Effects of Amounts of Blowing Agent and Contained Gases on Porosity
and Pore Structure of Porous Aluminum Fabricated
from Aluminum Alloy Die Casting by Friction Stir Processing Route
Takao Utsunomiya
1, Kazuya Takahashi
2;*, Yoshihiko Hangai
2and Soichiro Kitahara
31Research Organization for Advanced Engineering, Shibaura Institute of Technology, Saitama 337-8570, Japan
2Department of Mechanical System Engineering, Graduate School of Engineering, Gunma University, Kiryuu 376-8515, Japan 3Gundai Co., Ltd., Isesaki 372-0854, Japan
Porous aluminum is expected to be applied as a multifunctional material in various industrial fields because of its light weight and high energy absorption. When aluminum alloy die castings are used as a starting material in the fabrication of porous aluminum, it can be expected that, by effectively using the gases intrinsically contained in the die casting to generate pores, porous aluminum can be fabricated by adding a small amount of blowing agent. In this study, while systematically varying the amount of blowing agent from 0 to 1.4 mass% that is added to ADC12 aluminum alloy die castings containing three different amounts of gases, porous aluminum is fabricated by the FSP (friction stir processing) route precursor method. Titanium(II) hydride powder is used as the blowing agent. The variations of porosity and pore structure with the amount of added blowing agent are investigated for each amount of gases contained in the die castings. On the basis of the results, it is shown that, by using a blowing agent of approximately 0.6 mass%, ADC12 porous aluminum with high porosity can be fabricated regardless of the amount of gases contained in the die casting. [doi:10.2320/matertrans.MBW201016]
(Received October 26, 2010; Accepted February 28, 2011; Published April 20, 2011)
Keywords: porous metals, friction stir processing, die casting, ADC12, porosity, pore structure, foam, gas porosity
1. Introduction
Porous aluminum is expected to be applied as a multi-functional material in various industrial fields because of its light weight and its properties of high specific strength, high energy absorption and high sound insulation.1)For example,
by applying porous aluminum to automobile components and structures, lightweight automobiles with high collision safety and higher fuel efficiency can be realized.
The authors proposed a method called the FSP (friction stir processing) route precursor method2,3)for fabricating porous
aluminum. Moreover, using various kinds of aluminum alloy plates as starting materials, we have investigated the conditions suitable for fabricating porous aluminum with high porosity and high quality (i.e., a uniform pore size distribution and highly spherical pores) by the FSP route precursor method.4–9) In these attempts, it was found that when an aluminum alloy die casting was used as a starting material, the gases contained intrinsically in the die casting can be used to generate pores. Also, using ADC12 aluminum alloy die castings (called ‘‘ADC12’’ hereinafter) containing a large amount of gases, we fabricated porous aluminum of porosity up to 57% with small and highly spherical pores without the use of a blowing agent.4,5)Moreover, by adding
1 mass% of blowing agent to ADC12 containing a small amount of gases, porous aluminum with a porosity of approximately 70% was fabricated with comparatively large pores.6,7) From these results, it is considered that, by effectively using both blowing agent and the gases contained in an aluminum alloy die casting, porous aluminum with higher porosity can be fabricated by adding a small amount of blowing agent and, moreover, the pore structure (i.e., the
size and sphericity of pores) can be controlled by varying the amount of blowing agent. However, the effects of the amounts of blowing agent and gases contained in aluminum alloy die castings on the porosity and pore structure of porous aluminum have not been yet examined systematically.
In this study, we used ADC12 containing three different amounts of gases5) as starting materials and, varying the
amount of blowing agent added to each ADC12, porous aluminum is fabricated by the FSP route precursor method. First, the variation of porosity with the amounts of added blowing agent and gases contained in ADC12 is evaluated. Next, the pore structure in the porous aluminum for each amount of added blowing agent is observed on the cross section of the porous aluminum. Moreover, in addition to the porosity, the area and circularity of pores are evaluated as quantitative parameters of the pore structure. On the basis of the results, we determined the ranges of the amounts of added blowing agent and gases contained in ADC12 required to fabricate porous aluminum with high porosity. Moreover, the varying states of size and sphericity of pores was examined with the amount of added blowing agent.
