FORMULATION AND IN VITRO EVALUATION OF IN-SITU
GELLING LIQUID SUPPOSITORY OF PROMETHAZINE HCL
Iman S. Jaafar and Ameera A. Radhi*
Assistant Lecturer Department of Pharmaceutics, College of Pharmacy, University of
AlMustansiryah, Baghdad, Iraq.
ABSTRACT
The aim of this study was to formulate and in vitro evaluate a
thermogelling mucoadhesive liquid suppositories of promethazine
HCl(PMZ HCl) in order to improve the bioavailability, and decreases
the side effects as the absorbed drug avoids liver’s first pass
metabolism. Poloxamers P407 and P188 were used as thermo- gelling
agents and Hydroxypropyl methyl cellulose (HPMCK4M) as
mucoadhesive polymer. Thirteen formulas were prepared using cold
method and were evaluated for gelation temperature, gel strength, and
bioadhesive force and In-vitro release rate. Gelation temperature was
significantly increased with incorporation of 12.5 % w/v PMZ HCl
while decreased with addition of mucoadhesive polymer. The polymer
increased the gel strength and the mucoadhesive force of the prepared
solutions but significantly decreased the release rate. Among the
formulations examined, the P407/P188/HPMC (16/4/0.5 % w/v) and P407/ P188/ HPMC
(16/4/0.75 %w/v) that were used in formulas F9 and F10, respectively, were selected as the
bases of choice for PMZ HCl liquid suppository since they exhibited optimum physical
properties of gelation temperature, gel strength, high mucoadhesive force and a moderate
PMZ HCl in-vitro release. Therefore, these gels represent a promising alternative to
conventional suppositories and might be applicable for the development of in situ gelling and
mucoadhesive liquid suppository for humans as a more convenient and effective rectal
dosage form.
KEYWORDS: Thermogelling mucoadhesive, Poloxamers, Hydroxypropyl.
Volume 4, Issue 11, 273-286. Research Article ISSN 2277– 7105
*Correspondence for Author
Dr. Ameera A. Radhi
Assistant Lecturer
Department of
Pharmaceutics, College of
Pharmacy, University of
AlMustansiryah, Baghdad,
Iraq.
Article Received on 14 Sept. 2015,
INTRODUCTION
Promethazine Hydrochloride is an antiallergic, antihistaminic agent. It mediates its effect
through antagonizing the physiological and pathophysiological H1 mediated effect of
histamine.[1] It may also block the action of acetylcholine and this may explain the benefit in
reducing nausea and vomiting.[2]
It is used in treatment of allergic conditions, prevent or treat motion sickness, treat or avoid
nausea and vomiting in certain type of anesthesia and surgery and inducing sedation.[3]
Promethazine is well absorbed after oral or intramuscular dose with a peak plasma
concentration of 2 to 3 hours after a dose by these routes, although low systemic
bioavailability after oral doses due to high first pass metabolism in liver. It undergoes
extensive metabolism, predominantly to promethazine sulfoxide and also to N –desmethyl
promethazine.[4]
Nowadays one of the basic tasks of drug formulation is to develop an already existing dosage
form in a way which makes drug release the best possible under the given circumstances, that
is to enhance bioavailability in this way.[5]
The rectal route for drug administration was proven to be advantageous over other routes
because of the reduced side effects such as gastrointestinal irritation and the avoidance of
both disagreeable taste and first pass effect.[6]
A conventional suppository is a solid dosage form that melts or softens in the rectum at body
temperature. However solid suppository gives a feeling of alien, discomfort, and refusal to
the patients, possibly lowering patient compliance. Furthermore, if the solid suppositories
reach the end of the colon, the drugs delivered by the suppositories might undergo the
first-pass effect.[7]
In order to overcome these problems, a trail was made to establish a liquid rectal dosage form
which converts to a gel at body temperature which has a suitable gel strength in order not to
be leaked out from the anus after administration; and has a suitable bioadhesive force so as
not to reach the end of the colon.[8]
As a base of liquid suppository, Poloxamers as surfactants, are copolymers of poly
exhibit the phenomenon of reverse thermal gelation, remaining as solutions at low
temperature and gelling upon increasing the temperature.[10]
By modulating the gelation temperature of different poloxamer solutions, liquid bases for
rectal use can be formulated which form a gel in the rectum at body temperature with suitable
gel strength so as not to leak out from the anus after administration.[11] Furthermore, the
addition of mucoadhesives in these bases would reinforce the drug sustained action, and may
help in adhering the formed gel to the lower part of the rectum offering further protection
from the first pass effect, hence improving its bioavailability.[12]
The aim of this study was to formulate and in vitro evaluate a thermogelling mucoadhesive
system of promethazine HCl in order to improve the bioavailability, and decreases the side
effects as the absorbed drug avoids liver’s first pass metabolism. Physicochemical properties
such as gelation temperature, gel strength, and bioadhesive force of various formulations
composed of promethazine HCl, Poloxamers, and HPMCK4M as mucoadhesive polymer
were investigated.
