Technology of Making Tablets
Advantages
• Production aspectProduction aspect
– Large scale production at Large scale production at lowest cost
lowest cost
– Easiest and cheapest to Easiest and cheapest to package and ship
package and ship – High stabilityHigh stability
• User aspect (doctor, User aspect (doctor, pharmacist, patient) pharmacist, patient) – Easy to handlingEasy to handling
– Lightest and most compactLightest and most compact – Greatest dose precision & Greatest dose precision &
Disadvantages
• Some drugs resist Some drugs resist
compression into dense compression into dense compacts
compacts
• Drugs with poor wetting, slow Drugs with poor wetting, slow dissolution, intermediate to dissolution, intermediate to large dosages may be difficult large dosages may be difficult or impossible to formulate and or impossible to formulate and manufacture as a tablet that manufacture as a tablet that provide adequate or full drug provide adequate or full drug bioavailability
bioavailability
• Bitter taste drugs, drugs with Bitter taste drugs, drugs with an objectionable odor, or an objectionable odor, or
Types of tablets
• 1)compressed tablets
• 2)sugar coated tablets
• 3)film coated tablets
• 4)enteric coated tablets
• 5)effervescent tablets
• 6)chewable tablets
• 7)dispersible tablets
• 9)multilayer tablets
• 10)sublingual tablets
• 11)toroches
• 12)buccal tablets
• 13)implant tablets
• 14)hypodermic tablets
• 15)solution tabletc
EXCIPIENTS FOR COMPRESSED
TABLETS
A. DILUENTS
Diluents increase the volume to a formulation to
prepare tablets of the desired size. Widely used
fillers are lactose, dextrin, microcrystalline
cellu-lose starch, pregelatinized starch, powdered
• The diluent is selected based on various factors, such as the experience of the manufacturer in the preparation of other tablets, its cost, and compatibility with other
formulation ingredients. For example, in the preparation of tablets or capsules of tetracycline antibiotics, a
calcium salt should not be used as a diluent since
B.BINDERS
Examples of Binders Carboxymethylcellulose, sodium Karaya gum
Cellulose,microcrystalline(Avicel®) Starch, pregelatinized
Ethylcellulose Tragacanth gum
Hydroxypropyl methylcellulose Poly(acrylic acid)
Methylcellulose Polypvinylpyrrolidone
Acacia gum Gelatin
Agar Dextrin
Algin acid Glucose
C. LUBRICANTS
• Lubricant is a substance capable of reducing or
preventing friction, heat, and wear when introduced as a film between solid surfaces. It works by coating on the surface of particles, and thus preventing adhesion of the tablet material to the dies and punches.
• 1. Lubricants improve the flow of granules in the hopper to the die cavity.
• 2. Lubricants prevent sticking of tablet formulation to the punches and dies during formulation.
• 3. Lubricants reduce the friction between the tablet and the die wall during the tablet’s ejection from the tablet machine.
