1. Introduction
Owing to their high melting points, low density and out-standing mechanical properties, Nb-Si based alloys have been extensively studied in the past few years as a kind of promising ultrahigh temperature structural materials1–4). These alloys mainly consist of niobium solid solution (Nbss), (Nb,X)5Si3 and/or (Nb,X)3Si ( X represents Ti, Hf or Mo etc.), where the intermetallics supply excellent high temperature strength and the Nbss guarantees enough room temperature fracture toughness. Up to now, researchers mainly synthesize this kind of alloys by melting methods such as electron beam melting, vacuum arc melting, direc-tional solidification and so on5–8). However, the melting products are always subject to compositional segregation. To solve this problem, a few researchers have fabricated Nb-Si based ultrahigh temperature alloys by powder metallurgy methods such as spark plasma sintering, hot pressed sinter-ing and so on9–11). Besides, powder metallurgy can synthe-size products with fine microstructure in micrometer level, which shows excellent high-temperature plastic deformation ability and even superplasticity. In the powder metallurgy process, mechanical alloying (MA) is always employed to fabricate the powder mixtures used for subsequent sintering. This process has an important influence on the sintering be-havior of powder mixtures. First of all, mechanical energy stored in the powder particles in the form of different kinds of defects is the significant driving force for sintering. Sec-ondly, MA can promote the intimate mixing of each constit-uent, which is an important prerequisite for the microstruc-tural homogeneity of sintered products.
Based on the previous research results6,12–15), an opti-mized Nb-Si based ultrahigh temperature alloy powders with a composition of Nb-20Ti-15Si-5Cr-3Hf-3Al (hereafter all compositions are given in at.% unless otherwise stated) will
be fabricated by MA. Such high content (85%) of ductile components (except for Si) in this alloy implies that it is necessary to add process control agent (PCA) to the powder mixtures to avoid excessive cold welding that would lead to an increase in particle size and considerable sticking of the powders to the milling tools during MA process16). Although almost all organic compounds can be used as PCAs, their different natures have important effects on the efficiency of MA, powder size and morphology, the types and quantity of the contaminants, powder yield and so on16). To choose a suitable kind of PCA for the specific powder mixtures, the interactions between the PCAs and the powders should have been learnt about first. To our best knowledge, up to now there is no systematic research on the effect of PCA on the MA behavior of the multicomponent Nb-Ti-Si based ultra-high temperature alloy powder mixtures. The aim of the present work is to study the effects of types and amount of PCAs on the MA behaviors of Nb-20Ti-15Si-5Cr-3Hf-3Al powder mixtures in term of the efficiency of MA, particle size, morphology and internal structure and the contami-nants.
2. Experimental Procedures
Nb, Ti, Si, Cr, Hf and Al elemental powders were weighed according to the composition of Nb-20Ti-15Si-5Cr-3Hf-3Al. The total mass of powder mixtures was 35 g. MA was per-formed in a planetary ball mill using stainless steel jars and milling balls under Ar atmosphere. The milling speed and ball-to-powder ratio (BPR) were fixed at 400 rpm and 15:1 respectively. MA processes were interrupted at specified time (2, 5, 10, 20, 40 and 70 h) so as to take a few powders for analysis. Methyl alcohol, normal hexane and stearic acid were used as PCAs. The amount used and the corresponding physical properties of PCAs are listed in Table 117).