2. Fabrication of Porous Aluminum by FSP Route
2.1 Materials
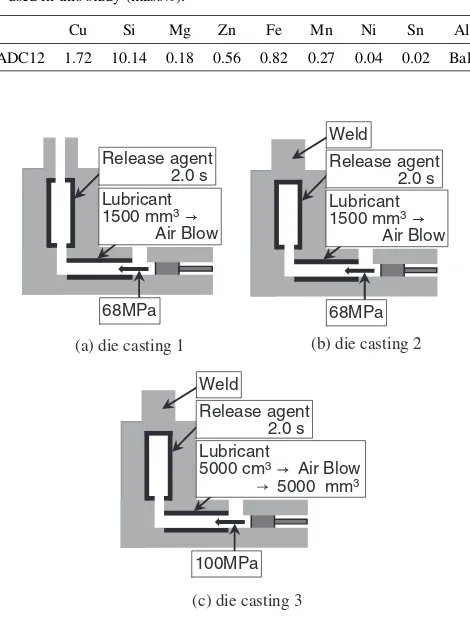
The materials used in the experiments were Al-Si-Cu aluminum alloy ADC12 (equivalent to A383.0 aluminum alloy) die casting, whose chemical composition is shown in Table 1. The dimensions of the ADC12 plates were 3 mm thickness, 70 mm width and 130 mm length. The plates were manufactured conventionally using a cold chamber die-casting machine. The die die-casting conditions used in the experiments are shown in Fig. 1 and same as those used in the ref. 5). The die-casting conditions shown in Fig. 1(a)
are the usual conditions for mass production (die casting 1). As shown in Fig. 1(b), air existing in the metal mold and the gases generated by the decomposition of a release agent and lubricant were intentionally mixed into plates by welding an air bent part (die casting 2). For the conditions in Fig. 1(c), in addition to the conditions of die casting 2, a large amount of lubricant was spread twice over the sleeve part, before and after air blowing. Moreover, the casting pressure was raised so that more gas was mixed into the plate alloy (die casting 3). As the release agent and lubricant, PW60 (Matsumura Oil Co., Ltd.) and Yushiron Form (AZ7150, Yushiro Chemical Industries Co., Ltd.) were used, respectively.
The amounts and types of gases in the three types of ADC12 plates are shown in Table 2.4)The values in this table
were measured by gas chromatographic analyses after cleaning and melting the three entire plates in a vacuum and were average values for two measurements. The source of N2 gas is considered to be air in the metal mold. H2 and
other gases are considered to be generated by the decom-position of the release agent and lubricant when the melted aluminum alloy encounters these agents. In the previous study, it was shown that the total amount of gases has a strong correlation with the porosity and pore structure, although the efficiency of each gas in generating pores is unclear.4,5)So, the discussions in this study are carried out using the total amount of gases. However, we consider that further studies are necessary to examine which types of gases are effective to generate pore and how much amount of contained gases contributes to generate pores.
2.2 FSP procedure
Figure 2 shows a schematic illustration of the FSP route for fabricating the precursors used in the experiments. Two aluminum plates were stacked with blowing agent powder and stabilization agent powder distributed between them. FSP was carried out using an FSW machine (SHH204-720, Hitachi Setsubi Engineering Co., Ltd). FSP10,11)is a
solid-state process generating friction heat and intense plastic flow by inserting a rotating tool with a shoulder and a probe and allowing it to traverse through the aluminum alloy matrix. The tool used for FSP has a columnar shape with a screw probe. The tool material is SKH51 high-speed tool steel. The diameter of the tool shoulder is 17 mm, the diameter of the probe part is 6 mm and its length is 5 mm. The traversing speed of the tool and its tool rotating rate were 100 mm/min and 1000 rpm throughout the experiments. A tilt angle of 3 was used. Titanium(II) hydride (TiH2,<45mm) powder and
alumina (-Al2O3,1mm) powder were used as the blowing
agent and stabilization agent, respectively. The stabilization agent is used to prevent the release of gases from porous aluminum and to stabilize the pore structure during the foaming process by increasing the viscosity of alumi-num.12,13) The powders were placed along the path of the
FSP tool, as shown in Fig. 2(a), over an area of 10 mm width and 110 mm length. The amount of Al2O3 powder
was 5 mass% relative to the mass of the aluminum with dimensions of110mm10mm5mm (i.e., the area over which the powders were distributed and the length of the tool probe). The amount of TiH2powderVbwas varied from
0 to 1.4 mass% relative to the mass of the aluminum with the above dimensions.