D. DISINTEGRATORS
•
The breakup of the tablets to smaller particles is important for dissolution of the drug and subsequent bioavailability.Disintegrators promote such breakup. To rupture or breakup of tablets, disintegrating agents must swell or expand on exposure to aqueous solution. Thus, the most effective disintegrating agents in most tablet systems are those with the highest wa-ter uptake
E. WETTING AGENTS
• Water molecules attract each other equally in all directions. Water
molecules on the surface, however, can only be pulled into the bulk water by water molecules underneath, since there are no water molecules to pull in the opposite direction. The surface tension of water is strong
enough to support the weight of tiny insects such as water striders. The surface ten-sion in action can be visualized by placing a small drop of alcohol on a thin layer of water. Alcohol with lower surface tension mixes with water causing reduction in the surface tension in the local region. Owing to the higher surface tension of water in the neighbor, water is
Compressed tablet manufacture
•The classification of manufacturing methods
wet granulation: suitable for drugs that are stable to moistur e and heat
dry granulation: suitable for drugs that are sensitive to moi sture and heat
powder compression : suitable for drugs that are sensitive to moisture and heat, fill material possessing, good flowabilit
granulation
drug
excipients
smash sieving mix
adhesive
prilling
dry processing granule
lubricant
mix press
dry granulation
drug
excipient
smash sieving mix press
cake smash
processing granule
adhesive
drugs
excipients
smash sieving mix
adhesive
mix press
•
crystal compression
drugs
excipients
smash sieving
mix mix press
• wet granulation technology
• ( 一 )wet granulation methods and equipment:
Compressed tablet manufacture
—— wet granulation
• The steps of wet granulation
weighing and blending the ingredients(disintegrant)
preparing a damp mass