MATERIALS
Promethazine HCl was purchased from Samara Drug Industry, Iraq. Poloxamer 407 and
Poloxamer 188 were purchased from M/s Provizer Pharma, Gujarat, India. Hydroxypropyl
methyl celleluse K4M was purchased from Alladine Industrial Co, Shanghai, China.
METHODS
Preparation of PMZ HCl liquid Suppositories
Poloxamer-based liquid suppositories were prepared by the cold method described by Choi et
al. (1998). 12.5 mg of PMZ HCl was dissolved in the calculated amount of distilled water at
room temperature. The mucoadhesive polymers were incorporated in PMZ HCl solutions.
P407/P188 was slowly added with continuous agitation using a magnetic bar. The mixtures
were left at 4°C in refrigerator until clear solutions were obtained.[13]
The medicated rectal solutions showing satisfactory gelation temperature (30-37oC) and
lower total poloxamer content were mixed with additional amounts of mucoadhesive
polymers namely: HPMC, in concentrations 0.5, 0.75, 1 and 2%, w/v.[11] Table 1 shows the
Table 1: Composition of Prepared Rectal Solutions
Formula No. P 407 % w/v P 188 % w/v HPMCK4M % w/v
F1 12
F2 14
F3 16
F4 18
F5 20
F6 16 4
F7 16 8
F8 16 12
F9 16 4 0.5
F10 16 4 0.75
F11 16 4 1
F12 16 4 2
F13 16 4 Without drug
Evaluation of In-situ Gelling Liquid Suppositories Measurement of Gelation Temperature
A 20-ml transparent vial containing a magnetic bar and 10 g of the liquid suppository was
placed in a low-temperature thermostat water bath. The liquid suppository was heated at a
constant stirring "50 (rpm)".When the magnetic bar stopped moving due to gelation, the
temperature was determined as the gelation temperature.[14] Only those liquid suppository
preparations that pass the gelation temperature test were subjected to the next tests.
Measurement of Gel Strength
Poloxamer solution (50 g) was put in a 100 ml-mass cylinder and gelled in a thermostat at
36.5°C. The apparatus for measuring gel strength (weight: 35 g) was then placed onto the
gelled poloxamer. The gel strength was determined by the time in (seconds) it took to move
the apparatus 5 cm down through the poloxamer gel (Schmolka, 1972). In cases that took
more than 10 min to drop the apparatus into the gel, various weights were placed on top of
the apparatus and gel strength was described by the minimal weights that pushed the
apparatus 5 cm down through the gel.[15]
Determination of the Mucoadhesive Force
The mucoadhesive force, the detachment stress of the liquid suppositories was determined
using a modification of the mucoadhesive force measuring device used by Choi et al. A
section was cut from the fundus of sheep rectum and instantly secured with the mucosal side
out into each glass vial. The vials were stored at 36.5°C for 10 min. 1 vial connected to the
that the gel is placed between the mucosal sides of both vials. Water was added to beaker.
