects, 5-H T neurons appear to be alm ost exclusively
susceptible to dam age by M D M A in prim ates and rats (Bankson & Cunningham, 2001;
Colado
et a l, 2004; Green
et a l, 2003; Gudelsky & Yam am oto, 2008; Shankaran &
Chapter 1: Introduction
following M D M A administration. Battaglia and colleagues (1987) and others (M cCann
et
a l, 1998; Reneman
et al., 2001) have reported significant reductions (up to 60 - 70%) in 5-
H T uptake sites following M DM A (20 mg/kg; s.c., twice daily for 4 consecutive days)
administration to rats in comparison to vehicle treated control animals, indicative o f a
reduction in 5-HT nerve terminal integrity. Immunoreactive 5-HT axon density was
quantified by Hatzidimitriou and colleagues (1999) in various brain regions following
M D M A administration (5 mg/kg; s.c., twice daily for 4 consecutive days) to non-human
primates. 83 - 95% reductions in 5-HT immunoreactive (5-HT-lR) axon density were
reported in cerebral cortex two weeks following M D M A administration. Seven years after
treatment with M D M A , reductions in 5-HT-IR were still evident but significant recovery
had occurred in comparison to the two week response. There are two major 5-HT
projections from the raphe nuclei to forebrain areas and immunocytochemistry studies in
animals have shown a differential vulnerability to the neurotoxic effects o f M DM A . Fine 5-
HT axons arising from the dorsal raphe nucleus display an enhanced vulnerability while
beaded 5-HT axons originating from the median raphe nucleus are spared (M am ounas &
Molliver, 1988; Molliver
et al., 1990). Retrograde degeneration does not seem to occur,
leaving cell bodies in the raphe nuclei intact and there is evidence that damaged terminals
can recover (Battaglia
et a l, 1988; M ayerhofer
et a l, 2001). M D M A administration to
mice also results in changes, to a lesser extent, in the concentration o f the catecholamines,
dopam ine (DA) (Bankson & Cunningham, 2001; Colado
et al., 2004; Green
et a l, 2003;
Gudelsky & Y am am oto, 2008; Shankaran & Gudelsky, 1998) and noradrenaline (NA)
(Green et a l, 2003; Rothman
et a l, 2001).
showed a 50 - 80% depletion o f 5-HT and 5-HIAA in the brain o f a chronic M D M A user,
while dopamine concentrations were unaffected. In addition, hum an studies using
neuroimaging techniques have indicated 5-HT neuronal dam age following M D M A
administration (M cC ann
et al, 1998; Obrocki
et al., 2002; Semple
et ai, 1999) but caution
is advised in relation to the interpretation o f these findings (de Win
et al, 2004; Kish, 2002;
Thom asius
et al, 2003). Impairment o f 5-HT function is also supported by blunted
responses to challenge with the 5-HT releasing agent D-fenfluram ine (Gerra
et al, 1998;
2000) and reduced 5-HlAA in the cerebrospinal fluid o f abstinent M D M A users (M cCann
et al, 1994). There has been some speculation that M D M A itself does not mediate the
neurotoxicity (Esteban
et al, 2001; Paris & Cunningham, 1992) and that it may, in fact, be
the products o f metabolism which are taken into the 5-HT neuron which are responsible
(Bai
et al, 2001; Cadet & Brannock, 1998; Capela
et al, 2007; Carvalho
et al, 2004;
Colado et al, 1995; de la Torre
et al, 2004; Jones et al, 2005). In addition oxidative stress
has also been implicated in M DM A -induced neurotxicity (Puerta
et al, 2010; Steinkellner
et al, 2011; Yam am oto & Raudensky, 2008).
1.2 M agnetic Resonance Imaging
Magnetic resonance imaging (MRI) is an imaging technique which m ay be employed in
both clinical and pre-clinical investigations to obtain a high quality image o f the interior o f
the brain.