The Nb-Ti-Si based alloy powders, milled for 20 h with 1.25 mass% stearic acid as the PCA, were uniaxially hot
* Corresponding author, E-mail: xpguo@nwpu.edu.cn
©2017 The Japan Institute of Metals and Materials
Effects of Process Control Agents on the Mechanical Alloying Behavior of Nb-Ti-Si
Based Alloy
Lijing Zhang and Xiping Guo
*State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi an 710072, China
Powder mixtures with the composition of Nb-20Ti-15Si-5Cr-3Hf-3Al (at.%) were mechanically milled in a planetary ball mill. Normal hexane (5 mass%), methyl alcohol (5 mass%) and different amount of stearic acid (0.625, 1.25, 2.5 and 5 mass%) were used respectively as the process control agent (PCA). The effects of the type and amount of PCAs on the mechanical alloying (MA) behavior of the powder mix-tures have been investigated systematically. It has been shown that, compared with using 5 mass% methyl alcohol or stearic acid, ball milling with the same amount of normal hexane shows the fastest MA progress. However, the final powder particles have a coarse size as a result of severe cold welding. Ball milling with 5 mass% stearic acid shows a hyperslow MA progress due to its excessive lubrication effect. Milling with 5 mass% methyl alcohol yields a moderate MA rate and much fine particle size. However, HfC formed during milling due to the high carbon contamination of methyl alcohol. Moreover, it is found that decreasing the amount of stearic acid could accelerate the MA progress significantly. A suitable amount of PCA is necessary to restrain the excessive cold welding and avoid the formation of HfC. Finally, 1.25 mass% stearic acid is adopted as the PCA to fabricate the powder mixtures used for hot pressed sintering.
[doi:10.2320/matertrans.MJ201609]
(Received April 25, 2017; Accepted June 26, 2017; Published September 25, 2017)
pressed to bulk samples. To eliminate the influences of the residual PCA on the as-sintered microstructure, the MA powders were first heated up to 500 C (far above the boiling point of the PCA) and then kept for 30 min under vacuum for the complete volatilization or decomposition of the resid-ual PCA. Thereafter, the temperature was raised to 1500 C and hold for 1 hour at a pressure of 50 MPa under an argon atmosphere.
The phase constituents of powders were characterized by X-ray diffractometer (Panalytical X Pert PRO) with a Cu tar-get. Pearson-VII function was used to fit the XRD profiles for extracting the full width at half maximum (FWHM) and diffraction peak position of Nbss. Cohen method was used to calculate the lattice parameter of Nbss. The morphology and internal structure of powder particles were observed us-ing a scannus-ing electron microscope (SEM, Tescan MIRA 3) with an energy-dispersive X-ray spectrum analyzer (EDS, Inca X-sight). The powder particles were firstly blended with bakelite powders and then the mixtures were hot pressed into bulk samples. Conventional sample preparation method for metallographic analysis was then used to section the powder particles. Carbon-sulfur analyzer (Leco CS844) and oxygen-nitrogen-hydrogen analyzer (Leco TC500) were
used to measure the contents of carbon and oxygen contami-nations, respectively. To eliminate the influences of residual PCAs on the mersurements, samples were heated to 500 C (far above the boiling points of these three PCAs) and kept for 30 min under vacuum for the complete volatilization or decomposition of residual PCAs.
3. Results and Discussions
3.1 Effects of the PCA types
Methyl alcohol, normal hexane and stearic acid are indi-vidually added to the powder mixtures as PCA at a fixed content of 5 mass% to illuminate the effect of PCA type on the MA behavior of powder mixtures.
