Original Article
Effects of
Jiangtang Xiaozhi
tablets on expression of
NFκB, CYP
2E
1, PPARγ and ABCA
1inobese rat livers
Xiaoxia Dong1*, Wenting Song2*, Zhengyan Ge2, Long Jin2, Bin Yang1, Hongkun Li1, Yujie Guo2, Xiaohua Hong2, Yanghui Wang2, Jianxun Liu2
1Department of Pathology, 2Institute of Basic Medical Sciences, Xiyuan Hospital CACMS, Beijing, China. *Equal contributors and co-first authors.
Received March 7, 2016; Accepted June 5, 2016; Epub September 15, 2016; Published September 30, 2016
Abstract: To explore effects of Jiangtang Xiaozhi Tablets (JTXZ) on expressions of NF-κB, CYP2E1, PPARγ and ABCA1
inobese rats’ liver. Obese rats were established by high-fat fodder feeding for 18 weeks. Then, the obesity rats were divided randomly into: control model group, metformin group (30 mg/kg), simvastatin group (4 mg/kg), and 3 JTXZdosage groups (2, 4 and 8 g/kg, crude herb mass equivalent) (n=10). Drugs were administered orally once
daily for 8 weeks. Subsequently, rats were sacrificed and bloods were collected from the abdominal aorta to deter -mineserum total cholesterol (TC) and triglyceride (TG). In addition, livers were collected to determine expressions of
NFκB, CYP2E1, PPARγ and ABCA1 using immunohistochemical assays. Our results showed that after treatment with JTXZ, body weight (BWs, P<0.01) and TC (P<0.05) are obviously decreased compared with the model rats, and TG was also decreased by treatment with JTXZ at the doses of 8 and 4 g/kg (P<0.05); importantly, the JTXZ (2, 4 and 8
g/kg) decreased the expressions of NF-κB and CYP2E1 significantly (P<0.01), while expressions of PPARγ and ABCA1 (P<0.01) are significantly increased compared with model rats. These results above suggested that JTXZ reduce
body weight and blood lipid, and improve the liver steatosis of obese rats. The potential mechanism might be related
to down-regulating NF-κB & CYP2E1 and up-regulating PPARγ & ABCA1.
Keywords:Jiangtang Xiaozhi Tablet, obese rats, NF-κB, CYP2E1, PPARγ, ABCA1
Introduction
Increasing reports have indicated that obesity have become one of the most prevalent diseas-es of the world [1, 2]. Obdiseas-esity could cause a number of metabolic diseases, and in particu-lar obesity is closely associated with Type 2 Diabetes Mellitus (T2DM), which insulin resis-tance exists in both conditions [3, 4]. Fur- thermore, obesity is also the most direct rea-son of fatty liver disease [5]. Chinese herbal medicine and its prescriptions have been used in China for thousands years to treat various diseases [6, 7]. Jiangtang Xiaozhi tablet (JTXZ) is a standardized herbal formulation containing the following herbal medicines: Glossy Privet fruit (12 g), Astragalus membranaceus (6 g), Coptis chinensis (3 g), Semen Litchi (6 g), Euonymus alatus (4.5 g) and Curcuma longa (4.5 g). JTXZ was commonly used in clinically for treating T2DM and metabolic syndrome. Previous studies have found that JTXZ has
obvi-ous hypoglycemic effects on diabetes animals induced by alloxan and streptozotocin [8, 9]. Further experiments have also shown that JTXZ
has significant hypoglycemic and hypolipidemic
effects in KK-Ay obese diabetic mice and type 2 diabetic rats, and can markedly increase the numbers of pancreas islets and reduce adipo-cytes [10]. However, the detail mechanisms of JTXZ are still not clear. Therefore, our present study is designed to explore the effects of JTXZ
on the expressions of NF-κB, CYP2E1, PPARγ
and ABCA1 in livers of obese rats.
