organic papers
Acta Cryst.(2006). E62, o1751–o1753 doi:10.1107/S1600536806011962 Vicarelet al. C
8H8N4O3S
o1751
Acta Crystallographica Section EStructure Reports
Online
ISSN 1600-5368
4-Acetamidobenzenesulfonyl azide
Monica L. Vicarel, Peter Norris and Matthias Zeller*
Department of Chemistry, Youngstown State University, 1 University Plaza, Youngstown, OH 44555-3663, USA
Correspondence e-mail: mzeller@cc.ysu.edu
Key indicators
Single-crystal X-ray study T= 100 K
Mean(C–C) = 0.003 A˚ Rfactor = 0.039 wRfactor = 0.099
Data-to-parameter ratio = 16.8
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 27 March 2006 Accepted 2 April 2006
#2006 International Union of Crystallography All rights reserved
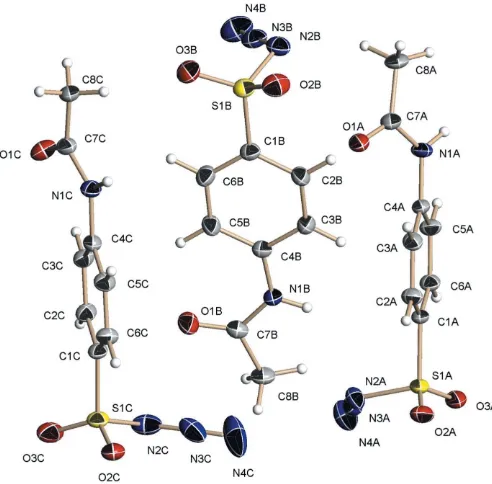
The title compound, C8H8N4O3S, is planar chiral in the solid state. It crystallizes with three independent molecules in the asymmetric unit, two of which have the same chirality and the third is the opposite enantiomer. Partial inversion twinning is present in the crystal which was examined. All three molecules are connectedviahydrogen bonds between the amide groups, forming a helix around thebaxis of the unit cell.
Comment
4-Acetamidobenzenesulfonyl azide (p-ABSA), (I), formed by the reaction of the corresponding sulfonyl chloride with sodium azide (Baumet al., 1987), is a commercially available reagent widely used for the base-assisted transfer of the diazo group to active methylene compounds, such as-keto esters (Davieset al., 1987; Baumet al., 1987; Taberet al., 2005).
Compound (I) crystallizes in the chiral monoclinic space group P21 with three crystallographically independent mol-ecules in the asymmetric unit. With two prochiral groups (the amide and the SO2N3groups) bonded to a planar core, thep -ABSA molecules are planar chiral. In solution, thepRandpS
enantiomers can interconvert rapidly by rotation of either of the substituents. In the solid state, however, this equilibration is frozen. Of the three independent molecules,AandChave the same sense of chirality; moleculeB, on the other hand, is in the opposite enantiomeric form, as expressed by the orienta-tion of the amide groups with respect to the posiorienta-tion of the azide units (Fig. 1). Not only the general orientation of the substituents between the two enantiomeric forms, but also the overall geometrical features of all three molecules are distinctly different from each other, as expressed mainly by the torsion angles of both the amide as well as the SO2N3 groups with respect to the aromatic six-membered rings.
The orientation of the S—N3units for moleculesA,Band
C, calculated as the dihedral angle between the aromatic ring and the plane formed by atoms C1, S1 and N2, are 83.60 (8), 69.25 (8), and 59.68 (8), respectively. Thus, for moleculesC
six-membered ring, but for moleculeA, the two S O bonds are rotated out of this plane by 13.1 (2) and 31.31 (2)
(see Table 1 for a more exhaustive list of torsion angles). The tilt of the amide units also varies significantly. For moleculeB, it is 9.77 (10), thus allowing for significant overlap between
theorbitals of the aromatic ring and the amide group. This is significantly diminished for molecule C, with a tilt angle of 18.65 (9), and for moleculeA, with a tilt angle of 32.99 (8), it
has to be assumed that the overlap and delocalization is even less pronounced.
The loss of delocalization energy seems to be compensated for by the formation of hydrogen bonds between the amide units of all three molecules (Fig. 2 and Table 2). The hydrogen bonds observed here are in the usual range for strong amide-to-amide hydrogen bonds, and, as is often observed for amides and formamides and other related systems, the ability to form strong hydrogen bonds is the determining factor behind the type of packing realised in the solid state (Zelleret al., 2005). Forp-ABSA, the hydrogen bonds connect all three molecules, forming an infinite chain arranged in a helix-like fashion around the direction of thebaxis of the unit cell (Fig. 2). This type of hydrogen-bonding interaction also allows moleculesA
andCto pair up to form-stacked dimers with a centroid-to-centroid distance of 3.781 (1) A˚ , thus further energetically favouring the complicated molecular packing observed forp -ABSA (symmetry code for moleculeC: 1x,1
2+y, 1z).