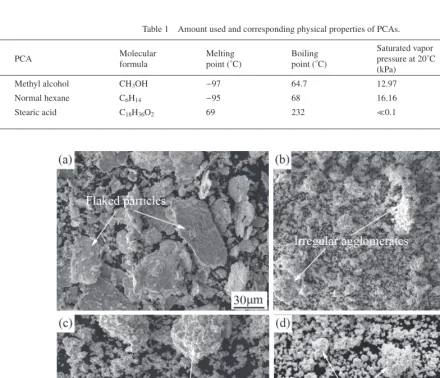
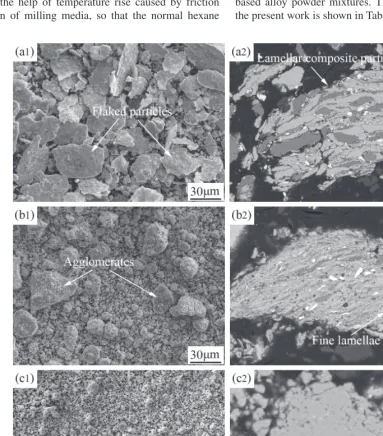
Figure 1 shows the morphological evolution of powder particles with ball milling time at the addition of 5 mass% methyl alcohol as PCA. It can be seen that after ball milling for 5 h there appear some large flaked particles (Fig. 1(a)), which are the typical particle morphology of ductile constit-uents during milling16). During ball milling, the ductile con-stituents are micro-forged to the flaked shape. The addition of methyl alcohol restrains the cold welding action and re-stricts the increase of the flake thickness. Besides, during the
[image:2.669.63.504.392.770.2]Fig. 1 Secondary-electron (SE) morphological images of powder mixtures milled with 5 mass% methyl alcohol for (a) 5 h, (b) 10 h, (c) 40 h and (d) 70 h. Table 1 Amount used and corresponding physical properties of PCAs.
PCA Molecular
formula
Melting point ( C)
Boiling point ( C)
Saturated vapor pressure at 20 C (kPa)
Amount (mass%)
Methyl alcohol CH3OH −97 64.7 12.97 5
Normal hexane C6H14 −95 68 16.16 5
Stearic acid C18H36O2 69 232 ≪0.1 0.625, 1.25,
subsequent micro-forged process, due to the presence of methyl alcohol which provides a lubricating effect, the trapped flaked powder particles are deflected under the ac-tion of milling balls, resulting in the force applied by milling balls to be perpendicular to the flat horizontal surfaces of particles. This will lead to a further decrease in the flake thickness. After ball milling for 10 h, the powder particles are fragmented to smaller ones and these small particles tend to agglomerate together. At this time, some irregular ag-glomerates form (Fig. 1(b)). After ball milling for 40 h, these irregular agglomerates transform into near-spheriodal ones with a wide particle size distribution (Fig. 1(c)). With further prolonging the milling time, the agglomerates de-crease and exhibit more homogeneous particle size (Fig. 1(d)).
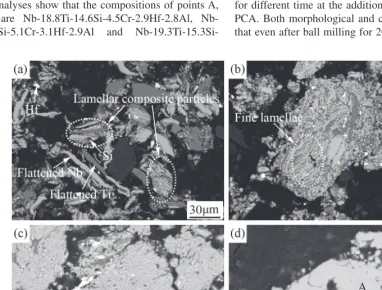
Figure 2 shows the internal microstructural evolution of powder particles during milling with the addition of 5 mass% methyl alcohol as PCA. It can be seen that after ball milling for 5 h (Fig. 2(a)), in accordance with the mor-phological observation (Fig. 1(a)) the ductile constituents are micro-forged to flaked plates. Besides, some composite particles with lamellar structure form. With increasing ball milling time to 10 h, the composite particles still remain lamellar structure, but the lamellar spacing decrease remark-ably (Fig. 2(b)). Figure 2(c) shows that after ball milling for 40 h the composite particles still retain the trace of lamellar structure, but the lamellae are too thin to recognize their boundary. After ball milling for 70 h, there is no any contrast difference in the cross sectional BSE image of powder parti-cles. EDS analyses show that the compositions of points A, B and C are 18.8Ti-14.6Si-4.5Cr-2.9Hf-2.8Al, Nb-19.1Ti-14.6Si-5.1Cr-3.1Hf-2.9Al and
Nb-19.3Ti-15.3Si-4.9Cr-2.9Hf-2.9Al, respectively, which all approach the nominal composition of the powder mixture (Nb-20Ti-15Si-5Cr-3Hf-3Al). This means that each constituent has been mixed uniformly after ball milling for 70 h using 5 mass% methyl alcohol as PCA.