Increasing weight of water added gradually would detach the two vials. Mucoadhesive force,
the detachment stress (dyne/cm²), is determined from the minimal weights of water that
detached the 2 vials.[16]
In Vitro Dissolution Test
Studies of PMZ HCl release from liquid suppository formulations were performed for
formulas (F6 ,F9-F12) using the in vitro dialyzing method in which 5ml of the prepared
liquid suppository formulation was syringed into a glass container closed at one end and the
open end covered by a 1.8 cm cap in diameter of hydrophilic membrane of regenerated
cellulose Millipore 0.45μ (soaked for 1 hr. before the test in the phosphate buffer), [17]
and
heated at 33 ± 1°C for 10 min to gelate completely.[18]This glass apparatus was fixed on the
paddle with cable ties, on dissolution apparatus type 2 and 500ml phosphate buffer pH 6.8
was used as a dissolution medium, within a period of 3hr and 50 rpm at 37°C. Aliquots (5ml)
were taken at regular intervals and analyzed spectrophotometerically at 248 nm.[17]
Kinetics Analysis of Drug Release
To analyze the mechanism of drug release from the prepared formulas, In vitro dissolution data were fitted to zero order, first order, Higuchi release model, and Korsmeyer Peppas
model and the model with the higher correlation coefficient was considered to be the best
model.[19]
RESULTS AND DISCUSSION
Evaluation of PMZ HCl Liquid Suppositories Gelation Temperature
Gelation temperature is the temperature at which the liquid phase makes a transition to gel. A
gelation temperature range suitable for liquid suppository would be 30–36°C. If the gelation
temperature of liquid suppository is lower than 30°C, gelation occurs at room temperature
leading to difficulty in manufacturing, handling and administering. If the gelation
temperature is higher than 36°C, the suppository still stays as a liquid at body temperature,
resulting in leakage from the anus.
Therefore, liquid suppository must have the suitable gelation temperature between 30–36°C,
Gelation temperatures of various preparations (F1-F13) and the obtained results are
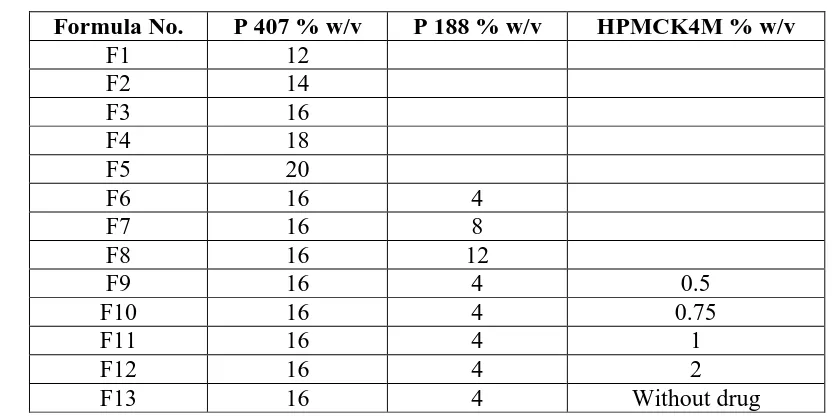
[image:6.595.161.434.144.350.2]summarized in table 2.
Table 2: Gelation Temperature of Prepared Formulas
Formula No Gelation Temperature oC
F1 50
F2 50
F3 28
F4 26
F5 19
F6 34
F7 32
F8 30
F9 32
F10 31
F11 30
F12 29
F13 18
PMZ HCl liquid suppository formulae (F1-F5) were prepared to study the effect of increasing
the concentrations of poloxamer P407 alone on gelation temperature. While formulae (F6-F8)
were prepared to study the effect of increasing poloxamer P188 concentration from 4 to 12 %
w/v, while fix the concentration of poloxamer P407 (16 % w/v) on gelation temperature.
.Formulas (F9-F12) were prepared to study the effect of addition and increasing the
concentrations of HPMCK4M from 0.5 to 2 %, while fix the concentration of poloxamer
P407 (16 % w/v) and poloxamer P188 (4 % w/v) on gelation temperature. Finally F13 were
prepared to study the effect of the addition of drug on gelation temperature.