Experimental
Compound (I) was purchased from Aldrich Chemicals and single crystals were grown from a solution in ethanol as large colourless blocks by slow evaporation of the solvent.
Crystal data C8H8N4O3S Mr= 240.24
Monoclinic,P21 a= 8.0529 (5) A˚
b= 22.988 (1) A˚
c= 8.3123 (5) A˚
= 93.534 (1) V= 1535.85 (15) A˚3
Z= 6
Dx= 1.559 Mg m
3
MoKradiation
= 0.31 mm1 T= 100 (2) K Block, colourless 0.530.450.40 mm
Data collection
Bruker SMART APEX CCD diffractometer
!scans
Absorption correction: multi-scan (SADABSinSAINT-Plus; Bruker, 2003)
Tmin= 0.810,Tmax= 0.882
14335 measured reflections 7326 independent reflections 7096 reflections withI> 2(I)
Rint= 0.021 max= 28.3
Refinement Refinement onF2 R[F2> 2(F2)] = 0.039 wR(F2) = 0.099
S= 1.07 7326 reflections 437 parameters
H-atom parameters constrained
w= 1/[2
(Fo 2
) + (0.0655P)2 + 0.1628P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.65 e A˚
3
min=0.23 e A˚
3
Absolute structure: Flack (1983), 3450 Friedel pairs
[image:2.610.46.292.71.313.2]Flack parameter: 0.33 (4)
Table 1
Selected geometric parameters (A˚ ,).
C7A—O1A 1.232 (3) C7A—N1A 1.363 (3) C7B—O1B 1.220 (3) C7B—N1B 1.360 (3) C7C—O1C 1.217 (3) C7C—N1C 1.369 (3) N2A—N3A 1.244 (3) N2A—S1A 1.7034 (19) N3A—N4A 1.125 (3) N2B—N3B 1.251 (3) N2B—S1B 1.706 (2)
N3B—N4B 1.129 (3) N2C—N3C 1.246 (4) N2C—S1C 1.695 (2) N3C—N4C 1.112 (4) O2A—S1A 1.4266 (17) O3A—S1A 1.4287 (18) O2B—S1B 1.4266 (18) O3B—S1B 1.4320 (18) O2C—S1C 1.4284 (16) O3C—S1C 1.4217 (18)
N3A—N2A—S1A 112.86 (15) N4A—N3A—N2A 173.8 (2) N3B—N2B—S1B 112.81 (17)
N4B—N3B—N2B 173.5 (3) N3C—N2C—S1C 112.69 (18) N4C—N3C—N2C 174.1 (3)
C2A—C1A—S1A—O2A 13.1 (2) C6A—C1A—S1A—O3A 31.31 (19) C2B—C1B—S1B—O2B 40.0 (2)
C6B—C1B—S1B—O3B 6.3 (2) C2C—C1C—S1C—O3C 48.4 (2) C6C—C1C—S1C—O2C 5.1 (2)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
N1B—H1B O1Ai
0.88 2.06 2.913 (2) 162 N1A—H1A O1Cii 0.88 2.03 2.907 (2) 172 N1C—H1C O1Biii
0.88 2.07 2.929 (2) 165
Symmetry codes: (i)x1;y;z; (ii)xþ1;y1
2;z; (iii)xþ1;y;z.
Partial inversion twinning is present in the crystal which was examined; the Flack parameter (Flack, 1983) refined to 0.33 (4). All H atoms were placed in calculated positions (N—H = 0.88A˚ and C— H = 0.95–0.98A˚ ). They were refined with isotropic displacement parameters of 1.5 (methyl) or 1.2 (all others) times that of the
organic papers
o1752
Vicarelet al. C8H8N4O3S Acta Cryst.(2006). E62, o1751–o1753
Figure 1
[image:2.610.313.566.390.571.2]equivalent isotropic displacement parameter of the parent C or N atom. Methyl H atoms were allowed to rotate to best fit the observed electron density. The s.u. values of the cell parameters are taken from the software, recognizing that the values are unreasonably small (Herbstein, 2000).
Data collection:SMART(Bruker, 2002); cell refinement: SAINT-Plus(Bruker, 2003); data reduction:SAINT-Plus; program(s) used to solve structure:SHELXTL(Bruker, 2000); program(s) used to refine structure:SHELXTL; molecular graphics:SHELXTL; software used to prepare material for publication:SHELXTL.
Funding for this research came from the American Chemical Society Petroleum Research Fund (No. PRF 43948-B1) and the diffractometer was funded by NSF grant No. 0087210, by Ohio Board of Regents grant CAP-491, and by YSU.