screening the damp mass into pellets or granules
drying the granulation
sizing the granulation by dry screening
adding lubricant and disintegrant, and blending
tableting by compression
(liquid binder)
Internal( 内加
法 )
External( 外加
The classification of tablet presses
• Tablet presses:
a. single-punch presses
The main components of single-punch
tablet presses
h o p p e r
f e e d s h o e
l o w e r p u n c h d i e
u p p e r p u n c h
t a b l e t w e i g h t a d j u s t o r
t a b l e t e j e c t o r a d j u s t o r
t a b l e t h a r d n e s s a d j u s t o r
S c h e m a t i c d i a g r a m o f t h e m a i n
c o m p o n e n t s o f s i n g l e - p u n c h p r e s s d i e
c a v i t y
Core components: die
The basic mechanical process of tableting
with single-punch presses
a) filling material
b) scraping away the excessive granulation
c) forming a tablet by compression
d) pushing up the tablet to stage surface
e) shoving the tablet aside
u p p e r p u n c h
d ie lo w e r
p u n c h
ta b le t
fill m a te ria l
a ) b ) c )
A picture of multi-station rotary press
Z P 1 9 r o t a r y t a b l e t p r e s s
hopper feed-frame
head: upper turret, lower turret, die table
The core components and compression
cycle of rotary presses
A: upper punch B: die cavity C: die
D: lower punch
The compression is applied by both the upper punch and the lower punch.
Compressed tablet manufacture
—— Direct compression tableting
Suitable for
1) granular chemicals possessing free flowing and cohesive properties
e.g. potassium chloride
2) chemicals added with special pharmaceutical
The direct compression tableting excipients include:
a) fillers, as spray-dried lactose, microcrystals of alphamonohydrate lacto
se, sucroseinvert ,sugar – corn starch mixtures, microcrystalline cellulos
e, crystalline malt and dicalcium phosphate;
d) disintegrants, as direct-compression starch, sodium carboxymethyl star
ch, cross-linked carboxymethylcellulose fiber, and cross-linked polyvinyl