For formulas (F1-F5) which prepared by increasing the concentration of poloxamer P407
from 12 % to 20%, the gelation temperatures were determined. The results as illustrated in
figure 1 show that as the concentration of poloxamer P407 increases the gelation temperature
decreases significantly (p<0.05). The decrease in the gelation temperature (from 50 to 19 oC)
with increasing P407 concentration may be attributed to the higher number and volume
occupied by micelles at lower temperatures. As the concentration increases, the gel structure
Figure 1: Effect of p 407 concentration on gelation temperature
It is important to note that PMZ HCl liquid suppositories composed of 16% P407 showed
intermediate gelation temperatures (28oC) so it is good candidate for gelation temperature
modulation using P188.
The previous results indicate that P407 alone could not provide gelation at the physiological
temperature. A modulation of the gelation temperature to reach the desired range (30-36oC)
could be accomplished by the use of a combination of the two poloxamer grades P407 and
P188, so we use mixture of poloxamers P407 and P188 in different ratios (16/4, 16/8 and
16/12) which showed a gelation temperature of 34°C, 32 oC and 30 oC, respectively.
[image:7.595.141.456.522.727.2]By studying the effect of increasing the concentration of P188 on constant concentration of
P407 solution, it was found that the increasing in P188 concentration was accompanied by a
significant (p<0.05) decrease in the gelation temperature of the P407 solutions as shown in
figure 2. This is mainly attributed to the participation of P188 in the micellization process.[21]
In the present study, a combination of 16% P407 and 4% P188 was used for further
formulations with the addition of different concentrations of HPMC (0.5, 0.75, 1, and 2%
w/v).
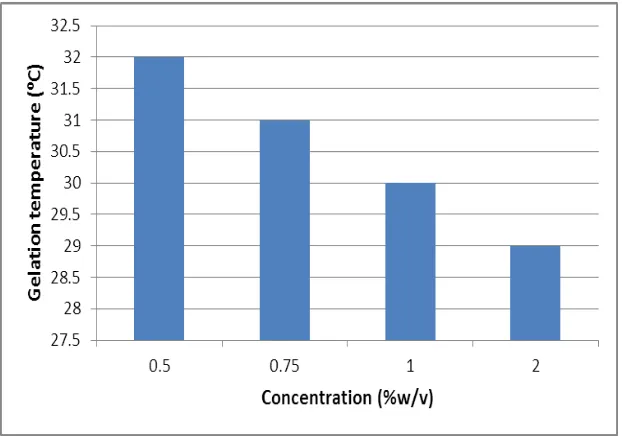
The results showed that the addition of HPMC results in lowering the gelation temperature of
the resultant liquid suppositories. The impact of HPMC on the gelation temperature and gel
strength was found to be depending on their concentration. Increasing the concentration of
HPMC from 0.5 to 2 % w/v as shown in figure 3 produced a significant (p<0.05) decrease in
the gelation temperature and increase in the gel strength of the corresponding liquid
[image:8.595.143.454.381.599.2]suppositories.
Figure 3: Effect of HPMCK4M concentration on gelation temperature
The gelation temperature lowering and gel strength increasing effect of mucoadhesive
polymers as HPMC could be explained by their ability to bind to the polyoxyethylene chains
present in the poloxamer molecules through hydrogen bonds. This will promote dehydration
causing an increase in entanglement of adjacent molecules and extensively increasing
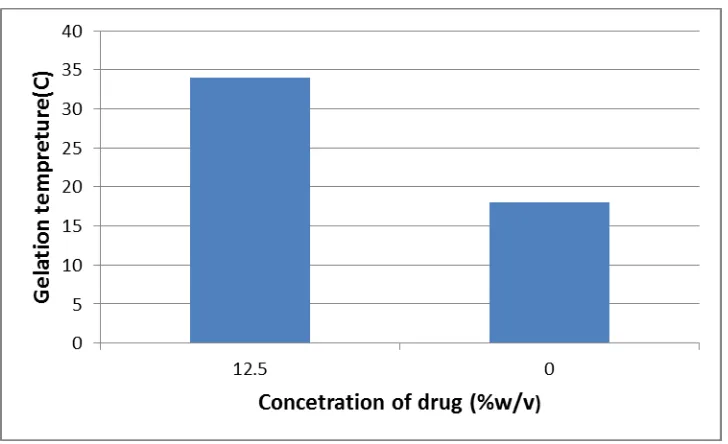
The incorporation of 12.5 % w/v PMZ HCl increased the gelation temperature. This is due to
that water soluble substances cause modification of the process of micellar association of
[image:9.595.117.481.144.366.2]poloxamer solutions leading to an increase in gelation temperature.[11]
Figure 3: Effect of drug on gelation temperature
Gel Strength
In the development of liquid suppository, the gel strength is important in finding the
condition which allows the easy insertion of the suppositories and no leakage from the anus.