References
Baum, J. S., Shook, D. A., Davies, H. M. L. & Smith, H. D. (1987).Synth. Commun.17, 1709–1716.
Bruker (2000). SHELXTL. Version 6.14. Bruker AXS Inc, Madison, Wisconsin, USA.
Bruker (2002). SMART for WNT/2000. Version 5.630. Bruker AXS Inc, Madison, Wisconsin, USA.
Bruker (2003). SAINT-Plus. Version 6.45. Bruker AXS Inc, Madison, Wisconsin, USA.
Davies, H. M. L., Smith, H. D. & Korkor, O. (1987).Tetrahedron Lett.28, 1853– 1856.
Flack, H. D. (1983).Acta Cryst.A39, 876–881. Herbstein, F. H. (2000).Acta Cryst.B56, 547–557.
Taber, D. F., Sheth, R. B. & Joshi, P. V. (2005).J. Org. Chem.70, 2851–2854. Zeller, M., Wilcox, R. J., Snyder, F. G., Seibel, H. A., Takas, N. J. & Hunter,
A. D. (2005).J. Chem. Crystallogr.35, 723–729.
organic papers
Acta Cryst.(2006). E62, o1751–o1753 Vicarelet al. C
8H8N4O3S
o1753
Figure 2supporting information
sup-1 Acta Cryst. (2006). E62, o1751–o1753
supporting information
Acta Cryst. (2006). E62, o1751–o1753 [https://doi.org/10.1107/S1600536806011962]
4-Acetamidobenzenesulfonyl azide
Monica L. Vicarel, Peter Norris and Matthias Zeller
4-(Acetylamino)benzenesulfonyl azide
Crystal data
C8H8N4O3S
Mr = 240.24
Monoclinic, P21
Hall symbol: P 2yb
a = 8.0529 (5) Å
b = 22.988 (1) Å
c = 8.3123 (5) Å
β = 93.534 (1)°
V = 1535.85 (15) Å3
Z = 6
F(000) = 744
Dx = 1.559 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 6077 reflections
θ = 2.6–30.5°
µ = 0.31 mm−1
T = 100 K Block, colourless 0.53 × 0.45 × 0.40 mm
Data collection
Bruker SMART APEX CCD diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan
(SADABS in SAINT-Plus; Bruker, 2003)
Tmin = 0.810, Tmax = 0.882
14335 measured reflections 7326 independent reflections 7096 reflections with I > 2σ(I)
Rint = 0.021
θmax = 28.3°, θmin = 1.8°
h = −10→10
k = −30→30
l = −11→10
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.099
S = 1.07 7326 reflections 437 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0655P)2 + 0.1628P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.65 e Å−3
Δρmin = −0.23 e Å−3
Absolute structure: Flack (1983), 3450 Friedel pairs
supporting information
sup-2 Acta Cryst. (2006). E62, o1751–o1753
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C1A 0.3370 (3) 0.31132 (8) 0.5921 (2) 0.0224 (4)
C2A 0.5081 (3) 0.31502 (10) 0.6216 (2) 0.0263 (4)
H2A 0.5546 0.3210 0.7281 0.032*
C3A 0.6107 (3) 0.30992 (9) 0.4949 (2) 0.0255 (4)
H3A 0.7281 0.3120 0.5137 0.031*
C4A 0.5397 (3) 0.30171 (8) 0.3389 (2) 0.0235 (4)
