I N THISPAPERI shall describe some of the information we have recently obtained regarding the enzymic and molecular or ganization of the mitochondria, which as you know are very small particulate or ganelles in the cytoplasm of all aerobic cells. These bodies have been found to cata lyze one of the most fundamental activities of the cell, namely, the transformation of the energy yielded by oxidation of food stuffs into the so-called phosphate-bond en ergy of adenosine triphosphate. This proc ess of respiration and phosphorylation is extremely complex and involves the inter action of at least seventy different enzymes and coenzymes in an integrated fashion.
The mitochondria have a characteristic ultrastructure in which these enzymes are embedded, and it is now possible to con sider in some detail the intramitochondrial location and function of these important energy-transforming molecules.
First, let us consider the organization of oxidative metabolism in purely biochemical terms. Figure 1 shows the usual text-book representation of the final common pathway of biologic oxidation in animal tissues. You will recall that all three of the major food stuffs of the cell (carbohydrate, fat and protein) ultimately are degraded in the tis sues to a two-carbon unit, namely, acetyl coenzyme A. The acetate group then un dergoes oxidation by the Krebs citric acid cycle, and in this process the two carbon atoms of acetate become oxidized to car bon dioxide. The oxidation of acetate is finally completed when pairs of hydrogen atoms are removed from certain of the in termediates of the Krebs cycle by dehydro genases. These hydrogen atoms, or their equivalent in electrons, pass along the respi
ratory chain via the cytochromes until they meet molecular oxygen and reduce it to form water.
The oxidation of these foodstuffs releases large amounts of energy. But this energy is not released simply as heat. In the cell the energy of biologic oxidation is largely re covered as chemical energy, as the so-called phosphate-bond energy of adenosine tri phosphate (ATP).
Several years ago we were able to prove that the site of this conversion of oxidative energy into ATP energy is the respiratory chain. Figure 2 shows this chain in more de tail, and you can see that electrons pass from substrate to oxygen via a series of elec tron carriers, including pyridine nucleotide, a flavoprotein, and the four cytochromes. Along this chain there are three energy transforming mechanisms which use the en ergy lost when a pair of electrons passes from a specific carrier to the next to cause the formation of ATP from adenosine di phosphate (ADP) and phosphate. ATP is thus the charged form of the energy carry ing system and it is charged at the expense of the energy lost during electron transport.
The enzymic mechanism of this energy conversion is one of the great unsolved mys teries of contemporary biochemistry. How ever, Figure 3 provides a picture of what we believe to be the probable form, in princi ple, of the energy-coupling mechanisms in the respiratory chain. I will not discuss these principles and mechanisms in detail; I want merely to impress you with the great complexity of the energy-coupled respira tory chain. It consists of a series of cycles, and cycles within cycles.
Some 10 years ago Kennedy and I dis covered that the entire Krebs cycle complex
Presented at the IX International Congress of Pediatrics, Montreal, Canada, July 20, 1959. ADDRESS:Baltimore 5, Maryland.
PEDIATRICS, September 1960 466
THE ENZYMIC AND MORPHOLOGIC ORGANIZATION
OF THE MITOCHONDRIA
Albert L. Lehninger
SPECIAL ARTICLES
CITRATE 4'
CIS —¿ACONITATE
.4'
ISO-CITRATE CYTOCHROME
4, ‘¿ SYSTEM
OXALOSUCCINATE \
\ H@O
@-KETOGLUTARATE ÷ @jJ \
S@CCINATE + ::@®—®—@
FUMARATE t
/
MALATE /
4' 2&
OXAL ACE TAT E
L KREBS
TRICARBOXYLIC@LELECTRON
TRANSPORT]
ACID CYCLE
Ftc.1.Krebstricarboxylicacidcycle.