The procedure of multipass FSP14,15) used in the
experi-ments is as follows. FSP is carried out to the right of the
(c) die casting 3
(a) die casting 1 (b) die casting 2
Lubricant
1500 mm3
Air Blow Release agent
2.0 s
68MPa 68MPa
Lubricant
1500 mm3
Air Blow Release agent
2.0 s Weld
Lubricant
5000 cm3 Air Blow
5000 mm3
100MPa Release agent
2.0 s Weld
[image:2.595.51.286.87.398.2]Fig. 1 Schematic illustration of die-casting conditions for manufacturing ADC12 aluminum alloy die casting plates.5)
Table 2 Amounts and types of gases in ADC12 aluminum alloy die castings (cm3/100 g).4Þ
H2 N2 CH4 CO CO2 C2H4 C2H6 Total
Die casting 1 1.2 6.1 0.9 — 1.6 — — 9.7 Die casting 2 0.8 42.1 0.6 — 1.5 — — 44.9 Die casting 3 84.5 1.9 26.0 23.1 44.5 13.5 — 195.2
(a)
(c) (d)
ADC12 Plate
TiH2 Alumina
Cutting
Precursor (b)
Fig. 2 Schematic illustration of the manufacturing process of a precursor by FSP.
Table 1 Chemical composition of ADC12 aluminum alloy die castings used in this study (mass%).
[image:2.595.308.544.96.348.2]region where the powders were placed (Figs. 2(b) and (c); ‘‘1st FSP’’). Next, the FSP tool is shifted by approximately the diameter of the tool probe in the direction perpendicular to the FSP direction. FSP is then carried out to the left (retreating side) of the 1st FSP, i.e., to the left of the region where the powders were placed (Fig. 2(d); ‘‘2nd FSP’’). Then, the traversing direction of the FSP tool is reversed, and 3rd FSP and 4th FSP are carried out in the regions immediately above the 2nd FSP and 1st FSP, respectively. Moreover, the 1st FSP to the 4th FSP are then repeated identically. Thus, the FSP region was stirred four times. In this multipass FSP process, TiH2powder and Al2O3powder
are almost uniformly mixed,8) and gas pores intrinsically contained in ADC12 are distributed finely and uniformly in the FSP region.4) We consider that these fine gas pores
formed by FSP contribute to generate pores as nuclei of pores in foaming process. Precursors of 6 mm thickness, 13 mm width and 13 mm length were machined from the stirred FSP region (Fig. 2(d)). On the cross sections of precursors, few gas pores were observed by visual inspection.
2.3 Foaming procedure
The precursor samples containing 0–1.4 mass% TiH2and
5 mass% Al2O3 were heat-treated in a preheated electric
furnace to induce foaming. For the precursor samples manufactured using die casting 2 containing various amounts of TiH2, the holding temperature (equal to the preheated
temperature) and holding time during the heating process were varied from 973 to 1003 K in steps of 15 K and from 6 to 8 min in steps of 1 min, respectively. Two samples were foamed under each set of foaming conditions (holding temperature and holding time). For the precursor samples manufactured using die castings 1 and 3, the holding temperature and holding time used were those that gave the highest porosity of the precursor samples manufactured using die casting 2, and two samples were also foamed for each amount of TiH2. After the heating process, the samples were
cooled to room temperature.
2.4 Evaluation of pore structures
The porosity p (%) of the foamed precursor (porous aluminum), including the skin, was calculated by the following equation:
p¼NPf
NP 100; ð1Þ
whereNPis the density of the precursor without pores andf
is the density of the porous aluminum. In this study, as the difference betweenNPand the density of the precursor with fine pores before heating i was very small, i was used
instead of NP in eq. (1). The densities were evaluated by Archimedes’ principle.