C5A 0.3682 (3) 0.29655 (9) 0.3112 (2) 0.0269 (4)
H5A 0.3214 0.2896 0.2052 0.032*
C6A 0.2655 (3) 0.30150 (9) 0.4384 (2) 0.0242 (4)
H6A 0.1483 0.2982 0.4206 0.029*
C7A 0.7833 (3) 0.32513 (10) 0.1856 (3) 0.0294 (4)
C8A 0.8579 (3) 0.31486 (12) 0.0270 (3) 0.0357 (5)
H8A 0.8401 0.3492 −0.0417 0.054*
H8B 0.8048 0.2810 −0.0261 0.054*
H8C 0.9775 0.3077 0.0453 0.054*
C1B 0.3907 (2) 0.48812 (9) 0.0297 (2) 0.0245 (4)
C2B 0.3381 (3) 0.43565 (9) 0.0934 (3) 0.0270 (4)
H2B 0.4003 0.4010 0.0807 0.032*
C3B 0.1943 (3) 0.43488 (9) 0.1750 (3) 0.0278 (4)
H3B 0.1571 0.3993 0.2187 0.033*
C4B 0.1024 (2) 0.48576 (9) 0.1942 (2) 0.0237 (4)
C5B 0.1593 (3) 0.53834 (9) 0.1359 (3) 0.0290 (4)
H5B 0.1006 0.5734 0.1534 0.035*
C6B 0.3036 (3) 0.53906 (9) 0.0514 (3) 0.0294 (4)
H6B 0.3420 0.5746 0.0086 0.035*
C7B −0.1563 (3) 0.52171 (9) 0.3099 (2) 0.0262 (4)
C8B −0.3134 (3) 0.49950 (11) 0.3806 (3) 0.0311 (4)
H8D −0.3062 0.5060 0.4974 0.047*
H8E −0.3255 0.4578 0.3587 0.047*
H8F −0.4099 0.5203 0.3316 0.047*
C1C 0.2330 (2) 0.66284 (9) 0.5771 (2) 0.0234 (4)
C2C 0.1854 (3) 0.67538 (9) 0.4179 (2) 0.0253 (4)
H2C 0.0732 0.6854 0.3885 0.030*
C3C 0.3013 (3) 0.67338 (9) 0.3012 (2) 0.0256 (4)
supporting information
sup-3 Acta Cryst. (2006). E62, o1751–o1753
C4C 0.4670 (3) 0.65910 (8) 0.3472 (2) 0.0237 (4)
C5C 0.5135 (3) 0.64594 (9) 0.5082 (3) 0.0262 (4)
H5C 0.6254 0.6357 0.5382 0.031*
C6C 0.3967 (3) 0.64786 (10) 0.6241 (2) 0.0264 (4)
H6C 0.4277 0.6391 0.7335 0.032*
C7C 0.5923 (3) 0.68068 (9) 0.0876 (2) 0.0268 (4)
C8C 0.7509 (3) 0.67167 (10) 0.0028 (3) 0.0322 (4)
H8G 0.7267 0.6738 −0.1140 0.048*
H8H 0.7976 0.6334 0.0312 0.048*
H8I 0.8313 0.7020 0.0362 0.048*
N1A 0.6380 (2) 0.29638 (8) 0.2051 (2) 0.0275 (4)
H1A 0.6025 0.2725 0.1278 0.033*
N2A 0.1472 (2) 0.38905 (8) 0.7433 (2) 0.0298 (4)
N3A 0.0025 (3) 0.39598 (8) 0.6851 (2) 0.0290 (4)
N4A −0.1280 (3) 0.40705 (10) 0.6390 (3) 0.0438 (5)
N1B −0.0471 (2) 0.47950 (8) 0.2718 (2) 0.0248 (3)
H1B −0.0732 0.4438 0.2990 0.030*
N2B 0.5014 (2) 0.45126 (9) −0.2554 (2) 0.0327 (4)
N3B 0.4002 (2) 0.47963 (10) −0.3445 (2) 0.0343 (4)
N4B 0.3078 (3) 0.50072 (13) −0.4341 (3) 0.0506 (6)
N1C 0.5929 (2) 0.65573 (8) 0.2372 (2) 0.0259 (4)
H1C 0.6817 0.6354 0.2681 0.031*
N2C −0.0633 (3) 0.62023 (11) 0.6648 (2) 0.0398 (5)
N3C −0.0303 (3) 0.56989 (11) 0.7122 (3) 0.0435 (5)
N4C −0.0145 (4) 0.52391 (13) 0.7518 (5) 0.0750 (10)
O1A 0.8469 (2) 0.35857 (7) 0.2876 (2) 0.0315 (3)
O2A 0.3014 (2) 0.31341 (8) 0.90065 (18) 0.0345 (4)