ATP ATP ATP
‘¿1' 1 1
Substrates—¿@DPN
@ c —¿@Cyta--@Cyta—¿@0
I
Ftc. 3. Schematicrepresentationof probablereactionpatternof electrontransportand coupled phosphorylation.
of enzymes, together with the respiratory chain and these energy-transforming mech anisms, are located in the mitochondria of
the cell. Since then it has been found that the mitochondnia of all cell types which have been examined, whether animal or
[GLUCOSE I
PYRUVATE
FATTY ACIDS —¿*1ACETYL—CoiJ
7
[@@NO ACIDSJ
+
ADP+P ADP+P
Overall equation: DPNH + H@ + 3 P@ + 3ADP + 0 —¿@DPN + 3ATP + H20
Energy liberated by electron transport = 55 KCAL.
Energy recovered as ATP = 36 KCAL.
Efficiency = 65%
Ftc. 2. Schematic representation of electron transport and coupled oxida tive phosphorylation mechanisms, showing the major energy relationships.
DP@@NH2-®
SUBSTRATES@'
DPN@ DPN-@-(X@
@ADP
AlP-F®
AD? +P
©
ATP +
jADP
phate, which represents the discharged or “¿spent―form of the energy carrier system. This, then, is the biochemical picture.
Now let us consider the ultrastructure of the mitochondria as revealed by the elec tron microscope. Figure 5 is a picture of rat heart mitochondria made by Palade. You
will see that the mitochondria have an outer double membrane and a number of septa running across the mitochondrion, termed
the cristae. Each mitochondrion is about 3
microns long and less than a micron in
thickness;there is some variationin shape.
Figure 6 shows diagrammatic representa
tions of mitochondrial structure. At the top is a longitudinal section of a mitochondrion, which shows an outer membrane surround
ing it and an inner membrane that more or
less periodically invaginates into the lumen
to from the so-called cristae. Within the inner membrane is a relatively structureless
matrix. The lower half of the Figure shows a three-dimensional representation of a mi
tochondrion.
Figure 7 indicates the dimensions of the mitochondrial membranes as deduced from
electron micrographs. In all kinds of mito
chonciria, regardless of cell types of origin
or whether they are from animal or plant
cells, tile mitochondrial membrane con
ENERGY-REQUIRING FUNCTIONS OF CELL
,—< MOTILITY, CONTRACTION @—¿< BIOSYNTHESIS OF CELL @
MATERIAL
@—¿< ACTIVE TRANSPORT
TRANSMISSION OF IMPULSES @
BIOLUMINESCENCE
ADP + ENERGY TRANSPORT IATP
SPENT FORMJ SYSTEM [@@RGED FORM
02ff
cARBOHYDRATE CO2 + -420
I 111
FUELS EXHAUST
E@L(;.4. The central role of mitochondria in energy
conversion. It is seen that the AlP energy is used
to drive the different energy-requiring functions of
the cell.
plant, contain this enzymic energy-convert
ing system. As Figure 4 shows, the mito
chondria may thus be looked upon as the
power plants of the cell. They oxidize food stuff molecules with molecular oxygen and
in so doing, the energy of oxidation is har
nessed to cause the coupled synthesis of ATP from ADP and phosphate. The ATP
becomes the energy donor for the energy requiring functions of the cell as is shown;
and in the process of energy conversion the
ATP is ultimately split to ADP and phos
Fic. 6. Three-dimensional aspects of mitochondrial structure (after Palade).
180A
sistsof two layers about 60 Angstrom units
(A) in thickness, separated by a light layer
of about 60A. For comparison, in the mid
dle of tile Figure, are shown some data on molecular sizes: A typical protein molecule, hemoglobin, has a length of about 63A and
a thickness of 45A. Hemoglobin has a mo
lecular weigiit of 68,000, which is in the mid dle range of molecular weights of globular
0
1@OA
Proteills. A phospholipid molecule is about 30A long.