Materials and methods
Animals
animals were housed individually in an air-con-ditioned room (22-25°C; relative humidity: 45-60%) with free access of food and water within the Laboratory Animals Center of Xiyuan Hospital, China Academy of Chinese Medical Sciences, which is a Grade 2 animal breeding facility [License No.: SYXK (Beijing) 2009-0001]. Standard diet was provided by Beijing Keaoxieli Fodder Co., LTD [License No.: Beijing Fodder (ingredient) No. 238, executive standard GB14924, 3-2001]. High fat diet formula was prepared by Beijing Huafukang Biological Te- chnology Co., LTD consisting 10% of lard oil, 10% of sucrose, 1.5% of cholesterol, 0.5% of bile salt, 5% of egg yolk powder 5% and 73% of standard diet. All experiments conducted in this project were approved by the local animal eth-ics committee of our Hospital.
Chemicals and materials
Jiangtang Xiaozhi tablets (Batch number:
20100308) were provided by the Department of Pharmaceutical Chemistry, Basic Medical Institute, Xiyuan Hospital, China Academy of
[image:2.612.89.528.86.178.2]Chinese Medical Sciences. Dry powder was used in the experiments; each gram of dry pow-der consists of 5.4 g raw herbs. Metformin Hydrochloride Tablets: was obtained from Bei- jing Union Pharmaceutical Factory (0.25 g/tab-let; batch number: 101205). Simvastatin ob- tained from the Hangzhou MSD Pharmaceutical Company Limited (20 mg/tablet; batch num-ber: 09547). Biochemical kits were obtained from Biosino Bio-Technology and Science Incorporation (TC batch number: 111221, TG batch number: 114751). Other immunohisto-chemical reagents used were summarized in
Table 1.
Animal models and experimental design
70 SD male rats were randomly allocated to seven experimental groups (n=10): Control rats were fed standard diets, while the obese rats were established by high-fat fodder feeding for 18 weeks. Then obese rats were divided into Model group, Metformin group, Simvastatin group and 3 doses JTXZ (2, 4 and 8 g/kg, crude herb mass equivalent). Drugs were adminis-tered orally once a day for 2 months.
Body weight and blood lipid
Rats body weight changes of before and after modeling were recorded, and blood samples of before and after modeling were recorded were taken via cardiac puncture in animals to deter-mine the serum total cholesterol (TC) and tri-glyceride (TG).
Determination of NF-κB, CYP2E1, PPARγ and
ABCA1
Rats in each group were dissected for liver,
which was made into paraffin section with 4 μm thickness via fixation with 10% neutral formal -dehyde, dehydration with ethanol step by step,
hyalinization by xylene and paraffin embedding.
Then the immunohistochemical process was
Table 1. Immunohistochemical reagents used in our present study
Name Product code Batch number Provider
NF-κB ab31481 GR78568-1 Abcam Co.
CYP2E1 A628146 GA72736-1 Abcam Co.
NF-κB and CYP2E1 secondary antibody (MaxVisionTM) KIT-5020 1303144707E Fujian Maixin Biotech. Co.
DAB DAB-2031 1305312031 Fujian Maixin Biotech. Co.,
PPARγ ab27649 GR82388-1 Abcam Company
ABCA1 ab125064 GR82846-7 Abcam Company
PPARγ and ABCA1 secondary antibody and DAB (DaKo REALTM EnVisionTM) K5007 00091392 Beijing Xiya Jinqiao Biol. Tech. Co.
Table 2. Body weight changes in rats with/with-out high-fat diets feeding
Group n Baseline (g) After 18 weeks modeling (g) Control rats 10 129.74 ± 9.78 575.19 ± 46.43 High lipid rats 60 130.64 ± 6.16 635.60 ± 51.28**
Data are expressed as mean ± SD, **P<0.01, compared with
the control rats.
Table 3. Blood lipid changes in rats with/with-out high-fat diets feeding
Group n TC (mmol/L) TG (mmol/L)
Control rats 10 1.45 ± 0.21 1.16 ± 0.22
High lipid rats 60 2.15 ± 0.36** 1.31 ± 0.32*
Data are expressed as mean ± SD, *P<0.05, **P<0.01,
performed by staining with each antibody (Dilution 1:100). Then, the tissue sections were observed under microscope. Integral optical density (IOD) = Average optical density (AOD) × positive area (A).