The porous aluminum samples were scanned by a micro-focus X-ray CT system (SMX-225CT, Shimadzu Corpora-tion). The X-ray source was tungsten in this system. The X-ray tube voltage and current used in the inspection were 80 kV and 30mA, respectively. The resolution of the X-ray CT image was512512pixels and the length of one pixel was approximately 60mm. An appropriate threshold was set for the two-dimensional cross-sectional X-ray CT images to
distinguish the aluminum and the pores to obtain binarized X-ray CT images of the pore structures. In the evaluations of pore structures, pores with area less than 1 mm2 were
excluded owing to the resolution of the X-ray CT images. From the binarized X-ray CT images, in addition to the number of pores, the areaAand circularitye16)of each pore
were evaluated using image-processing software. The circu-larity is defined by the following equation:
e¼4A
l2 ; ð2Þ
wherelis the circumference of a pore in the cross-sectional X-ray CT image. A value of e close to 1.0 indicates that the pore is highly circular. The area and circularity were evaluated at three slicing positions as shown in Fig. 3, i.e., at positions 20, 50 and 80% from the bottom relative to the sample height, for each porous aluminum sample.
3. Results and Discussion
3.1 Foaming conditions
As an example of the relationship between porosity and holding time for each holding temperature of the precursor manufactured from die casting 2, Fig. 4 shows the result when the amount of TiH2 is 1.0 mass%. From this figure,
it was found that the porosity is highest when the holding temperature and holding time are 988 K and 7 min, respec-tively. This tendency is similar for other amounts of TiH2.
On the basis of these results, we used a holding temperature and holding time of 988 K and 7 min, respectively, as the optimum foaming conditions for the three types of ADC12 (die castings 1, 2 and 3). The liquidus temperature of ADC12 is approximately 853 K. This temperature is lower than the holding temperature (the preheated temperature of the electric furnace) used in this study.
3.2 Porosity for each amount of blowing agent
Figure 5 shows the relationship between porositypand the amount of TiH2Vbfor the three types of ADC12. Each mark
plotted in this figure is an average value for the two porous aluminum samples fabricated under the same foaming conditions. When the amount of TiH2 is 0 mass%, the
porosity increased with increasing total amount of gases in ADC12, as also shown in previous studies.4,5)The differences among the porosities of die castings 1, 2 and 3 rapidly became small with increasing amount of TiH2. Thus, when
0.8H
0.5H
0.2H
H
Top
Bottom
10 mm
[image:3.595.336.517.74.191.2]the amount of TiH2 was small, the gas generated by the
decomposition of TiH2 greatly affected the porosity. When
the amount of TiH2 was 0.6 mass%, little difference in the
porosity could be observed among the three types of die casting. When the amount of added TiH2was 0.6 mass% or
larger, the porosities were approximately 70% and remained constant with increasing amount of TiH2. This is because the
gas in the pores generated by the decomposition of TiH2was
released from the precursor. Here, the porosity for die casting 1, for which the total amount of gases is smallest, is slightly smaller than those for die castings 2 and 3. However, the optimum foaming condition for TiH2 was essentially
differ-ent from that for the gases contained in ADC12; thus, it is considered that the foaming conditions for ADC12 contain-ing different amounts of gases should be set accordcontain-ing to both the amount of TiH2and the amount of gases contained
in ADC12. Although we consider that further studies are necessary, it is expected that, by optimizing the foaming conditions for die casting 1, the porosity can be increased from the present values.
From the above results, it is found that to fabricate porous aluminum with high porosity without the use of TiH2,
ADC12 should contain a large amount of gases. On the other
hand, the amount of added TiH2required to fabricate porous
aluminum with a porosity of approximately 70% was 0.6 mass% regardless of the total amount of gases contained in ADC12.
3.3 Pore structure for each amount of blowing agent As examples of the pore structures obtained in the experiments, Figs. 6(a)–(c) show those of die casting 1 with 0, 0.1 and 0.6 mass% TiH2and Figs. 6(d)–(f) show those of
die casting 3 with 0, 0.1 and 1.0 mass% TiH2, respectively.