These dimensions are significant because
chemical analysis of tile mitochondriai
membranes shows they are composed
largely of phospholipo-protein, having about 65% protein and 35% phospholipid. These proportions and the thickness of the membranes as revealed by tile electron mi
PROTEIN
MOLECULE
*
IFA
o130A
IJL
PHO5PHO
LIPID MOLECULE
SECTION OF MITOCHONDRIAL
DOUBLE MEMBR ANE
POSSIBLE MOLECULAR CONFIGURATION
OF
MEMBRANES
470 THE CELL
flavoproteins and the different cytochromes together with the coupling enzymes which form ATP during electron transport) are associated with the membranes alone. Fur thermore, it appears most probable from other lines of evidence that the respiratory chain enzymes are located specifically in the inner mitochondrial membrane as shown.
Now let us consider in more detail the
structure and localization of the respiratory chain enzymes in the mitochondrial mem branes. One of the first questions is this: Are the respiratory chains in intact mito chondria made up of a specific and constant ratio of the different individual electron car rier molecules, suggesting a high degree of
molecular organization and design; or, are
there widely different molar ratios of the separate electron carriers, suggesting a more random organization? We have determined the relative molecular proportions of the flavoproteins and cytochromes in fragments of the mitochondrial membranes by using a highly sensitive spectroscopic method, sim ilar to that first described by Britton Chance. We have found that, within experimental error, the different electron carriers compris ing the respiratory chain occur in nearly equimolar proportions in the mitochondrial membranes. This is highly suggestive evi dence, but not necessarily proof, that these catalytically active protein molecules of the
MEMBRANES
RESPIRATORY CHAIN ENZYMES DPN(BOUND) FLAVOPROTEIN CYTOCHROME C CYTOCHROME C1 CYTOCHROME B CYTOCHROME A CYTOCHROME A3 SUCCINIC DEHYD. CHOLINE DEHYD. @-HYDROXYBUTYRATE DEHYD. PHOSPHORYLATING ENZYMES croscope are consistent with a “¿sandwich―
construction, in which the double mem branes consist of two monolayers of protein molecules each perhaps 60A thick, sepa rated by a double layer of oriented lipid molecules of about 60A thickness.
Now let us consider the intramitochon
drial location of enzymes and enzyme sys tems concerned in respiration. How can such information be arrived at? Isolated mi tochondria can be disrupted either mechan ically (by sonic oscillation for example) or by application of chemical agents (such as detergents). Then, by differential ultracen trifugation of fragmented mitochondria, fractions corresponding to the mitochon drial membranes and the matrix may be iso lated. Such fractions may then be subjected to chemical analysis, as well as enzymic
analysis.
Figure 8 indicates the gross intramito chondrial localization of the respiratory en zymes. Most of the Krebs-cycle enzymes are located in the inner matrix of the mitochon drion in a soluble form. Similarly, enzymes of the fatty acid-oxidation cycle are also located in the matrix of the mitochondrion. It therefore appears that the first stages of biologic oxidation of pyruvate and fatty acids occur in the inner matrix of the mito chondrion.
On the other hand, it is seen that the en
zymes making up the respiratory chains (the
MAIR:X
?:R:es CYCLE ENZYMES .%CONITASE
\IAL:C DEHYDROGENASE F LMAR ASE
:SOCITRIC DEHYD. CONDENSING ENZYME pYRUVIC AND KETOGLUT.
DEHYD. ETC. FATTY ACID CYCLE ENZYMES
CROTONASE ACYL DEHYD.
ETC. ETC.
respiratory chain are grouped together geo metrically in an ordered sequence, presum ably to permit rapid and efficient passage of electrons along the chain.
This finding now permits us to define
what we term a “¿respiratoryassembly― (Fig. 9). Such an assembly consists of one mole cule of each of the six electron-carrier pro teins, together with perhaps nine additional enzyme molecules which are specifically concerned with the formation of ATP at
each of the energy transformingsitesin the
respiratory chain. Thus the complete respi ratory assembly is made up of perhaps 15 separate specific enzyme molecules. If each
has a molecular weight of 100,000, the as sembly would have a particle weight of
about 1,800,000. Such an assembly would represent the basic molecular machine for respiratory energy conversion.