Statistical analysis
All Data are presented as mean ± S.D, and were evaluated with one-way ANOVA and two-tailed Student’s t test between different groups.
The statistical significance of differences
be-tween groups was analyzed by using SPSS soft-ware (SPSS for Windows 18.0, SPSS Inc., USA),
and the differences were considered significant
at P<0.05.
Results
Results of the model establishment
As can be seen from Table 2, there is no signifi -cant difference in body weights between the control group and high lipid group before model establishment. However, after 18 weeks’ feed-ing with high-fat fodder, the rats’ body weights
obese rats
After the obese model rats were prepared, rats were treated with JTXZ at the doses of (2, 4 and 8 g/kg, crude herb mass equivalent) for 8 weeks. Before drug treatments, there was no difference in body weights among all the test-ing groups. Interesttest-ingly, our results showed th- at similar to the metformin (0.3 g/kg), the JTXZ
at the doses of 4 and 8 g/kg could significantly
decrease the body weight of obese rats (P< 0.05), compared with the model rats (Table 4).
Effects of JTXZ on blood lipid of obese rats
We can see from the Table 5 that our results
revealed that JTXZ (4 and 8 g/kg) significantly
decreased the TC of obese rats (P<0.05), com-pared with the model rats. In addition, the TG of the obese rats could be also show a decreasing trend by treating with JTXZ.
Effect of JTXZ on expressions of NF-κB and CYP2E1
Our present results showed that expressions of
NF-κB and CYP2E1 increased significantly
(P<0.001) in the model rats, compared with the rats in control group. Interestingly, as shown in
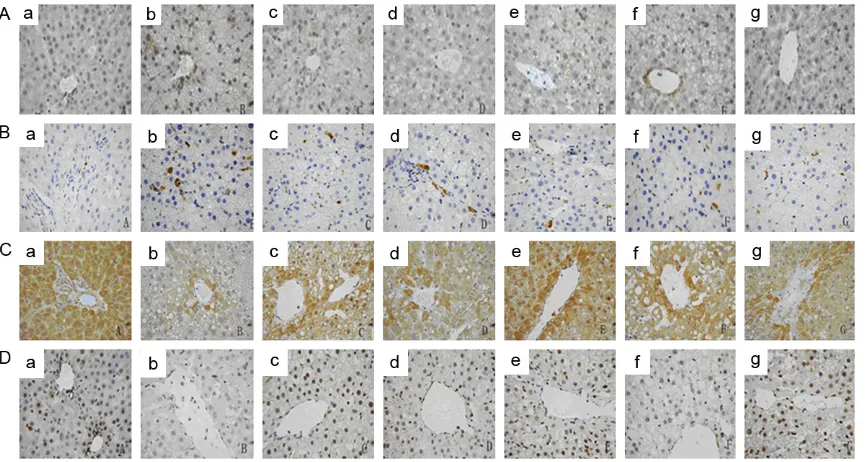
Table 6 and Figure 1A, 1B, JTXZ (2, 4 and 8 g/
kg, crude herb mass equivalent) significantly decreased the expressions of NF-κB (P<0.01)
and CYP2E1 (P<0.01), compared with the model rats, with a doses-dependent manner. Our
results suggested JTXZ possesses significant inhibitory effects on NF-κB and CYP2E1 in livers of obese rats.