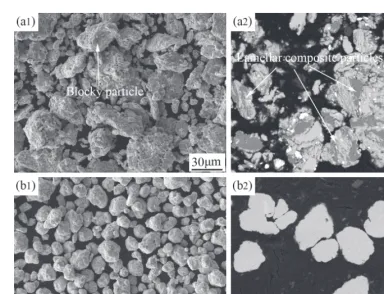
Figure 3 shows the morphological and corresponding cross sectional images of powder particles after ball milling for different time at the addition of 5 mass% normal hexane as PCA. It can be seen that after ball milling for 5 h there form some large blocky particles (Fig. 3(a1)) instead of flaked particles formed at the addition of 5 mass% methyl alcohol as PCA (Fig. 1(a)). From the corresponding cross sectional image, it can be found that the lamellar composite particles form more extensively than those as shown in Fig. 2(a). After ball milling for 40 h, the particles have gran-ular morphology and are separated from each other (Fig. 3(b1)), which is obviously different form that using the same amount of methyl alcohol as PCA (Fig. 1(c)). It can be inferred that excessive cold welding takes place at this time. After ball milling for only 40 h, there is no any contrast dif-ference in the cross sectional BSE image of powder particles (Fig. 3(b2)). EDS analyses also show that each constituent has been mixed uniformly. While, as shown in Fig. 2(d), it takes 70 h to achieve the same result using 5 mass% methyl alcohol as PCA. This indicates that milling with 5 mass% normal hexane shows faster microstructural evolution than that with 5 mass% methyl alcohol.
Figure 4 shows the morphological and corresponding cross sectional images of powder particles after ball milling for different time at the addition of 5 mass% stearic acid as PCA. Both morphological and cross sectional images show that even after ball milling for 20 h each constituent still
[image:3.669.108.490.471.761.2]mains as individual particle. Only after increasing the mill-ing time to 70 h, there form lamellar composite particles. The microstructural evolution using 5 mass% stearic acid lags far behind that using equal amount of methyl alcohol,
not to mention that using normal hexane.
Figures 5(a), (b) and (c) respectively show the XRD pro-files of powder mixtures after ball milling for different time using 5 mass% methyl alcohol, normal hexane and stearic Fig. 3 SE morphological and corresponding cross-sectional BSE images of powder mixtures milled with 5 mass% normal hexane for (a1)–(a2) 5 h and
(b1)–(b2) 40 h.
[image:4.669.106.490.67.361.2] [image:4.669.105.492.404.695.2]acid. It can be seen that with normal hexane and stearic acid as PCAs there is no any new phase formed during the whole milling process. While for methyl alcohol, HfC is detected by the XRD (Fig. 5(a)) analysis. Table 2 also shows that the powders after ball milling for 20 h with 5 mass% methyl al-cohol have the highest C contamination. The only carbon source for the formation of HfC is methyl alcohol. During ball milling, methyl alcohol itself or its breakdown product reacts with the very active Hf to form HfC. This is consistent with the research of R. Juárez et al.18), which reveals that the ball milling with methyl alcohol as PCA produces the high-est carbon contamination compared with cyclohexane and stearic acid under the same milling condition. For three PCAs, the diffraction peak intensity decreases gradually with the increase of milling time in the XRD patterns. At the addition of normal hexane as PCA, after ball milling for 40 h only Nb peaks can be detected. For methyl alcohol, Al or/and Cr can still be found after ball milling for 40 h. When using 5 mass% stearic acid as PCA, all the constitu-ents can still be detected even after 70 h milling in the XRD
patterns.
Grain size and lattice strain of major phases in the MA products are always calculated in accordance with the FWHM of diffraction peaks in XRD patterns using William-son-Hall method19,20). Both increase in lattice strain and crease in grain size are indicative of the extent of plastic de-formation21). As a result, the changes of FWHMs of Nb(110) diffraction peaks are plotted in Fig. 5(d) to indicate the plas-tic deformation extent of Nb parplas-ticles qualitatively, where for three PCAs the FWHMs of Nb(110) diffraction peaks in-crease gradually with inin-crease in ball milling time. While
[image:5.669.107.498.320.765.2]af-Fig. 5 XRD patterns of powder mixtures after ball milling for different time using 5 mass% methyl alcohol (a), normal hexane (b) and stearic acid (c) as PCAs. FWHM of Nb(110) diffraction peak (d) and Nb lattice parameter (e) evolution as a function of ball milling time for different kinds of PCAs.