O3A 0.0612 (2) 0.28276 (7) 0.7193 (2) 0.0338 (4)
O1B −0.1314 (2) 0.57361 (7) 0.2926 (2) 0.0362 (4)
O2B 0.6906 (2) 0.45011 (9) −0.0149 (2) 0.0393 (4)
O3B 0.6056 (2) 0.54647 (8) −0.1286 (2) 0.0370 (4)
O1C 0.4759 (2) 0.70885 (8) 0.0289 (2) 0.0351 (4)
O2C 0.1595 (2) 0.65156 (7) 0.87722 (18) 0.0299 (3)
O3C 0.0018 (3) 0.72287 (8) 0.7077 (2) 0.0443 (5)
S1A 0.20593 (6) 0.31786 (2) 0.75080 (6) 0.02521 (11)
S1B 0.56587 (6) 0.48809 (2) −0.08468 (6) 0.02667 (11)
S1C 0.08692 (6) 0.66879 (2) 0.72344 (6) 0.02662 (11)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C1A 0.0267 (10) 0.0207 (9) 0.0212 (8) −0.0005 (7) 0.0116 (7) 0.0000 (7)
C2A 0.0272 (10) 0.0303 (10) 0.0219 (8) −0.0021 (8) 0.0052 (7) −0.0030 (8)
C3A 0.0212 (9) 0.0269 (10) 0.0290 (9) −0.0033 (8) 0.0064 (7) −0.0036 (8)
C4A 0.0267 (10) 0.0216 (8) 0.0233 (9) −0.0032 (7) 0.0116 (7) −0.0010 (7)
C5A 0.0292 (11) 0.0328 (10) 0.0193 (9) −0.0039 (8) 0.0058 (8) −0.0015 (7)
C6A 0.0203 (9) 0.0284 (9) 0.0246 (9) −0.0012 (7) 0.0066 (7) −0.0005 (7)
supporting information
sup-4 Acta Cryst. (2006). E62, o1751–o1753
C8A 0.0368 (12) 0.0398 (12) 0.0329 (10) −0.0041 (10) 0.0218 (9) −0.0004 (10)
C1B 0.0222 (9) 0.0285 (10) 0.0233 (8) −0.0022 (8) 0.0041 (7) −0.0006 (8)
C2B 0.0273 (11) 0.0237 (9) 0.0308 (10) 0.0021 (8) 0.0070 (8) −0.0027 (8)
C3B 0.0306 (11) 0.0229 (9) 0.0308 (10) −0.0012 (8) 0.0086 (8) 0.0017 (8)
C4B 0.0229 (9) 0.0266 (9) 0.0219 (8) 0.0003 (8) 0.0041 (7) −0.0010 (7)
C5B 0.0261 (11) 0.0230 (9) 0.0387 (11) 0.0025 (8) 0.0085 (9) −0.0019 (8)
C6B 0.0265 (11) 0.0255 (10) 0.0369 (11) −0.0019 (8) 0.0078 (9) 0.0037 (8)
C7B 0.0244 (10) 0.0317 (10) 0.0227 (9) 0.0021 (8) 0.0032 (7) −0.0020 (8)
C8B 0.0254 (10) 0.0400 (12) 0.0289 (10) 0.0010 (9) 0.0098 (8) −0.0002 (9)
C1C 0.0205 (9) 0.0247 (9) 0.0260 (9) 0.0023 (7) 0.0098 (7) 0.0024 (7)
C2C 0.0193 (9) 0.0284 (10) 0.0288 (10) 0.0027 (8) 0.0050 (7) 0.0042 (8)
C3C 0.0257 (10) 0.0280 (9) 0.0234 (9) 0.0015 (8) 0.0037 (7) 0.0031 (8)
C4C 0.0245 (10) 0.0221 (9) 0.0252 (9) −0.0001 (7) 0.0077 (7) −0.0009 (7)
C5C 0.0195 (10) 0.0306 (10) 0.0290 (10) 0.0049 (8) 0.0042 (8) 0.0009 (8)
C6C 0.0259 (10) 0.0326 (10) 0.0210 (9) 0.0051 (8) 0.0042 (7) 0.0018 (7)
C7C 0.0284 (10) 0.0277 (10) 0.0251 (10) −0.0057 (8) 0.0086 (8) −0.0024 (7)
C8C 0.0381 (12) 0.0284 (10) 0.0321 (10) −0.0030 (9) 0.0189 (9) 0.0001 (9)
N1A 0.0296 (9) 0.0317 (9) 0.0226 (8) −0.0046 (7) 0.0121 (7) −0.0056 (7)
N2A 0.0247 (9) 0.0242 (8) 0.0409 (10) 0.0005 (7) 0.0051 (8) −0.0069 (7)