The use o f MRI, in hum ans and animals, is possible due to the fact that body tissues are
Chapter I: Introduction
large n u m b e rs o f h y d ro g e n a to m s w h ic h c o m p ris e u npaire d protons. T h e se unpaired
pro to n s po ssess a p h e n o m e n o n k n o w n as “s p in ” . T h e spin o f an u n p aire d proton allo w s
pro to n s to line up w ith (parallel fo rm a tio n ) o r ag a in st (anti-parallel fo rm a tio n ) a m ag n etic
field, fo llo w in g ap p lica tio n o f a m a g n e tic field. In M R l, a m ag n etic field is generated b y a
m ag n etic field gra d ie n t coil (F igure 1.2).
Figure 1.2 Protons, or hydrogen ions, aligning parallel and anti-parallel to a magnetic
fie ld
T h e s e p ro to n s po ssess d iffe rent e n e rg y states and a proton has the ability to m o v e from on e
en e rg y state to an o th e r e n e rg y state fo llo w in g the a b so rp tio n o f a p h oton. W h e n the en e rg y
o f th e p h o to n m a tc h e s the en e rg y d iffe re n c e b etw e en the tw o spin states, ab so rp tio n o f
en e rg y occurs. In M R I, the fre q u e n c y o f the p h oton falls w ithin th e radio fr eq u en c y (R F )
range ( w w w .c is .rit.e d u /h tb o o k s /m r i/in s id e .h tm ) and this fre q u e n c y m a y be applied by a
ra d io fre q u e n c y coil. W h e n the RF pulse is tu rn ed off, the h y d ro g e n p ro to n s return to th e ir
natural a lig n m e n t w ith in th e m a g n e tic field and re le ase th e ir e x c e s s stored energy. W h e n
[image:30.529.42.487.104.759.2]subsequently integrated and converted through the use o f a Fourier transformation into an
M R image (Huettei
et a l, 2008).
1.3 A rterial Spin Labelling
Arterial spin labelling (ASL) is a method used to assess for functionality within an MRl
scan. It acts to assess cerebral blood flow (CBF) or cerebral blood volum e (CBV ) in the
brain without the use o f neuronal activation, it is a technique originally introduced by
Alsop & Detre (1996) and it is the only MRl technique that can directly and absolutely
quantify regional CBF (rC BF) (Beckmann, 2006). An MR image can becom e sensitive to
C B F changes if the m agnetic state o f blood water spins is different to that o f the tissue
water spins (Thom as
et al., 2000). This ASL technique uses magnetically labelled arterial
blood water as an endogenous tracer for the assessment o f perfusion changes (Jahng
et al.,
2007). In this way ASL M Rl is a non-invasive imaging technique that assesses for cerebral
blood perfusion changes. This method is advantageous as it causes minimal disturbance to
the system being imaged (Beckm ann, 2006). T w o separate sets o f M R images are generated
following an A SL scan. The first image contains blood and tissue w ater magnetisations that
are different (the
labelled image) and the second image contains blood and water
magnetisations that are the same (the
control image). Subtraction o f the
labelled from the
control image generates a perfusion weighted image with an intensity that is directly related
to perfusion (Thom as et al., 2000).
Recently, a new quantitative bolus-tracking A SL (btASL) MRl technique was developed
C h apter I: Introdu ction
rodent brain. The technique assesses cerebral perfusion through the calculation o f two
transit times: the mean transit time (M T T) which represents the time taken for labelled
arterial blood water to traverse the vasculature and reach the imaging plane and the
capillary transit time (CTT) which represents the time taken for the arterial blood water to
disperse at the imaging plane. M TT is inversely proportional to CBF, while C T T is
inversely proportional to C B F squared. A third quantifiable output is the btASL signal
amplitude, which is derived from the area under the signal-time ASL curve and has been
interpreted as being proportional to CB V (Figure 1.3).
j-2 r
|CTT
MTT
F igure 1.3 S ch em a tic depictin g th e btA SL M R I techn ique
The schematic depicts ASL as it occurs. Briefly:
1. The inflowing arterial blood water is magnetised.
2. An image o f this magnetised blood is taken at the imaging plane (the
la b elled
image).