Table 2 C and O contents in samples ball milled for 20 h.
Sample C (mass%) O (mass%)
5 mass% methyl alcohol/20 h 1.02 0.67
5 mass% normal hexane/20 h 0.11 0.28
1.25 mass% stearic acid/20 h 0.13 0.31
ter the same milling time, the Nb diffraction peak using nor-mal hexane as PCA shows the broadest FWHM, namely the severest plastic deformation extent, followed by methyl al-cohol and then stearic acid.
Figure 5(e) shows the Nb lattice parameters as a function of ball milling time for different kinds of PCAs. It can be seen that the Nb lattice parameters for both methyl alcohol
and normal hexane as PCAs decrease gradually with the in-crease of ball milling time. For the present alloy composi-tion, only Hf atom has larger atomic size than Nb atom17). But its content is as low as 3 at.%. As a result, the reducing effects of other solute atoms (Ti, Si, Cr and Al) dissolution on the Nb lattice parameter play a dominant role. Mean-while it can be found that after the same ball milling time,
[image:6.669.106.492.178.762.2]the Nb lattice parameter obtained using normal hexane as PCA shows a smaller value than that obtained using methyl alcohol, which means a higher dissolution content for the former. While for 5 mass% stearic acid, the Nb lattice pa-rameter hardly changes during milling, implying negligible dissolution of solute atoms.
In conclusion, with the same addition amount, milling with normal hexane as PCA shows the fastest MA progress. However, the particle size is so big due to excessive cold welding, which might be detrimental to the following sinter-ing behavior. When ussinter-ing stearic acid as PCA, the powders are still not alloyed after ball milling for 70 h. For ball mill-ing with methyl alcohol as PCA, it shows a moderate MA rate and much fine particle size. The above difference can be attributed to the different physical properties of these three organics, as shown in Table 1, where normal hexane has the highest saturated vapor pressure. As a result, the liquid nor-mal hexane is easy to transform to gas during milling, espe-cially with the help of temperature rise caused by friction and collision of milling media, so that the normal hexane
content adsorbed on the powder particles is too low to re-strain the excessive cold welding. As for stearic acid, due to its high stability, there always exists a stearic acid film on the surface of powder particles. Its accompanying lubrica-tion effect can significantly reduce the milling efficiency. For methyl alcohol, although its moderate volatility guaran-tees the MA efficiency on one hand and restrains the exces-sive cold welding on the other hand, it is not the most appro-priate PCA for the present system due to its high carbon contamination.
3.2 Effect of the amount of stearic acid
Stearic acid is one of the most common solid PCAs for the ball milling process22–24). The above analyses have indi-cated that 5 mass% additive amount is too high for the pres-ent milling process due to the high stability of stearic acid. In view of this, this section will be focused on the effect of the amount of stearic acid on the MA behavior of Nb-Ti-Si based alloy powder mixtures. The additive amount used in the present work is shown in Table 1.