N3A 0.0340 (11) 0.0265 (9) 0.0271 (9) −0.0026 (7) 0.0062 (8) −0.0038 (7)
N4A 0.0365 (12) 0.0380 (11) 0.0553 (14) 0.0031 (9) −0.0088 (10) −0.0104 (10)
N1B 0.0254 (8) 0.0236 (8) 0.0263 (8) −0.0011 (7) 0.0085 (6) 0.0002 (6)
N2B 0.0293 (10) 0.0384 (10) 0.0313 (10) −0.0007 (8) 0.0078 (8) −0.0049 (8)
N3B 0.0306 (10) 0.0449 (11) 0.0281 (9) −0.0089 (9) 0.0077 (8) −0.0012 (8)
N4B 0.0442 (13) 0.0708 (17) 0.0362 (11) −0.0101 (12) −0.0034 (10) 0.0075 (11)
N1C 0.0233 (9) 0.0282 (9) 0.0270 (8) 0.0036 (7) 0.0087 (7) 0.0005 (6)
N2C 0.0261 (10) 0.0624 (15) 0.0311 (10) −0.0050 (9) 0.0041 (8) −0.0051 (9)
N3C 0.0248 (10) 0.0494 (14) 0.0574 (14) −0.0062 (9) 0.0099 (9) −0.0252 (11)
N4C 0.0433 (16) 0.0376 (15) 0.145 (3) −0.0110 (12) 0.0122 (18) −0.0275 (16)
O1A 0.0323 (9) 0.0286 (7) 0.0352 (8) −0.0064 (6) 0.0149 (6) −0.0034 (6)
O2A 0.0431 (9) 0.0380 (8) 0.0237 (7) 0.0035 (8) 0.0117 (6) −0.0003 (6)
O3A 0.0363 (9) 0.0304 (8) 0.0368 (9) −0.0072 (7) 0.0188 (7) −0.0048 (6)
O1B 0.0318 (9) 0.0282 (8) 0.0502 (10) 0.0040 (7) 0.0144 (7) −0.0018 (7)
O2B 0.0260 (9) 0.0507 (10) 0.0416 (9) 0.0080 (7) 0.0054 (7) 0.0056 (8)
O3B 0.0307 (9) 0.0372 (9) 0.0439 (9) −0.0079 (7) 0.0097 (7) 0.0006 (7)
O1C 0.0323 (9) 0.0441 (9) 0.0295 (8) −0.0046 (7) 0.0053 (6) 0.0095 (7)
O2C 0.0285 (8) 0.0367 (8) 0.0254 (7) 0.0000 (6) 0.0084 (6) 0.0005 (6)
O3C 0.0467 (11) 0.0444 (10) 0.0442 (10) 0.0197 (8) 0.0216 (9) 0.0101 (8)
S1A 0.0292 (3) 0.0244 (2) 0.0234 (2) −0.00106 (19) 0.01256 (18) −0.00172 (17)
S1B 0.0198 (2) 0.0318 (3) 0.0288 (2) −0.00115 (19) 0.00476 (17) 0.00076 (19)
S1C 0.0237 (2) 0.0319 (2) 0.0253 (2) 0.0045 (2) 0.00933 (18) 0.00261 (19)
Geometric parameters (Å, º)
C1A—C2A 1.387 (3) C1C—C2C 1.385 (3)
C1A—C6A 1.387 (3) C1C—C6C 1.395 (3)
C1A—S1A 1.7459 (18) C1C—S1C 1.7490 (19)
supporting information
sup-5 Acta Cryst. (2006). E62, o1751–o1753
C2A—H2A 0.95 C2C—H2C 0.95
C3A—C4A 1.397 (3) C3C—C4C 1.405 (3)
C3A—H3A 0.95 C3C—H3C 0.95
C4A—C5A 1.392 (3) C4C—C5C 1.400 (3)
C4A—N1A 1.409 (2) C4C—N1C 1.408 (3)
C5A—C6A 1.386 (3) C5C—C6C 1.388 (3)
C5A—H5A 0.95 C5C—H5C 0.95
C6A—H6A 0.95 C6C—H6C 0.95
C7A—O1A 1.232 (3) C7C—O1C 1.217 (3)
C7A—N1A 1.363 (3) C7C—N1C 1.369 (3)
C7A—C8A 1.501 (3) C7C—C8C 1.511 (3)
C8A—H8A 0.98 C8C—H8G 0.98
C8A—H8B 0.98 C8C—H8H 0.98
C8A—H8C 0.98 C8C—H8I 0.98
C1B—C6B 1.383 (3) N1A—H1A 0.88
C1B—C2B 1.393 (3) N2A—N3A 1.244 (3)
C1B—S1B 1.749 (2) N2A—S1A 1.7034 (19)
C2B—C3B 1.377 (3) N3A—N4A 1.125 (3)
C2B—H2B 0.95 N1B—H1B 0.88
C3B—C4B 1.399 (3) N2B—N3B 1.251 (3)