Effect of JTXZ on expression of PPARγ and
ABCA1
In our present study [(Table 7; Figure 1C, 1D],
the expressions of PPARγ and ABCA were
sig-Table 4. Effects of JTXZ on body weight of obese rats
Groups Doses Before treatment (g) After treatment (g)
Control 576.58 ± 41.57 605.50 ± 41.39
Model 635.42 ± 62.38* 734.20 ± 66.61**
Metformin 0.3 g/kg 636.08 ± 58.76 667.10 ± 42.28#
Simvastatin 4 mg/kg 636.83 ± 54.41 704.90 ± 58.68
JTXZ 2 g/kg 635.58 ± 39.85 695.80 ± 53.17
4 g/kg 633.42 ± 43.69 672.00 ± 54.97#
8 g/kg 634.17 ± 57.69 641.90 ± 25.70##
Dose of JTXZ was represented as crude herb mass equivalent. Data are expressed as mean ± SD (n=10), *P<0.05, **P<0.01, compared with the
control rats; #P<0.05, ##P<0.01, compared with the model rats.
Table 5. Effects of JTXZ on blood lipid of obese rats
Groups Doses TC (mmol/L) TG (mmol/L)
Control 1.15 ± 0.21 0.43 ± 0.06
Model 1.64 ± 0.40** 0.61 ± 0.18**
Metformin 0.3 g/kg 1.38 ± 0.22* 0.49 ± 0.14
Simvastatin 4 mg/kg 1.24 ± 0.25# 0.58 ± 0.13**
JTXZ 2 g/kg 1.48 ± 0.24** 0.63 ± 0.13**
4 g/kg 1.30 ± 0.24# 0.59 ± 0.11**
8 g/kg 1.28 ± 0.33# 0.47 ± 0.13
Dose of JTXZ was represented as crude herb mass equiva-lent. Data are expressed as mean ± SD (n=10), *P<0.05, **P<0.01, compared with the control rats; #P<0.05.
of high lipid rats were significantly
greater than that of the control rats (P<0.001) (Table 2).
Our results, presented in Table 3, showed that there was no obvious dif-ference in rats between the two group; however, after 18-week high-fat diets’ feeding, TC (P<0.01) and TG (P<0.05)
levels were significantly higher than
that of the control rats. The results above suggested that the obese model rats were successfully established.
Table 6. Grayscales of NFκB and CYP2E1
Groups Doses NF-κB (IOD) CYP2E1 (IOD)
Control 61688.44 ± 34057.09 9867.88 ± 3437.17
Model 167297.44 ± 45886.61** 29349.73 ± 10955.71**
Metformin 30 mg/kg 109302.03 ± 38047.52## 17618.89 ± 5180.88##
Simvastatin 4 mg/kg 108050.35 ± 35325.91## 19917.70 ± 7366.51##
JTXZ 2 g/kg 96104.76 ± 32033.91## 16389.65 ± 4757.06##
4 g/kg 135679.87 ± 55025.26## 24203.79 ± 6827.15##
8 g/kg 117037.68 ± 34849.37## 19700.33 ± 5681.56##
Integral optical density (IOD) = Average optical density (AOD) × positive area (A). Dose of JTXZ was represented as crude herb mass equivalent. Data are expressed as mean ± SD (n=10), **P<0.01, compared with the control rats; #P<0.05, ##P<0.01,
[image:4.612.93.527.248.479.2]compared with the model rats.
Figure 1. Effects of JTXZ on expressions of NF-κB (A), CYP2E1 (B), PPARγ (C) and ABCA1 (D) in obese rats’ liver (×40). (a-g) represented the Control group, Model group, Metformin group, Simvastain group and JTXZ groups (2, 4 and 8 g/kg, crude herb mass equivalent), respectively.