3. The inflowing arterial blood water has no magnetic pulse applied to it.
[image:32.529.32.503.197.785.2]1.4 Cerebral Blood Flow
1.4.1 Introduction
C B F is the blood supply to the brain at any given tim e. The brain is dependent on a
continuous supply o f oxygenated blood and it has the ability to control the blood delivery by
sensing pressure changes in its main arteries and by m onitoring respiratory gas levels. The
m ajor arteries supplying the brain are the internal carotid arteries which divide into the anterior
and middle cerebral arteries. The basilar artery divides into the tw o posterior cerebral arteries at
the upper border o f the pons and the Circle o f Willis links all o f these arteries. The arteries
which arise from this structure branch out into sm aller pial vessels that bring blood to the brain
surface. The pial arteries (Figure 1.4.1) give rise to penetrating arteries and arterioles which
penetrate the substance o f the brain and as the arterioles become progressively smaller with
each branching, by losing their smooth muscle layer, they become cerebral capillaries. These
capillaries are also known as the intracerebral or intraparenchymal micro vessels (Cohen
et a l,
1996) and all the other vessels in the brain including the pial vessels and the major cerebral
arteries are known as the extracerebral vessels (Cohen
et a l,
1996). The endothelial cells o f
these capillaries are not fenestrated, as they are in the periphery, but are instead inter-connected
by focal adhesions (ladecola
et a l,
2004) known as tight junctions which, along with the
astrocytic end-feet, form the blood brain barrier (BBB). The BBB is extrem ely important in the
brain as it m odulates the entry o f m etabolic substances such as glucose, controls the m ovem ent
o f ions, and prevents the access o f toxins and peripheral neurotransm itters to the central
nervous system. The presence o f the BBB is one o f the m ajor differences that exists between
Chapter 1: Introduction
V
P ial a rte ry
G lo l
C en tral p a th w a y s from :
C a p i ll a r y
Figure 1.4.1 Schematic o f brain vasculature (ladecola et al., 2004)
T h e d ia g ra m s h o w s a pial arte ry b ra n c h in g into the intracerebral arte riole and finally the
capillary, in additio n , it s h o w s th e c o n n e c tio n s o f the v esse ls to cells.
1.4.2 Neurovascular Coupling
In the brain en e rg y m u s t be m a d e a v a ila b le w h e re n ee d ed q u ick ly and efficiently to e n su r e
p ro p e r fu n c tio n in g and this m e a n s b lo o d flo w m u st co rrelate clo sely w ith n euronal
ac tivation. A useful c o n c e p tu a l tool in d e s c rib in g this p h e n o m e n o n is th e n e u r o v a sc u la r
unit w h ic h is th e functional unit c o m p r is in g n eu ro n s, blo o d vessels, and glial cells that
w o r k in unison to e n su r e a d e q u a te blo o d flo w is c o u p led to neuronal activation (D ra k e &
ladec ola, 2007). N e urovascula r coupling enables cerebral blood flow to be increased in areas
[image:34.531.89.485.60.450.2]neurotransm itters such as acetylcholine, glutamate, GA BA , 5-HT, dopam ine and noradrenaline
as well as some non-conventional transm itters such as nitric oxide (NO).
O ur understanding o f the processes involved in the regulation o f CBF is evolving and
subject to debate (Paulson
et a l, 2010). it is not alw ays clear exactly how activation and
blood flow are coupled and this m ay be due to lim itations in the ability to m easure neuronal
activity or C B F and to correlate them accurately (Tan, 2009). The current paradigm is that
rather than being controlled by a negative feedback loop (an energy deficit signalling for
increased blood flow ), feed-forw ard m echanism s (increased neuronal firing am plifying
local and upstream blood flow ) are key in second-to-second changes in C B F (C auli &
H am el, 2010). H ow ever, it is becom ing clear that astrocytes (H aydon & C arm ignoto,
2006), pericytes (K am ouchi
et al., 2 0 1 1), local neurons (D rake & ladecola, 2007), and the
direct and indirect effects o f neurotransm itters (Cauli & H am el, 2010) play a key role in the
process.