C3B—H3B 0.95 N2B—S1B 1.706 (2)
C4B—C5B 1.391 (3) N3B—N4B 1.129 (3)
C4B—N1B 1.407 (3) N1C—H1C 0.88
C5B—C6B 1.394 (3) N2C—N3C 1.246 (4)
C5B—H5B 0.95 N2C—S1C 1.695 (2)
C6B—H6B 0.95 N3C—N4C 1.112 (4)
C7B—O1B 1.220 (3) O2A—S1A 1.4266 (17)
C7B—N1B 1.360 (3) O3A—S1A 1.4287 (18)
C7B—C8B 1.516 (3) O2B—S1B 1.4266 (18)
C8B—H8D 0.98 O3B—S1B 1.4320 (18)
C8B—H8E 0.98 O2C—S1C 1.4284 (16)
C8B—H8F 0.98 O3C—S1C 1.4217 (18)
C2A—C1A—C6A 121.59 (17) C1C—C2C—C3C 120.20 (19)
C2A—C1A—S1A 120.12 (15) C1C—C2C—H2C 119.9
C6A—C1A—S1A 118.29 (15) C3C—C2C—H2C 119.9
C3A—C2A—C1A 119.59 (19) C2C—C3C—C4C 119.09 (18)
C3A—C2A—H2A 120.2 C2C—C3C—H3C 120.5
C1A—C2A—H2A 120.2 C4C—C3C—H3C 120.5
C2A—C3A—C4A 119.28 (19) C5C—C4C—C3C 120.28 (18)
C2A—C3A—H3A 120.4 C5C—C4C—N1C 116.60 (18)
C4A—C3A—H3A 120.4 C3C—C4C—N1C 123.11 (18)
C5A—C4A—C3A 120.67 (18) C6C—C5C—C4C 120.3 (2)
C5A—C4A—N1A 117.53 (19) C6C—C5C—H5C 119.9
C3A—C4A—N1A 121.77 (19) C4C—C5C—H5C 119.9
C6A—C5A—C4A 119.94 (19) C5C—C6C—C1C 118.87 (19)
C6A—C5A—H5A 120.0 C5C—C6C—H6C 120.6
supporting information
sup-6 Acta Cryst. (2006). E62, o1751–o1753
C5A—C6A—C1A 118.88 (19) O1C—C7C—N1C 123.18 (19)
C5A—C6A—H6A 120.6 O1C—C7C—C8C 122.55 (19)
C1A—C6A—H6A 120.6 N1C—C7C—C8C 114.25 (19)
O1A—C7A—N1A 122.98 (19) C7C—C8C—H8G 109.5
O1A—C7A—C8A 122.1 (2) C7C—C8C—H8H 109.5
N1A—C7A—C8A 114.9 (2) H8G—C8C—H8H 109.5
C7A—C8A—H8A 109.5 C7C—C8C—H8I 109.5
C7A—C8A—H8B 109.5 H8G—C8C—H8I 109.5
H8A—C8A—H8B 109.5 H8H—C8C—H8I 109.5
C7A—C8A—H8C 109.5 C7A—N1A—C4A 125.46 (18)
H8A—C8A—H8C 109.5 C7A—N1A—H1A 117.3
H8B—C8A—H8C 109.5 C4A—N1A—H1A 117.3
C6B—C1B—C2B 121.02 (18) N3A—N2A—S1A 112.86 (15)
C6B—C1B—S1B 120.24 (16) N4A—N3A—N2A 173.8 (2)
C2B—C1B—S1B 118.71 (16) C7B—N1B—C4B 128.12 (18)
C3B—C2B—C1B 118.84 (19) C7B—N1B—H1B 115.9
C3B—C2B—H2B 120.6 C4B—N1B—H1B 115.9
C1B—C2B—H2B 120.6 N3B—N2B—S1B 112.81 (17)
C2B—C3B—C4B 120.84 (19) N4B—N3B—N2B 173.5 (3)
C2B—C3B—H3B 119.6 C7C—N1C—C4C 127.17 (18)
C4B—C3B—H3B 119.6 C7C—N1C—H1C 116.4
C5B—C4B—C3B 119.90 (18) C4C—N1C—H1C 116.4
C5B—C4B—N1B 123.95 (19) N3C—N2C—S1C 112.69 (18)
C3B—C4B—N1B 116.14 (18) N4C—N3C—N2C 174.1 (3)
C4B—C5B—C6B 119.28 (19) O2A—S1A—O3A 120.62 (10)
C4B—C5B—H5B 120.4 O2A—S1A—N2A 103.55 (10)
C6B—C5B—H5B 120.4 O3A—S1A—N2A 108.33 (10)
C1B—C6B—C5B 120.03 (19) O2A—S1A—C1A 109.56 (10)
C1B—C6B—H6B 120.0 O3A—S1A—C1A 109.72 (10)
C5B—C6B—H6B 120.0 N2A—S1A—C1A 103.57 (10)
O1B—C7B—N1B 123.8 (2) O2B—S1B—O3B 120.96 (11)
O1B—C7B—C8B 121.5 (2) O2B—S1B—N2B 101.81 (11)