The pore structure of die casting 2 was similar to that of die casting 3. When the amount of TiH2 was 0 mass%, the pore
size became large with increasing total amount of gases (cf. Figs. 6(a) and 6(d)). However, by adding a small amount of TiH2 such as 0.1 or 0.2 mass%, the differences between the
pore structures for die castings 1 and 3 became smaller as shown in Figs. 6(b) and 6(e). When the amount of TiH2was
0.6 mass% or larger and the porosities were almost the same, comparatively large pores were formed by the coalescence of pores and the pore structures were similar for both cases as shown in Figs. 6(c) and 6(f).
As examples of distributions of the area and circularity of pores, Fig. 7 shows the relationship between area of poresA
and frequency f and Fig. 8 shows the relationship between circularity of poreseand frequencyf for die casting 1. Points plotted in Figs. 7 and 8 indicate the sum of the evaluation results at the three slicing positions of porous aluminum (cf. Fig. 3). The distributions of the area and circularity of pores for die castings 2 and 3 were similar to those for die casting 1. From Fig. 7, it was found that the frequency for an area of 1–3 mm2 decreased with increasing amount of TiH
[image:4.595.51.288.280.467.2]2. From
Fig. 8, it was found that when the amount of TiH2 was 0–
0.2 mass%, the frequency for a circularity of 0.9–1 had a large value of approximately 60%, and when the amount of TiH2was 0.6 mass% or larger, the frequency for a circularity
of 0.9–1 decreased owing to the coalescence of pores. From these results, it is found that, although the porosity is low, porous aluminum with small pores can be fabricated by adding a small amount of TiH2of up to 0.2 mass% regardless
of the amount of gases contained in ADC12. On the other hand, porous aluminum with high porosity and compara-tively large pores can be fabricated when the amount of TiH2
is 0.6 mass% or larger regardless of the amount of gases contained in ADC12. Moreover, from the results in this and previous sections, it is considered that when the amount of TiH2 is almost 0 mass%, the porosity and pore structure can
be controlled by varying the total amount of gases contained in ADC12. In addition, the porosity and pore structure can also be controlled by varying the amount of TiH2 added to
ADC12 containing various amounts of gases. That is, by varying the amounts of TiH2and gases contained in ADC12,
it is expected that porous aluminum with various degrees of porosity, pore size and circularity can be fabricated depending on the application of the porous aluminum.
Here, as described above, the porosity and pore structure of porous aluminum for die casting 1, in which the total amount of gases is smallest, rapidly approach those for die castings 2 and 3 with increasing amount of TiH2, and when the amount
of TiH2is 0.6 mass% or larger, they are almost the same as
those of die castings 2 and 3. From this finding, we can
Fig. 4 Relationship between porosity and holding time for each holding temperature of the precursor manufactured from die casting 2 (amount of TiH2: 1.0 mass%).
Fig. 5 Relationship between porositypand amount of TiH2Vbfor holding
predict that when dense aluminum or aluminum alloy without intrinsically contained gases which generate pores is used as a starting material, the variations of porosity and pore
structure with the amount of TiH2will be similar to those for
die casting 1. Obviously, when the amount of TiH2 is
0 mass%, the porosity of dense aluminum and aluminum alloy will be almost 0% and no pores will be observed in the foamed precursor.
10 mm
(c) (a)
(b)
(f) (d)
(e)
Die casting 1 Die casting 3
Fig. 6 X-ray CT images of pore structures of fabricated porous aluminum: (a) die casting 1; 0 mass% TiH2;p¼12:6%; (b) die casting 1;
0.1 mass% TiH2;p¼43:2%; (c) die casting 1; 0.6 mass% TiH2;p¼61:6%; (d) die casting 3; 0 mass% TiH2;p¼42:1%; (e) die casting
3; 0.1 mass% TiH2;p¼53:6%; (f) die casting 3; 1.0 mass% TiH2;p¼70:6%.
Fig. 7 Relationship between area of poresAand frequencyf(die casting 1).