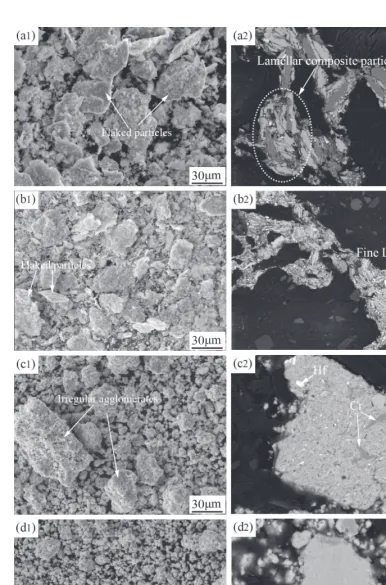
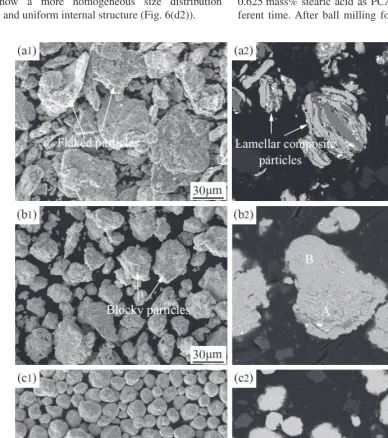
[image:7.669.107.491.326.763.2]Figure 6 shows the morphological and corresponding cross sectional images of powder particles at the addition of 2.5 mass% stearic acid as PCA after ball milling for differ-ent time. It can be seen that decreasing the amount of stearic acid from 5 mass% to 2.5 mass% will accelerate the MA progress significantly. After ball milling for 5 h, there appear some large flaked composite particles (Fig. 6(a1)). The cor-responding cross sectional BSE image shows the formation of lamellar composite particles (Fig. 6(a2)). After ball mill-ing for 20 h, some composite particles still have flaked shape (Fig. 6(b1)). The BSE image shows a finer lamellar spacing (Fig. 6(b2)). After ball milling for 40 h, the flaked composite particles are fragmented and vanished completely. There form some big and irregular composite particles (Fig. 6(c1)). For the internal microstructure, the lamellar structure has disappeared but the microstructure is not uniform. For exam-ple, as shown in Fig. 6(c2), Cr and Hf can still be observed in the BSE image. After ball milling for 70 h, the powder particles show a more homogeneous size distribution (Fig. 6(d1)) and uniform internal structure (Fig. 6(d2)).
Figure 7 shows the morphological and corresponding cross sectional images of powder particles at the addition of 1.25 mass% stearic acid as PCA after ball milling for differ-ent time. After ball milling for 5 h, there also form lots of flaked particles (Fig. 7(a1)). The internal microstructure of the particles is coarse lamellar (Fig. 7(a2)). Different from the ball milling at addition of 2.5 mass% stearic acid as PCA, after only ball milling for 20 h, the flaked composite particles have been fragmented and some big and irregular agglomerates form (Fig. 7(b1)). The lamellae are refined ob-viously, as shown in Fig. 7(b2). After ball milling for 40 h, the irregular agglomerates have disappeared and the powder particles show a more homogeneous size distribution (Fig. 7(c1)) and uniform internal structure (Fig. 7(c2)). It is worth noting that it will take 70 h to achieve this similar mi-crostructure when using 2.5 mass% stearic acid as PCA.
Figure 8 shows the morphological and corresponding cross sectional images of powder particles at the addition of 0.625 mass% stearic acid as PCA after ball milling for dif-ferent time. After ball milling for 5 h, the particles show a
[image:8.669.105.493.327.765.2]flaked shape (Fig. 8(a1)). While compared with Fig. 7(a1), the thickness-to-diameter ratio of the flaked particles is larg-er. The internal structure of the particles is coarse lamellar (Fig. 8(a2)). Increasing the ball milling time to 20 h, there forms big blocky composite particles (Fig. 8(b1)) instead of irregular agglomerates as shown in Fig. 7(b1). The internal structure of the particles (Fig. 8(b2)) is not homogeneous. In some regions, the lamellar structure has practically disap-peared (region A), while in other region the internal struc-ture is fine lamellar (region B). After ball milling for 40 h, the particles have a near-spheroidal morphology and are sep-arated from each other (Fig. 8(c1)). The corresponding inter-nal structure shows a homogeneous composition distribution (Fig. 8(c2)). A comprehensive comparison between Figs. 7 and 8 reveals that the addition amount of 0.625 mass% of stearic acid is too low to restrain the excessive cold welding.