N1B—C7B—C8B 114.65 (19) O3B—S1B—N2B 108.33 (10)
C7B—C8B—H8D 109.5 O2B—S1B—C1B 110.51 (10)
C7B—C8B—H8E 109.5 O3B—S1B—C1B 109.79 (11)
H8D—C8B—H8E 109.5 N2B—S1B—C1B 103.78 (10)
C7B—C8B—H8F 109.5 O3C—S1C—O2C 119.55 (11)
H8D—C8B—H8F 109.5 O3C—S1C—N2C 102.58 (13)
H8E—C8B—H8F 109.5 O2C—S1C—N2C 108.67 (10)
C2C—C1C—C6C 121.31 (18) O3C—S1C—C1C 110.20 (10)
C2C—C1C—S1C 119.35 (16) O2C—S1C—C1C 110.20 (10)
C6C—C1C—S1C 119.28 (15) N2C—S1C—C1C 104.30 (10)
C6A—C1A—C2A—C3A 1.2 (3) C8B—C7B—N1B—C4B 174.97 (19)
S1A—C1A—C2A—C3A −179.83 (16) C5B—C4B—N1B—C7B −3.2 (3)
C1A—C2A—C3A—C4A 0.7 (3) C3B—C4B—N1B—C7B 177.8 (2)
C2A—C3A—C4A—C5A −2.3 (3) O1C—C7C—N1C—C4C −1.7 (3)
supporting information
sup-7 Acta Cryst. (2006). E62, o1751–o1753
C3A—C4A—C5A—C6A 2.2 (3) C5C—C4C—N1C—C7C −161.0 (2)
N1A—C4A—C5A—C6A −179.91 (19) C3C—C4C—N1C—C7C 20.5 (3)
C4A—C5A—C6A—C1A −0.3 (3) N3A—N2A—S1A—O2A −141.30 (17)
C2A—C1A—C6A—C5A −1.4 (3) N3A—N2A—S1A—O3A −12.1 (2)
S1A—C1A—C6A—C5A 179.64 (15) N3A—N2A—S1A—C1A 104.34 (18)
C6B—C1B—C2B—C3B 1.8 (3) C2A—C1A—S1A—O2A −13.1 (2)
S1B—C1B—C2B—C3B −176.00 (17) C6A—C1A—S1A—O2A 165.87 (16)
C1B—C2B—C3B—C4B −0.1 (3) C2A—C1A—S1A—O3A −147.68 (17)
C2B—C3B—C4B—C5B −2.4 (3) C6A—C1A—S1A—O3A 31.31 (19)
C2B—C3B—C4B—N1B 176.7 (2) C2A—C1A—S1A—N2A 96.85 (18)
C3B—C4B—C5B—C6B 3.3 (3) C6A—C1A—S1A—N2A −84.16 (17)
N1B—C4B—C5B—C6B −175.7 (2) N3B—N2B—S1B—O2B −173.99 (16)
C2B—C1B—C6B—C5B −1.0 (3) N3B—N2B—S1B—O3B −45.47 (19)
S1B—C1B—C6B—C5B 176.83 (18) N3B—N2B—S1B—C1B 71.18 (18)
C4B—C5B—C6B—C1B −1.6 (3) C6B—C1B—S1B—O2B 142.18 (19)
C6C—C1C—C2C—C3C −0.2 (3) C2B—C1B—S1B—O2B −40.0 (2)
S1C—C1C—C2C—C3C 177.17 (16) C6B—C1B—S1B—O3B 6.3 (2)
C1C—C2C—C3C—C4C −0.7 (3) C2B—C1B—S1B—O3B −175.88 (17)
C2C—C3C—C4C—C5C 1.3 (3) C6B—C1B—S1B—N2B −109.35 (19)
C2C—C3C—C4C—N1C 179.69 (19) C2B—C1B—S1B—N2B 68.50 (19)
C3C—C4C—C5C—C6C −1.1 (3) N3C—N2C—S1C—O3C −160.66 (19)
N1C—C4C—C5C—C6C −179.59 (19) N3C—N2C—S1C—O2C −33.1 (2)
C4C—C5C—C6C—C1C 0.3 (3) N3C—N2C—S1C—C1C 84.4 (2)
C2C—C1C—C6C—C5C 0.4 (3) C2C—C1C—S1C—O3C −48.4 (2)
S1C—C1C—C6C—C5C −176.97 (17) C6C—C1C—S1C—O3C 128.95 (19)
O1A—C7A—N1A—C4A 1.8 (4) C2C—C1C—S1C—O2C 177.51 (17)
C8A—C7A—N1A—C4A −176.0 (2) C6C—C1C—S1C—O2C −5.1 (2)
C5A—C4A—N1A—C7A 146.9 (2) C2C—C1C—S1C—N2C 61.0 (2)
C3A—C4A—N1A—C7A −35.2 (3) C6C—C1C—S1C—N2C −121.58 (19)
O1B—C7B—N1B—C4B −6.4 (4)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N1B—H1B···O1Ai 0.88 2.06 2.913 (2) 162
N1A—H1A···O1Cii 0.88 2.03 2.907 (2) 172
N1C—H1C···O1Biii 0.88 2.07 2.929 (2) 165