The question now arises: How many of
these respiratory assemblies are present in
the membranes of a single mitochondnion? The answer can be arrived at rather easily,
at least approximately. We know the dry weight and proteincontent of the mitochon
drion, and from spectroscopic and chemical
e —¿ c 0 03 02
E―
\\
\\
\\
ATP ATP ATP
A T P + @i@_)
@ ci@ii@@ + A DP
MECHANOENZYME PRINCIPLE
Ftc.9. Diagram of a respiratoryassembly.Each
circle represents an individual enzyme protein. In
addition to the respiratory carriers arranged hori
zontally in the diagram, there are three additional
enzymes at each of the three coupling sites in the chain, which participate in the conversion of re
spiratoryenergy intoATP. It ispostulatedthatthe enzyme molecules E, E', and E―are mechano
enzymes which may change their configuration de
pending upon whether or not they are in the
phosphorylated state.
determination of the carrier molecules, it can be calculated that a single rat-liver mi tochondrion contains perhaps 5,000 such as semblies of electron carriers and a single rat-heart mitrochondrion con taitis perl@aps
as many as 20,000. Of course these assem
blies are present entirely in the mitochon drial membranes.
The next question is: Do these assemblies make up only a very tiny fraction of the
weight of the mitochondrial membrane, or
do they comprise a significant proportion of its substance. Calculations show that the catalytically active proteins of the respira tory-chain assemblies may make up as much as 40% of the mass of the inner mitochon drial membrane. From this figure, we can see at once that the molecular substance comprising the mitochondrial membrane is not made simply of inert building blocks
with purely a structural function, but is con
stituted of many different types of cataly
tically active protein molecules organized
geometrically to carry out electron transport and oxidative phosphorylation. In short, this membrane is not a dead wall, but is a very complex enzyme system.
Another approach has permitted us to make some deductions about the disposition of these respiratory-carrier assemblies in the membranes of the mitochondnion. We have subjected the mitochondnial membranes to sonic oscillation, which shatters the mem branes into an assortment of fragments ranging from quite large to small fragments having a particle weight of only a few mil lions. We have separated these fragments in the ultracentrifuge into fractions on the basis of size. These fractions were analyzed not only for their chemical composition, but also their content of the respiratory-carrier proteins. It was the remarkable finding that all the fragments of the membrane, regard less of size, contained complete sets of res piratory carriers, in which the individual carrier molecules were always a constant fraction of the total protein content. From this it follows that the respiratory assem blies are probably uniformly disposed over the molecular sheet which makes up the
CHAIN
LINES OF FRAGMENT@T ION @
LIPOPROTEIN @—¿ FABRIC
TOP VIEW
RESPIRATORY ASSEMBLIES
50 A •¿ss OYISSSSSScnIYDOS..SS CROSS SECTION
FIG. 10. Schematic representation of mitochondrial membrane.
membrane (Fig. 10). Because the smallest fragments also contained a complete set of carrier molecules, it is presumed that the mitochondrial membrane is made up of
many recurring units, each containing a
functionally complete respiratory assem bly. Each catalytic unit is separated from
the next by relatively fragile lines of cleav
age, which are susceptible to mechanical or chemical splitting. However, the chemical and physical bonds holding the respiratory carriers of the assembly together, within the recurring unit, are very strong.
With this picture of the enzymic consti tution of the mitochondrial membranes in hand, we can now proceed to consider still another property which lends a whole new dimension of complexity to the enzymic organization of these membranes.
In the past year, we obtained evidence that the mitochondrial membranes consti tute a reversible contractile system, which
is driven by ATP and in which the hormone
thyroxine plays a role. In this process, as
it occurs in liver and kidney mitochondria,
large amounts of water may be transported into or out of mitochondria in a reversible manner by the relaxation or contraction of the mitochondnial membranes.This investigation began with the finding that there are four substances of physiologic occurrence which will cause rapid swelling of isolated liver or kidney mitochondria sus pended in sucrose solutions through uptake
of water. These swelling agents are: phos phate, calcium ions, reduced glutathione, and the hormone thyroxine. A wide variety of other physiologic substances have been tested, but only these four cause mitochon drial swelling. Especially noteworthy is the swelling caused by thyroxine, which is by far the most potent agent, and is capable of swelling mitochondria in physiologic con centrations.