At high gel strength, it is difficult to insert the suppositories. On the other hand, at low gel
strength the suppositories would leak from the anus. It has been reported that the optimal in
situ gelling and mucoadhesive liquid suppositories must have suitable gel strength, in the
range of 10 to 50 seconds.[23]
The gel strength is related to the internal structure and the strength of the physical or
chemical bonds forming the interplaying network of the long chains of the polymers involved
in gel formation, it resembles consistency and is related to viscosity and depends on the type
of polymer used.[24]
The results of gel strength for formulas (F6 ,F9-F12) shown in table (3) reveal that the
addition of the mucoadhesive polymer (HPMCK4M) increased the gel strength of
poloxamer mixture significantly (p < 0.05) in a concentration dependent manner. The
mechanism of the increase might be related to hydrogen bonding between poloxamer and the
Table 3: Gel Strength of the Prepared Formulas
Formula No Gel strength(seconds)
F6 15
F9 32
F10 36
F11 40
F12 57
Mucoadhesive Force
Mucoadhesive force is known to be dependent on the nature and the concentration of
mucoadhesive polymers. The stronger the mucoadhesive force is, the more it can prevent the
gelled suppositories from reaching the end of the colon, the pathway for the first-pass effect.
But if the mucoadhesive force is too excessive, the gel can damage the rectal mucous
membrane.[26] Therefore, liquid suppository must have the suitable mucoadhesive force.
The results of mucoadhesive force for formulas (F6, F9-F12) are shown in table (4) and
reveled that F6 which has poloxamer mixture only has low mucoadhesive force.
The addition of mucoadhesive polymer (HPMC) in (F9-F12) reinforced the mucoadhesive
force of rectal suppositories which has increased significantly (p<0.05) as the concentration
of mucoadhesive polymer increased over the range of 0.5-2%. Such effects on the
mucoadhesive force might be due to the binding of these polymers with the oligosaccharide
chains of rectal mucous lining.[27]
Table 4: Mucoadhesive Strength of Prepared Formulas
Formula No Mucoadhesive strength ( dyne/cm2 x 103 )
F6 0.867
F9 5.85
F10 10.4
F11 15.61
F12 23.4
Release Studies
Five PMZ HCl liquid suppositories passed the gelation temperature and gel strength tests
(F6, F9-F12) were subjected to the dissolution test.
Compared to the control gel (F6) that showed the fastest release among other formulas ( 98
% in 120 minutes) , the release was decreased significantly by addition of mucoadhesive
mucoadhesive polymer could be attributed to their ability to increase the overall product
viscosity [28] as well as their ability to distort or squeeze the extra-micellar aqueous channels
of poloxamer micelles through which the drug diffuses thereby delaying the release
[image:11.595.142.454.167.385.2]process.[15]
Figure 4: Effect of addition of mucoadhesive polymer on drug release
Table 5: Release Kinetic of PMZ HCl from Different Suppository Frmulas Order of
reaction Formula
Number
Correlation coefficient (r2) Korsmeyer Pappas
Zero First Higuchi n r2
F6 0.9751 0.8978 0.9929 0.0139 0.9957
F9 0.862 0.9731 0.9314 0.4577 0.9377
F10 0.8384 0.966 0.9148 0.4598 0.9247
F11 0.7923 0.9169 0.881 0.453 0.9016
F12 0.8528 0.9521 0.9258 0.5192 0.9257
Analysis of Release Kinetic
Table (5) illustrated the correlation of dissolution data to different models of release kinetic,
where a good fitting to zero order model was observed with F6 while a good fitting to first
order model was observed with F9-F12, indicated by highest regression value (r2).