/. 4.3 Regulation o f Cerebral Blood Flow
(a) Endothelial Cells
The endothelial cells o f the m icrovessels are regulators o f vascular tone, vasculogenesis,
inflam m ation and throm bosis (A ndresen et a l, 2006). Stimulation o f endothelial cells leads to
Chapter I: Introduction
regulators o f endothelial nitric oxide synthase (eNOS) and so a decrease in the
concentration or inhibition o f the HSP-90 protein can lead to constriction o f cerebral blood
vessels. Three endothelium -derived constricting factor (ET-1) receptors have been found
expressed in the endothelium and sm ooth m uscle (Andresen
et a l,
2006). ET-I is a profound
2+
vasoconstrictor that increases intracellular C a
concentration and m ay also increase release
o f chloride (C1‘) from sm ooth muscle cells (A ndresen
et al,
2006).
E ndothelial-derived vasoactive com pounds are o f im portance because neurotransm itters
such as acetylcholine (H eistad
et a l,
1977), dopam ine (K rim er
et a l,
1998), 5-H T (C ohen
et a l,
1996) and noradrenaline (R aichle
et a l,
1975) have all been show n to have either
vasoconstrictor or vasodilatatory properties and endothelial vasoactive com pounds m ay
play a role in their m echanism s o f action.
(h) Pericytes
In addition, pericytes appear to have a m acrophage-like activity acting as first line defence in
the brain and having the ability to present antigen (Guillem in & Brew, 2004). The pericytes
have processes that surround the capillaries (Peppiatt
et al.^ 2006) and the prim ary processes
extend from the pericyte cell body along the capillary and subsequently branch into secondary
and tertiary processes (Hamilton
et a l ,
2010). Their importance within the nervous system is
further supported by the fact that there are more pericytes per endothelial cell here than in any
other area o f the body (Hamilton
et a l , 2010).
Chapter I: Introduction
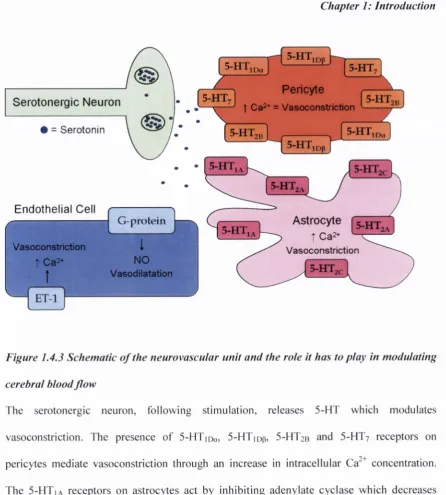
Astrocytic end-feet and neurons have both been shown to interact with pericytes suggesting they may also have a role within the neurovascular unit (Kamouchi et a l , 2004; Bergers & Song, 2005; D ore-D uffy, 2008; Hamilton e t a l , 2010). Pericyte-induced vasoconstriction may also be caused by an increase in intracellular Ca^^ concentration leading to an increase in resting membrane potential which subsequently activates L-type voltage-operated channels (VO CC s). In addition, activation o f non-specific cation channels, leading to the activation o f VOCC, and Ca^"^-activated Cl' channels can increase the activity o f the voltage- operated channels (Hamilton et al., 2010), and it is through these aforementioned m echanism s that pericytes can act to mediate CBF.fc) N eu ro tra n sm itters
5-HT neurons have been reported to modulate blood flow in the microcirculation (Cohen
et al,
1996). Ultrastructural analysis has revealed an intimate association between serotonergic
neurons and blood vessels in the brain (Cohen
et a l ,
1995; Milner
et al.,
1966). It was the
discovery o f this association that promoted the theory that 5-HT could modulate cerebral blood
flow. The vasoconstrictive actions o f 5-HT have been widely reported in both humans
(Kaumann
et al,
1993; Price
et al,
1997; Ullmer
et al,
1995) and animals (Cao
et al,
1992;
McBean
et al,
1991; Roberts
et al,
1997) however, 5-HT has also been reported to promote
vasodilatation under certain conditions (Cohen
et al,
1996). The predominant vasomotor effect
o f 5-HT on cerebral blood vessels is constriction and it has been suggested that the different
receptor subtypes, and possibly the vessel tone before exposure, mediate the opposing effects o f
5-HT on the microcirculation.