Table 7. Grayscales of PPARγ and ABCA1
Groups Dose PPARγ (IOD) ABCA1 (IOD)
Control 772644.30 ± 217462.79 110327.13 ± 48590.24
Model 510345.36 ± 239765.58** 68230.17 ± 43590.37**
Metformin 30 mg/kg 620230.73 ± 124459.16## 100453.50 ± 48839.81##
Simvastatin 4 mg/kg 650431.79 ± 161080.01## 95152.72 ± 44817.41##
JTXZ 2 g/kg 646398.05 ± 149053.07## 96827.80 ± 40401.22##
4 g/kg 641288.81 ± 108764.48## 93641.09 ± 43456.52##
8 g/kg 754298.41 ± 126515.12## 98970.79 ± 37725.83##
Integral optical density (IOD) = Average optical density (AOD) × positive area (A). Dose of JTXZ was represented as crude herb mass equivalent. Data are expressed as mean ± SD (n=10), **P<0.01, compared with the control rats; #P<0.05, ##P<0.01,
[image:4.612.90.521.558.666.2]nificantly decreased (P<0.001) in the model
group compared with those of the control group. However, JTXZ treatments (2, 4 and 8 g/
kg, crude herb mass equivalent) significantly up-regulated the expression of PPARγ (P<0.01)
and ABCA1 (P<0.01) compared with those of the model group (P<0.01), with a doses-depen-dent manner, suggesting that JRXZ has obvi-ous up-regulating effects on expressions of
PPARγ and ABCA1 in livers of obese rats.
Discussions
Obesity is defined as excessive fat tissue accu -mulation and is one of the important risk fac-tors of T2DM [3]. Obesity-induced nonalcoholic fatty liver disease (NAFLD) is most commonly, which reaches 50% of the morbidity in T2DM patients and nearly 100% in the crowd with dia-betes accompanied with obesity. Fatty liver is one of the clinical pathological syndromes given prior to diffuse macro-vesicular steatosis in lobular liver cell and hepatocell [5, 11].
NF-κB is a widespread kind of eukaryotic cells
transcription factors, which can be activated by oxidative stress via inducing expression of Fas ligand on liver cell membrane surface and plays a key control role in the induction of
inflammatory factor expression [12, 13]. In
addition, CYP2E1 is a main source for lipid per-oxide in vivo. Its high expression could enhance oxidation reaction in the liver cells, thereby causing liver cell membrane lipid peroxidation; therefore, it decreases the protection role of antioxidant system and damage hepatocyte [14, 15], thus causing hepatocyte fatty
degen-eration and necrosis. PPAR-γ is mainly
expressed in adipose tissue and liver, which can promote fat cell differentiation. It is acti-vated by a variety of fatty acid and its
deriva-tives. The activated PPAR-γ regulates the utili -zation of saccharides in the muscle tissue through affecting the translocase in the adi-pose tissue [16]. ABCA1 is a member of the ATP-binding cassette transporter superfamily, which is mainly involved in the reversal of the cholesterol. It undertakes an important task in cholesterol scavenging of macrophages, thus clearing the excessive cholesterol [17, 18]. The rats are fed with high fat fodder for 18 weeks in our experiment. The obese rat model is successfully established when the rat BWs,
TC and TG are significantly increased. After
8-week drugs treatment, high and medium
dose groups have significantly lower body
weight, obese symptoms, and TC and TG levels than those of the model group. In each dose
group, liver NF-κB and CYP2E1 expressions are
obviously decreased, while liver PPARγ and
ABCA1 expressions are obviously increased, suggesting JTXZ can inhibit the activation of
NF-κB and reduce lipid peroxidation, thus reducing inflammation and liver steatosis.
Up-regulation of ABCA1 and PPAR-γ expression
can also promote that JTXZ can active PPAR-γ,
inhibit expression of inflammatory cytokines,
enhance the transcription of ABCA1, thus elimi-nating excessive cholesterol in the organization and reducing liver lesions.
In conclusion, JTXZ can significantly reduce the
body weight and blood lipid and improve the liver steatosis of obese rats. Its mechanism may be related to the down-regulation
expres-sion of liver NF-κB and CYP2E1, and reduction of lipid peroxidation damage; it may also be
relat-ed with up-regulation expression of PPARγ and
ABCA1, promotion of adipocyte differentiation, increased insulin sensitivity and improvement of IR.
Acknowledgements
This work was supported by the National Major
Scientific and Technological Special Project for “Significant New Drugs Development” (No.
2010ZX09102-213, 2012ZX09103201-040) and Beijing Natural Science Foundation project (No. 7154233), and National Basic Research Program (No. 2015CB554400).
Disclosure of conflict of interest
None.