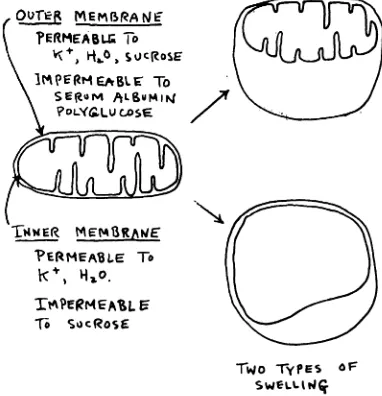
Figure 11 shows some pertinent facts on the swelling of mitochondria. First, mito chondria can swell in two general ways.
Since they have two membranes, which
EMr@r@,4@4
I,
i'ERMEABU
To
\
@
@j,O
SUC@toSE
\ it@ -iEi'@L@
To
\ SEI@OMALSUMIP4\ @OL'(@UGOSE
?ERMEA@L.E To h@, I@o.
It'@PERMEA%LE
1@o SocRos@
TWO TyPES oF
SWELLII4ç
P0=2.4
SPECIAL ARTICLES differ in permeability, two different con
figurations of the mitochondria are formed. Water, K@ and Na@ penetrate both mem branes very rapidly. Sucrose, on the other hand, penetrates the outer membrane very rapidly, but the inner membrane only
slowly. Serum albumin, polyvinyl pyrroli
done and polyglucose do not penetrate either membrane readily. We found that thyroxine causes a general increase in per meability of the inner mitochondrial mem brane to a variety of substances; for exam pie, sucrose. When mitochondria undergo
such swelling with a two- to three-fold in
crease in volume, they show increased rates of oxidation, but a greatly reduced ability to catalyze formation of ATP. Clearly the
volume and degree of swelling of the mito
chondria can dictate their metabolic ac
tivity.
Within the past year, we found that swelling of mitochondria caused by thyrox ine in this way may be reversed under con ditions which are close to physiologic. If the swelling takes place in a physiologic medium high in potassium chloride, then the swelling can be reversed with the ex trusion of water simply by addition of ATP (Fig. 12). In these experiments, the volume
Ftc.12.Swellingof rat-livermitochondriain the
presence of thyroxine and their contractionby
ATP. The decrease in optical density corresponds
to an increase in water content, and vice versa. It is also seen that the P:O ratio declines during swelling, but is restored again during the contrac tion stage. However, the mitochondria lose DPN and also their respiratory response to ADP after
drastic swelling. I--J U) a I-4 U) Li 0 D520 0.4
60 p MOLES
ATP
MOLES H2O EXTRUDED =
390 MOLES ATP SPLIT
60 20
MINUTES
FIG. 13. The relationship of AlP-splitting to water extrusion by isolated mitochondria. It is seen that hundreds of molecules of water may be extruded
per mole of ATP split.
of the mitochondrion is an inverse function of light transmission through the suspen sion. As can be seen, the volume of the mitochondrion quickly returns to its original value after addition of ATP.
The action of ATP in shrinking the mito chondrion is entirely specific; no other sub stance yet tested can replace it. Further more, it has been found that the degree of shrinking of the mitochondrion is deter mined by the concentration of ATP added. Another very important point shown in
Figure 12 is that ATP can restorenearly a
normal rate of oxidative phosphorylation when it causes contraction of the thyroxine swollen mitochondria. The swelling and con traction thus can produce changes in res piration and phosphorylation, implying that
this cycle may be important in metabolic
control.