[image:5.595.113.480.77.463.2] [image:5.595.59.276.517.718.2] [image:5.595.312.539.523.683.2]4. Conclusions
Porous aluminum was fabricated using ADC12 aluminum alloy die castings containing three different amounts of gases as starting materials by the FSP route precursor method. In this study, the variations of porosity and pore structure with the amounts of gases contained in ADC12 aluminum alloy die castings and added blowing agent were investigated. The experimental results led us to the following conclusions. (1) The differences in the porosity of ADC12 containing different amounts of gases rapidly became small with increasing amount of TiH2. The amount of added TiH2
required to fabricate porous aluminum with a porosity of approximately 70% was 0.6 mass% regardless of the total amount of gases contained in ADC12.
(2) When the amount of TiH2 was 0 mass%, the pore size
became large with increasing total amount of gases in ADC12. The differences among the pore structures of ADC12 containing different amounts of gases became small with increasing amount of TiH2.
(3) When the amount of TiH2 was small, such as 0–
0.2 mass%, although the porosity was low, porous aluminum with small pores of high circularity was fabricated, and when the amount of TiH2 was 0.6 mass% or larger, the circularity
decreases and the pore size increases owing to the coales-cence of pores, regardless of the amount of gases contained in ADC12.
(4) It is expected that, by varying the amounts of gases contained in ADC12 and added TiH2, porous aluminum
with various degrees of porosity, pore size distribution and circularity can be fabricated depending on the application of the porous aluminum.
Acknowledgments
The authors especially thank one-time Associate Professor
T. Yokota, Shibaura Institute of Technology, and Professor K. Saito, Gunma University, for their helpful advice on conducting the experiments, and one-time Professor H. Kumehara, Gunma University, for fruitful discussions on the experimental results. This work was partly financially supported by the Industrial Technology Research Grant Program (2009) from the New Energy and Industrial Tech-nology Development Organization (NEDO) of Japan and by the Grant-in-Aid for Scientific Research (C) (No. 22560705).
REFERENCES
1) J. Banhart: Prog. Mater. Sci.46(2001) 559–632.
2) Y. Hangai and T. Utsunomiya: Metall. Mater. Trans. A40(2009) 275– 277.
3) Y. Hangai and T. Utsunomiya: Metall. Mater. Trans. A 40(2009) 1284–1287.
4) Y. Hangai, H. Kato, T. Utsunomiya and S. Kitahara: Metall. Mater. Trans. A41(2010) 1883–1885.
5) Y. Hangai, H. Kato, T. Utsunomiya, S. Kitahara, O. Kuwazuru and N. Yoshikawa: J. Japan Inst. Metals74(2010) 592–597.
6) Y. Hangai, Y. Ozeki and T. Utsunomiya: Mater. Trans. 50(2009) 2154–2159.
7) T. Utsunomiya, K. Takahashi, Y. Hangai, S. Kawano, O. Kuwazuru and N. Yoshikawa: J. JILM60(2010) 590–595.
8) Y. Hangai, T. Utsunomiya and M. Hasegawa: J. Mater. Proc. Technol.
210(2010) 288–292.
9) T. Utsunomiya, K. Tamura, Y. Hangai, O. Kuwazuru and N. Yoshikawa: Mater. Trans.51(2010) 542–547.
10) R. S. Mishra and Z. Y. Ma: Mater. Sci. Eng. R50(2005) 1–78. 11) Z. Y. Ma: Metall. Mater. Trans. A39A(2008) 642–658.
12) A. R. Kennedy and S. Asavavisithchai: Adv. Eng. Mater.6(2004) 400– 402.
13) M. Haesche, J. Weise, F. Garcia-Moreno and J. Banhart: Mater. Sci. Eng. A480(2008) 283–288.
14) Y. S. Sato, S. H. C. Park, A. Matsunaga, A. Honda and H. Kokawa: J. Mater. Sci.40(2005) 637–642.
15) J. Q. Su, T. W. Nelson and C. J. Sterling: Scr. Mater.52(2005) 135– 140.
16) H. Seki, S. Sasaki, M. Otsuka and H. Nakajima: J. Japan Inst. Metals72