A high number o f perivascular serotonergic neurons synapse on astrocytes, implying an
important role for astrocytes in the regulation o f CBF. Astrocytes have been shown to
express a number o f 5-HT receptor subtypes including 5-HTia,
5-HT2a and
5-HT2c (Cohen
et a l ,
1996; Osredkar & Krzan, 2009). The 5-H Tia receptors act by inhibiting adenylate
cyclase which decreases cyclic adenosine monophosphate (cA M P) leading to
down-regulation o f particular genes. This can lead to reduced levels o f cyclooxygenase
eicosanoids (Volterra & M eldolesi, 2005) which can result in vessel constriction. The
5-HT2A receptors are associated exclusively with astrocytes in the human brain (Cohen
et a l ,
Seroton ergic Neuron
• = Seroton in
5-H T ,„,
5-H T,d3 h -
5-HT7
• 5 - H T 7 « •
Pericyte
t Ca2+ = Vasoconstriction 5 -H T , 5 -H T 5 -H T
5 -H T 5 -H T ,,,
5 -H T
Endothelial Cell
Astrocyte
t Ca2+G-protein
5 -H T 5 -H T
I
NO Vasodilatation V asoconstnctiont C a2+
t
V asoconstriction 5 -H T
Figure 1.4.3 Schematic o f the neurovascular unit and the rote it has to play in modulating
cerebral blood flo w
[image:41.529.54.501.58.554.2]Chapter 1: Introduction
oestrogen, adenosine diphosphate (ADP) and adenosine triphosphate (ATP) which activate
G-protein coupled receptors which can stimulate vasodilatation by the production o f nitric
oxide (NO). Endothelium-derived constricting factor (ET-1) receptors have been found
expressed on the endothelium and ET-1 is a profound vasoconstrictor that increases
intracellular Ca^^ concentration.
The catecholamine dopamine has been implicated in the regulation o f CBF. Dopam ine is a
catecholamine and acts as a neurotransmitter in the CNS. It is synthesised from the amino
acid tyrosine which is taken up into the nerve terminal and converted to dopam ine which is
subsequently stored in vesicles by the vesicular m onoam ine transporters (VM ATs). Release
o f dopamine from the nerve terminal leads to the activation o f dopamine receptors. To date
5 different dopamine receptors have been identified (Golan
et al., 2008) which have been
divided into 2 distinct classes D |-like and D
2-like. D |-like comprise D| and D
5receptors
whereas D
2-like comprise D
2, D
3and D
4receptors. Di-like receptors are positively coupled
to G-proteins leading to stimulation o f adenylate cyclase and D
2-like receptors are
negatively coupled to G-proteins leading to inhibition o f adenylate cyclase. Dopam ine is
removed from the synaptic cleft via the pre-synaptically located dopam ine transporter
(DAT).
substantia nigra to the limbic system and the neocortex and is implicated in behaviour. The
second is the nigrostriatal pathway which consists o f neurons projecting from the substantia
nigra to the caudate/putamen and is involved in voluntary m ovem ent. The third is the
tuberoinfundibular pathway, which links arcuate nuclei and periventricular neurons to the
hypothalam us and posterior pituitary and is involved in prolactin homeostasis. The fourth is
the medullary-periventricular pathway which consists o f cell projections in the motor
nucleus o f the vagus nerve. The fifth is the incertohypothalamic pathw ay which connects
the medial zona inccrta to the hypothalamus and the am ygdala (Golan
et al.,
2008).
Krimer and colleagues (1998) first speculated that observed changes in vessels innervated
by dopaminergic neurons were due to direct effects o f dopam ine on the vessel rather than
due to neuronal activation. Further studies have been carried out to elucidate a role for
dopamine in mediating CBF changes. Utilising an array o f selective dopam ine agonists,
antagonists, releasers and re-uptake inhibitors, and using both increased relaxation with
iron oxide nanoparticles (IRON) and blood-oxygen level dependent (B O L D ) techniques
investigators exam ined the effects o f dopam ine release on the cerebrovasculature to
elucidate the specific DA receptors underlying the observed changes, investigators reported
a strong correlation between dopamine concentration in the brain, as released by
am phetam ine, and relative cerebral blood flow (rCBF). Administration o f a dopamine
transporter (DAT) blocker produced com parable results (Krim er
et ai,
1998).