We also measured the amounts of water which are moved into and out of mitochon dna during this cycle, by direct gravimetric and isotopic procedures. At the beginning
of such an experiment (Fig.13),thyroxineis
added and the mitochondnia swell, as is shown by the drop in the optical trace. Di
rect measurement of the water uptake dur
ing swelling shows that 780 @moleof water
entered the mitochondria, which approxi mately doubled their volume. On addition
of ATP, the mitochondria promptly under
went contraction with extrusion of water,
as is shown by the optical trace. About 650
p.moles of water were extruded on adding
only 60 @moleof ATP.
Actually only a very small fraction of the added ATP undergoes splitting during con traction of the mitochondria. As can be seen, only 1 or 2 p.moles of ATP are split (luring the contraction, and as soon as con traction ceases the hydrolysis of ATP also
stops. These findings show that the mito chondria thus can extrude several hundred molecules of water for each molecule of
ATP split. These facts, together with others
which cannot be discussed here, are con
sistent with the existence of a contractile
system in the membranes that can literally
squeeze water and other small molecules
out of the mitochondria 1w mechanical
means.
These findings have encouraged us to
approach the biochemical and enzymic
basis of the contractile mechanism. It has been found that when ATP is added to fragments of the mitochondrial membrane, they do not absorb and extrude water, but they do change their shape in the presence of ATP. We have also found that the con tractile enzymes in the mitochondrial mem brane are probably identical with one or
more of the intermediate coupling enzymes
of the respiratory chain, because the con traction of the mitochondria is inhibited by certain agents which can also uncouple oxi
dative phosphorylation, such as azide, or
polyhydroxylic molecules such as sucrose. If we again look at a respiratory assembly (Fig. 9), this finding means that one of the phosphate-coupling enzyme molecules in
FIG. 14. Arrangement of mitochondria in the renal tubule cell
the assembly may be “¿mechano-enzyme― which changes shape depending on whether or not it is phosphorylated. This change of shape presumably causes contraction of the membrane.
The mitochondnial membrane is not only a complex electron-carrying and phosphory lation enzyme system capable of converting
energy of oxidation into ATP, but also is a mechanoenzyme system capable of changes of dimension and permeability.
These experiments also show that the meta bolic activities of the mitochondrion are greatly affected by its geometrical config uration and the degree of swelling. These interrelationships permit the possibility of a number of control mechanisms, of a feed back nature, governing the availability of fuel for the mitochondnia, its oxidative utili zation and the formation of ATP. The ex periments also suggest a physiologic role
for thyroxine, but I will not be so foolhardy as to insist that these effects are a full ex planation of the physiologic action of the hormone.
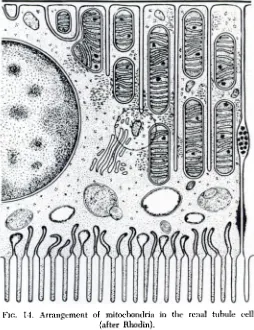
Figure 14 is a diagram of the configura tion of a renal tubule cell,as deduced by Rhodin from electron micrographs of kidney
sections. It is seen that the mitochondria are aligned in the direction of transport of water and solutes between blood and urine; this arrangement suggests the mitochondnia
are not only the power supply for active
transport, but they may also be active vehicles for the transport of water and elec trolytes. Although the research on mito chondnial structure and function I have de
scribed is perhaps far removed from the
clinic, yet the transport functions of the renal tubule cell are surely of daily sig
nificance in the practice of pediatric medi
1960;26;466
Pediatrics
Albert L. Lehninger
MITOCHONDRIA
THE ENZYMIC AND MORPHOLOGIC ORGANIZATION OF THE
Services
Updated Information &
http://pediatrics.aappublications.org/content/26/3/466 including high resolution figures, can be found at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or in its
Reprints
1960;26;466
Pediatrics
Albert L. Lehninger
MITOCHONDRIA
THE ENZYMIC AND MORPHOLOGIC ORGANIZATION OF THE
http://pediatrics.aappublications.org/content/26/3/466
the World Wide Web at:
The online version of this article, along with updated information and services, is located on
American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.