Figures 9(a), (b) and (c) respectively show the XRD pro-files of powder mixtures after ball milling for different time using 2.5, 1.25 and 0.625 mass% stearic acid as PCAs re-spectively. It can be seen that with addition of 2.5 and
1.25 mass% stearic acid as PCAs there is no any new phase formed during the whole milling process. While for 0.625 mass% stearic acid, HfC can be found after ball mill-ing for 10 h. S. Kleiner et al.25) studied the decomposition of stearic acid during the mechanical milling of Al-TiO2 system and found that a lower amount of stearic acid can not guar-antee enough lubrication and hence more heat evolution oc-curred due to the ball collisions. This leads to a faster de-composition rate of stearic acid and a consequent higher carbon contamination. This mechanism can be applied to the present system. Table 2 confirms that the powders after ball milling for 20 h with 0.625 mass% stearic acid show higher C contamination than that with 1.25 mass% stearic acid. For all three amounts of stearic acid, the diffraction peak intensi-ty of elemental powders decreases gradually with the in-crease in milling time. At the addition of 2.5 mass% stearic acid, Hf and Cr diffraction peaks can still be detected after ball milling for 40 h (Fig. 9(a)), which is also confirmed by EDS analysis as shown in Fig 6(c2). When using 1.25 or 0.625 mass% stearic acid as PCAs, only Nb diffraction
[image:9.669.107.489.326.760.2]peaks can be detected after 40 h milling, which is consistent with the BSE observations (Fig. 7(c2) and Fig. 8(c2)). To il-lustrate the plastic deformation extent of Nb particles, the FWHMs of Nb(110) diffraction peaks as a function of mill-ing time are shown in Fig. 9(d). It can be seen that the FWHMs of Nb(110) diffraction peaks increase gradually with the increase in milling time, and after the same milling time the FWHMs of Nb(110) diffraction peaks increase with the decrease in the amount of stearic acid. The Nb lattice pa-rameter as a function of milling time when different amount of stearic acid was employed as PCAs is shown in Fig. 9(e). It can be seen that for all three amounts of stearic acid the Nb lattice parameter decreases gradually with the increase in ball milling time. However, after the same milling time, the Nb lattice parameter decreases with the decrease in the amount of stearic acid, which means that decreasing the stearic acid amount will accelerate the dissolution of solute atoms. In conclusion, decreasing the amount of stearic acid could accelerate the MA progress. However, if the amount of stearic acid is too low, such as 0.625 mass% for the pres-ent system, the degree of cold welding will be severe and HfC will form.
Figure 10 shows the microstructure of the Nb-Ti-Si based alloy prepared by hot pressing at 1500 C for 1 h the MA powders milled for 20 h with 1.25 mass% stearic acid as PCA. The microstructure is comprised of (Nb, X)5Si3 and Nbss ( X represents Ti, Hf, Cr etc.). The carbide and oxide impurities are not found. This indicates that the addition of 1.25 mass% stearic acid as PCA during ball milling process do not result in such so high C and O contaminants to form carbide and oxide inclusions after hot pressing. The desired microstructure after hot pressing can be obtained.
4. Conclusion
The physical properties of PCA have a great effect on the MA behavior of Nb-Ti-Si based alloy powder mixtures. At the same additive amount of 5 mass%, milling with normal hexane as PCA shows the fastest MA rate due to its high volatility. However, the powder particles have a coarse size as a result of excessive cold welding, which might be detri-mental to the following sintering process. While for stearic acid, ball milling for 70 h still failed to realize the objective
for alloying. Milling with 5 mass% methyl alcohol as PCA shows a moderate MA rate and the particle size is also much fine. But its high carbon yield leads to the formation of HfC. The effect of the amount of stearic acid on the MA behavior is that decreasing the amount of stearic acid could accelerate the MA progress, but appropriate amount of PCA is still necessary to restrain the excessive cold welding and avoid the formation of HfC. 1.25 mass% stearic acid is adopted as the PCA to fabricate the suitable Nb-Ti-Si based alloy pow-der mixtures.