Address correspondence to: Dr. Jianxun Liu, In- stitute of Basic Medical Sciences, Xiyuan Hospital CACMS, 1 Xiyuan Playground, Beijing 100091, Chi- na. Tel: +86-10-62835601; Fax: +86-10-62874049; E-mail: jianxliu_xh@126.com
References
[1] Llewellyn A, Simmonds M, Owen CG and Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev 2015; 17: 56-67. [2] Vedovato GM, Surkan PJ, Jones-Smith J,
Git-telsohn J. Food insecurity, overweight and obe-sity among low-income African-American fami-lies in Baltimore City: associations with food-related perceptions. Public Health Nutr 2015; 6: 1-12.
[3] Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes 2013; 6: 327-338.
[4] Jenkins AB, Batterham M, Samocha-Bonet D,
Tonks K, Greenfield JR, Campbell LV. Segrega -tion of a Latent High Adiposity Phenotype in Families with a History of Type 2 Diabetes Mel-litus Implicates Rare Obesity-Susceptibility Ge-netic Variants with Large Effects in Diabetes-Related Obesity. PLoS One 2013; 8: e70435. [5] Chi JM, Wang Y, Zhou YS. Practical diabetes.
Beijing: People’s Medical Publishing House; 2009; 36-42: 533-554.
[6] Patwardhan B. Ethnopharmacology and drug discovery. J Ethnopharmacol 2005; 100: 50-52.
[7] Peng W, Hu CL, Shu ZH, Han T, Qin LP, Zheng CJ. Antitumor Activity of Tatariside F isolated from roots of Fagopyrum tataricum (L.) Gaertn against H22 hepatocellular carcinoma via up-regulation of p53. Phytomed 2015; 22: 730-736.
[8] Ge ZY, Jin L, Run AG. Study of Jiangtang Xiaozhi Tablet on Decreasing the Levels of Blood Glu-cose in Animal with Diabetes Mellitus. Chin J Exp Trad Med Form 2010; 16: 143.
[9] Yan AG, Ge ZY, Liu JX. Effect of Jiangtang Xiaozhi Capsule on Morphological Changes of Islet and Liver in Rat Model of Type 2 Diabetic Mellitus. Chin J Chin Materia Med 2008; 33: 1067.
[10] Ge ZY, Jin L, Guo YJ. Experimental Study of Ji-angtang Xiaozhi Tablet on Decreasing the Lev-els of Blood Glucose and Serum Lipids in Transgenic Mice with Diabetes Mellitus. Chin J Integr Trad West Med 2012; 32: 1095.
[11] Wanezaki S, Tachibana N, Nagata M. Soy
β-conglycinin improves obesity-induced meta -bolic abnormalities in a rat model of nonalco-holic fatty liver disease. Obes Res Clin Pract 2015; 9: 168-174.
[12] de la Fuente V, Federman N, Zalcman G, Salles
A, Freudenthal R, Romano A. NF-κB transcrip -tion factor role in consolida-tion and reconsoli-dation of persistent memories. Front Mol Neu-rosci 2015; 8: 50.
[13] Wang XW, Huang L, Chen W. Mechanism of iron metabolism in nonalcoholic fatty liver
dis-ease adjusted by TLR4/Nf-κB signal pathway.
Appl J Gener Prac 2014; 12: 384.
[14] Rouach H, Fataccioli V, Gentil M. Effect of chronic ethanol feeding oil lipid peroxidation and protein oxidation in relation to liver pathol-ogy. Hepatol 1997; 25: 351-355.
[15] van de Wier B, Balk JM, Bast A, Koek GH, Hae-nen GR. Chemical characteristics for optimiz-ing CYP2E1 inhibition. Chem Biol Interact 2015; 242: 139-144.
[16] Chen YX, Wang WM, Zhou T. Function of PPAR-γ
and Related Signaling Pathways. Chin J Cell Biol 2006; 28: 382.
[17] Oram JF. HDL apolipoprteins and ABCA1 part-ners in the removal of excess cellular choles-terol. Arterioscler Thromb Vasc Biol 2003; 23: 720-727.