A m phetam ine administration alone, and in combination, with a D
1/ D
5antagonist (SCH
23390) revealed that SCH 23390 produced a small negative rCBF change (approximately
5%) when administered alone, but blocked the rCBF changes normally induced by
am phetam ine and D A T blocker. D
tagonists (quinpirole and R(-)-2,IO,
Chapter I: Introduction
the regions where D2 receptors are present. The D3 agonist 7-OHDPAT also produces small
negative rCBF changes, but differed from D
2agonists in respect that no CBF changes were
observed in the caudate/putamen. The findings o f this study indicate that increases in CBF
are mediated by agonism o f D
1/D
5receptors, while decreases in rCBF are mediated by
agonism o f D2
/D
3 receptors (Choiet al,
2006). Ren and colleagues (2009) also reported a
strong relationship between amphetamine dose and dopamine release however, a negative
rCBF change was associated with low dose amphetamine. Microdialysis, performed to
assess dopamine release at low amphetamine concentration, indicates a dose-dependent
release o f dopamine. It was hypothesised that, because D2/D
3 receptors have a higheraffinity for dopamine than D
1/D
5receptors, at low concentrations, they have higher relative
occupancy and thus exert more net effect on the vasculature. As amphetamine, and thus
dopamine, concentration increases, D
1/D5 receptors dominate the vascular effect. Thesestudies provide evidence to imply a role for dopamine as a potent modulator o f CBF in the
microcirculation.
not aiD- adrenoceptors, was expressed in the cerebral microvesseis.
iodo-2-[p-(4-
hydroxyphenyl)-ethyl-aminomethyl]tetralone
(['^^1]HEAT)
binding
to
the
cerebral
microvesseis was inhibited by 5-methylurapidil, a selective aiA-adrenergic receptor
antagonist, in a dose-dependent manner.
1.5 Sum m ary
Chapter 1: Introduction
1.6 A im s and Objectives
T h e o v e ra ll aim o f the w ork d escrib e d in th is th e sis w a s to d eterm in e the acu te and lo n g
term e ffe c ts o f M D M A on cerebral b lo o d p erfu sio n and to e x p lo r e th e u n d erly in g
m e c h a n ism s in a rodent m o d el o f M D M A a b u se u sin g b tA S L M R I. S p e c ific a lly the
o b je c tiv e s w er e as fo llo w s;
(1 ) T o e m p lo y b tA S L M RI to d e term in e r eg io n a l, tim e and d o se -d e p e n d e n t c h a n g e s to
cerebral p erfu sio n in the rat f o llo w in g a cu te M D M A a d m in istration . It w a s a ls o d eem ed
n e c e ssa r y to cla rify i f an y c h a n g e s o b se r v e d w ere a sso c ia te d w ith B B B disru p tion as
su sta in ed in crea ses in B B B p erm ea b ility h a v e p r e v io u sly been reported fo llo w in g
a d m in istration o f h igh d o s e s o f M D M A to rats.
(2 ) G iv e n the e sta b lish e d role o f 5 -H T and d o p a m in e in the regu lation o f cerebral
p erfu sio n , attem p ts w e r e m ad e to d e term in e the m e c h a n ism s that m ed ia te the a b ility o f
M D M A to in crease cortical p erfu sio n and v o lu m e in rats. First, the a b ility o f M D M A to
g e n e r a lise to fe n flu r a m in e, a sy n th e tic a m p h eta m in e that s e le c t iv e ly in d u ce s th e relea se o f
central 5 -H T w a s d e term in ed , or i f the r e sp o n se to M D M A co u ld be sim u la ted by
a d m in istration o f the n o n -s e le c tiv e 5 -H T 2 recep tor a g o n ist 2 ,5 d im e th o x y -4 -io d o p h e n y
la m in o p ro p la n e h y d r o ch lo rid e (D O l). N e x t, the e ffe c ts o f 5 H T d e p le tio n on M D M A
-in d u ced c h a n g e s -in cortical p erfu sio n w e r e d eterm -in ed . In a d d itio n , -in h ib itio n o f 5-H T
tr a n sm issio n w a s a sse sse d by prior ad m in istration o f the n o n -s e le c tiv e 5 -H T receptor
a n ta g o n ist m e terg o lin e. T h e c o n s e q u e n c e s o f b lo c k in g the 5 -H T transporter and resultant