Acknowledgments
This work was financially supported by the National Nat-ural Science Foundation of China (Nos. 51371145, 51431003 and U1435201), the Research Fund of the State Key Laboratory of Solidification Processing (NWPU), China (Grant No. 143-TZ-2016) and the Doctorate Foundation of Northwestern Polytechnical University (CX201229).
REFERENCES
1) B.P. Bewlay, M.R. Jackson and H.A. Lipsitt: Metall. Mater. Trans., A
27 (1996) 3801–3808.
2) B.P. Bewlay, J.J. Lewandowksi and M.R. Jackson: JOM-US 49 (1997) 44–45.
3) B.P. Bewlay, M.R. Jackson, J.C. Zhao, P.R. Subramanian, M.G. Mendiratta and J.J. Lewandowski: MRS Bull. 28 (2003) 646–653. 4) B.P. Bewlay, M.R. Jackson, J.C. Zhao and P.R. Subramanian: Metall.
Mater. Trans., A 34 (2003) 2043–2052.
5) H.S. Guo and X.P. Guo: Scr. Mater. 64 (2011) 637–640. 6) S. Zhang and X.P. Guo: Intermetallics 64 (2015) 51–58.
7) N. Sekido, Y. Kimura, S. Miura, F.G. Wei and Y. Mishima: J. Alloy. Compd. 425 (2006) 223–229.
8) Y. Tang and X.P. Guo: Scr. Mater. 116 (2016) 16–20.
9) T. Fei, Y.X. Yu, C.G. Zhou and J.B. Sha: Mater. Des. 116 (2017) 92– 98.
10) X.L. Wang, G.F. Wang and K.F. Zhang: Mater. Sci. Eng. A 527 (2010) 3253–3258.
11) J.L. Yu, K.F. Zhang and G.F. Wang: Intermetallics 16 (2008) 1167– 1170.
12) S. Zhang and X.P. Guo: Mater. Sci. Eng. A 638 (2015) 121–131. 13) K. Zelenitsas and P. Tsakiropoulos: Intermetallics 13 (2005) 1079–
1095.
14) J. Geng, P. Tsakiropoulos and G. Shao: Intermetallics 14 (2006) 227– 235.
15) N. Vellios and P. Tsakiropoulos: Intermetallics 15 (2007) 1529–1537. 16) C. Suryanarayana: Prog. Mater. Sci. 46 (2001) 1–184.
17) J. G. Speight: Lange s Handbook of Chemistry, 16th ed., (Mc-Graw-Hill, New York, 2005) pp. 430–582.
18) R. Juárez, J.J. Suñol, R. Berlanga, J. Bonastre and L. Escoda: J. Alloy. Compd. 434–435 (2007) 472–476.
19) A. Fathy, A. Wagih, M.A. El-Hamid and A.A. Hassan: Adv. Powder Technol. 25 (2014) 1345–1350.
20) A. Molladavoudi, S. Amirkhanlou, M. Shamanian and F. Ashrafiza-deh: Intermetallics 29 (2012) 104–109.
21) L. Shaw, M. Zawrah, J. Villegas, H. Luo and D. Miracle: Metall. Ma-ter. Trans., A 34 (2003) 159–170.
22) A. Nouri, P.D. Hodgson and C.E. Wen: Acta Biomater. 6 (2010) 1630–1639.
23) R. Shashanka and D. Chaira: Powder Technol. 278 (2015) 35–45. 24) F. Hosseini-Gourajoubi, M. Pourabdoli, D. Uner and S. Raygan: Adv.
Powder Technol. 26 (2015) 448–453.
25) S. Kleiner, F. Bertocco, F.A. Khalid and O. Beffort: Mater. Chem. Phys. 89 (2005) 362–366.
[image:10.669.63.274.599.760.2]