For Korsmyer-Pappas model, the value of release exponent (n) defines the release
mechanism, the n value of F6 shows n value less than 0.45 pointing a Fickian diffusion
[image:11.595.81.516.446.578.2]polymeric chains.[30] F 9-F12 are between 0.45 to 0.89 indicating anomalous(non–Fickian)
transport which refer to combination of diffusion and erosion controlled drug release.[31]
CONCLUSION
In this study, an in situ gelling thermoreversible liquid suppository of PMZ HCl was
developed using poloxamer P407, poloxamer P188 and mucoadhesive polymer HPMCK4M.
In order to obtain liquid systems at room temperature which can be conveniently handled and
administered and that develop an intimate contact with the rectal mucosa, the gelation
temperatures of PMZ HCl rectal formulations was modulated to adjust the gelation just
below the body temperature. Based on the obtained results, it was concluded that
P407/P188/HPMC (16/4/0.5 % w/v) and P407/ P188/ HPMC (16/4/0.75 %w/v) that were
used in formulae F9 and F10, respectively, were selected as the bases of choice for PMZ HCl
liquid suppository since they exhibited optimum physical properties like gelation
temperature, gel strength, high mucoadhesive force and a moderate PMZ HCl in-vitro
release. Therefore, these gels represent a promising alternative to conventional suppositories
and might be applicable for the development of in situ gelling and mucoadhesive liquid
suppository for humans as a more convenient and effective rectal dosage form.
REFERENCES
1. Kourosh Saeb-Parsy. Instant Pharmacology. John Wiley and Sons, Jun 18, 1999. p286.
2. Barber P, Parkes J and Blundell D. Further Essentials of Pharmacology for Nurses.
McGraw-Hill International 2012: 68.
3. Marla J. De Jong , Amy M. Karch A M. Lippincott's Critical Care Drug Guide.
Lippincott Williams & Wilkins 2000: 506.
4. Sweetman S S, Martindale: The Complete Drug Refrance, 36th edition, 2009,
London:Pharmaceutical Press : 589.
5. Berkó S, Regdon Jr. G, Ducza E, Falkay G and Erős I .In vitro and in vivo study in
rats of rectal suppositories containing furosemide .European Journal of Pharmaceutics
and Biopharmaceutics 2002; 53(3): 311–315.
6. Tukker J., Rectal and vaginal drug delivery. In: Pharmaceutics, the Science of Dosage
Form Design (Aulton ME, ed.). Churchill Livingstone, Edinburgh, UK, 2009; 534-543.
7. Park Y J, Yong CS, Kim H M, Rhee J D, Oh Y K, Kim C K, et al. Effect of sodium
chloride on the release, absorption, and safety of diclofenac sodium delivered by
8. Lee J W, Park J H and Robinson J R. Bioadhesive-based dosage forms; the next
generation. J Pharm Sci. 2000; 89: 850–866.
9. Patel H R, Patel R P and Patel M M. Poloxamers: A pharmaceutical excipients with
therapeutic behaviors, International Journal of PharmTech Research 2009; 1(2): 299-303.
10. Dumortier G, Grosslord J L, Agnely F, et al. A review of poloxamer 407 pharmaceutical
and pharmacological characteristics, Pharm Res 2006; 23: 2709-2727.
11. Abd ElHady S, Mortada N D, Awad G A, Zaki N M and Taha R A Development of in
situ gelling and mucoadhesive mebeverine hydrochlorided solution for rectal
administration.Saudi Pharmaceutical Journal 2003; 11(4): 159-169.
12.Koffi A A, Agnelyb F, Ponchelb G and Grossiordb J L. Modulation of the rheological and
mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended forthe
rectal administration of quinine. european journal of pharmaceutical sciences 2006; 27:
328–335.
13. Ramadan EM, Borg TM and Elkayal MO. Formulation and evaluation of novel
mucoadhesive ketorolac tromethamine liquid suppository. African Journal of Pharmacy
and Pharmacology 2009; 3(4): 124-132.
14. Al-Wiswasi NN and Al-Khedairy E. Formulation and in vitro Evaluation of In-situ
Gelling Liquid Suppositories for Naproxen. Iraqi J.Pharm.Sci. 2008; 17(1): 31-38.
15.Han-Gon Choi a, Jae-Hee Jung b, Jei-Man Ryu b, Sung-June Yoon b, Yu-Kyoung Oh
a,Chong-Kook Kim. Development of in situ-gelling and mucoadhesive acetaminophen
liquid suppository. International Journal of Pharmaceutics, 1998; 165: 33–44.
16. Keny R V and Lourenco C F. Formulation and evaluation of thermoreversible in situ
gelling and mucoadhesive diltiazem hydrochloride liquid. International Journal of Pharma
and Bio Sciences 2010; 1(1): 1-17.
17. Kassab H J and Khalil Y I. 5-Flourouacil mucoadhesive liquid suppository formulation
and evaluation. World Journal of Pharmaceutical Research 2014; 3(9): 119-135.
18.Xu X., Shen Y., Wang W., Sun C., Li C., Xiong Y. and Tu J. Preparation and in vitro
characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of
phenylephrine hydrochloride .European Journal of Pharmaceutics and Biopharmaceutics
2014; 88: 998–1004.
19.Sachin S. Darekar s, Khadabadi S S and Shahi S R. Formulation and evaluation of bilayer
buccal tablet of sumatriptan. International Journal of Pharmacy and Pharmaceutical
20. Abu El-Enin A S M and El-Feky G S. Formulation and Evaluation of In-situ Gelling
Tenoxicam Liquid Suppositories. Journal of Life Medicine 2013; 1(2): 23-32.
21.Wei, G., Xu, H., Ding, P.T., Li, S.M., and Zheng, J.M., Stimuli-sensitive hydrogels in
controlled and sustained drug delivery, J. Control. Rel. 2002; 85: 1-15.
22.Gilbert C G, Richardson J L, Davies M C, Pallin K J, et al. The effect of solutes and
polymers on the gelation properties of Pluronic F-127 solution for controlled drug
delivery., J.Control.Rel. 1987; 5: 113-118.
23. Chaudhari P, Ajab A, Malpure P, Kolsure P and Sanap D. Development and in vitro
evaluation of thermoreversible nasal gel formulations of Rizatriptan benzoate. Indian J
Pharm Educ Res 2009; 43(1): 55-62.
24. Baloglu E, Ay Senyigit Z, Karavana S Y and Bernkop-Schnűrch A. Strategies to Prolong
the Intravaginal Residence time of Drug Delivery System J.Pharm.Pharmaceut. Sci 2009;
12(3): 317-336.
25.Kolsure P K and Rajkappoor B. Development of zolmitrptan gel for nasal. Asian J Pharm
Clin Res 2012; 5(3): 88-94.
26. Abbas Z,Aditya N,and swamy N G N. Fabrication and evaluation of Mucoadhesive
,thermoreversable in situ gelling liquid suppository of chloraquine Phosphate.Indian
Journal of Novel Drug Delivery 2013; 5(2): 60-70.
27. Barakat N S. In Vitro and In Vivo Characteristics of a Thermogelling Rectal Delivery
System of Etodolac. AAPS PharmSciTech. 2009; 10(3): 724–731.
28.Desai SD and Blanchard J. Evaluation of Pluronic F-127 based sustained-release ocular
delivery systems for pilocarpine using albino rabbit eye model. J. Pharm. Sci. 1998; 87:
1190-1195.
29.Islama M S, Rezab S and Rahmanb H. In vitro release kinetics study of diltiazem
hydrochloride from wax and kollidon SR based matrix tablets. Iranian Journal of
Pharmaceutical Research 2008; 7(2): 101-108.
30.Costa P and Lobo J M S. Modeling and comparison of dissolution profile. Eur. J. Pharm.
Sci., 2001; 13: 123-133.
31.Singhvi G and Singh M. In-vitro drug release characterization models. International