powder of sophora
AIopecuroides L . Seed mix 制软材
prilling 、
processing granule
mix
press
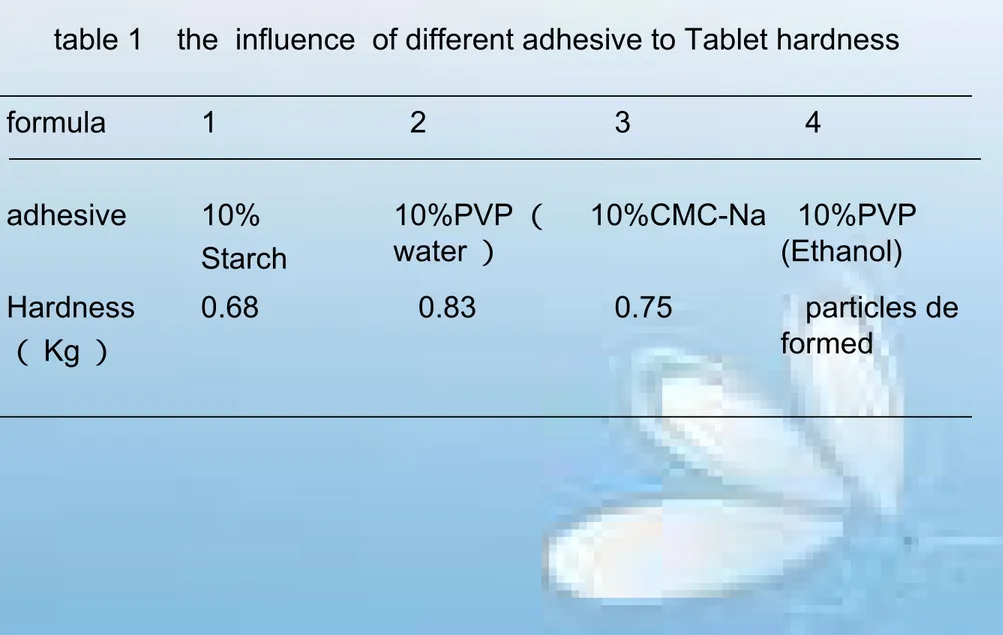
table 1 the influence of different adhesive to Tablet hardness
formula 1 2 3 4
adhesive 10%
Starch
10%PVP ( water )
10%CMC-Na 10%PVP (Ethanol)
Hardness
( Kg )
0.68 0.83 0.75 particles de
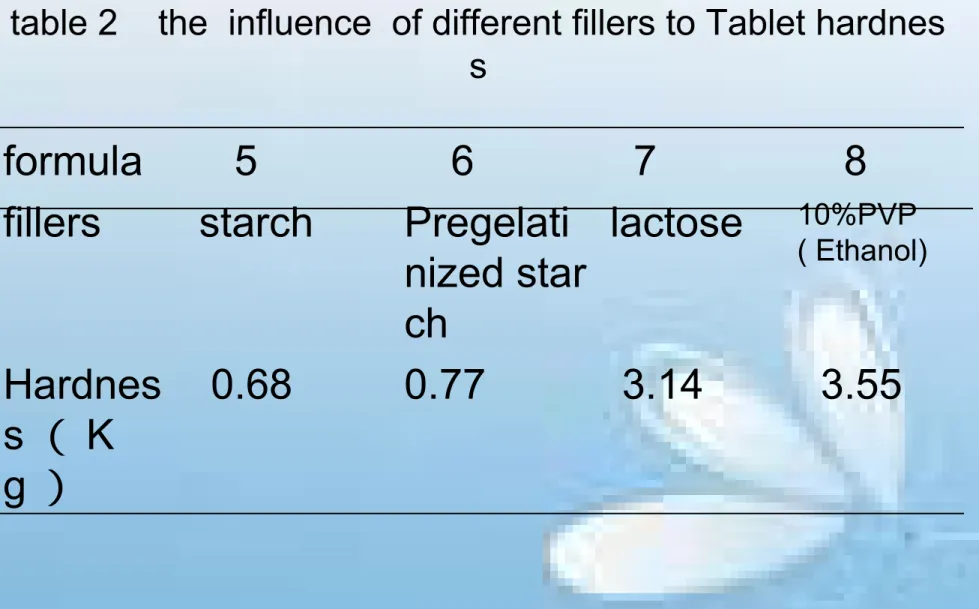
table 2 the influence of different fillers to Tablet hardnes s
formula
5
6
7
8
fillers
starch
Pregelati
nized star
ch
lactose
10%PVP( Ethanol)
Hardnes
s
(
K
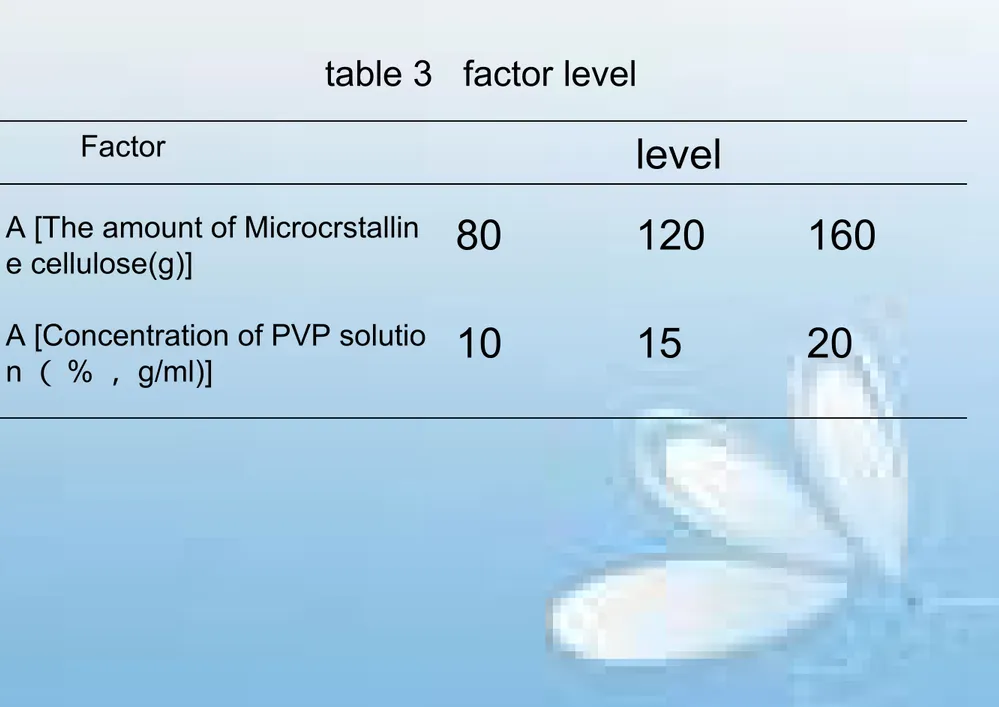
table 3 factor level
Factor
level
A [The amount of Microcrstallin
e cellulose(g)]
80
120
160
A [Concentration of PVP solutio
table 4 Result of Orthogonal test
Test NO. A B
A×B Result 1 2
Ⅰ Ⅱ Total
1 2 3 4 5 6 7 8 9 K1 K2 1 1 1 2 2 2 3 3 3 17.8 24.2 1 2 3 1 2 3 1 2 3 23.0 21.8 1 2 3 2 3 1 3 1 2 21.4 22.5 1 2 3 3 1 2 2 3 1 22.2 22.0
3.1 2.9 6.0 2.8 3.1 5.9 3.2 2.7 5.9 4.1 4.4 8.5 4.0 4.1 8.1 4.2 3.4 8.5 4.0 3.8 7.8
table5 Analysis of variance table
variance source SS V MS F P
total variance 5.658
A 4.698 2 2.349 33.562 0.0001
B 0.191 2 0.096 1.366 0.3034
A×B 0.139 4 0.035 0.993 0.4077
table 6 微晶纤维素用量影响苦豆子片硬度的q检验(n=6)
Comparison group
两均数之
差 standard value of q error
Number of
group α =0.05 α= 0.01
P
A1andA3 -1.1 0.1074 -10.241 3 4.34 6.33 <0.01
A1andA2 -1.0 0.1074 -9.310 2 3.46 5.24 <0.01
Tablet coating
The reasons for tablet coating
1) to protect the medicinal agent against destructive exposure to air and/or humidity;
2) to mask the taste of the drug;
3) to provide special characteristics of drug release;
4) to provide aesthetics or distinction to the product;
The general methods involved in coating tablets are as follows
1) sugarcoating tablets
2) film-coating tablets
3) enteric coating
4) pan coating
The sugarcoating of tablets may be divided into the following steps:
1) waterproofing and sealing (if needed)
2) subcoating
3) smoothing and final rounding
4) finishing and coloring (if desired)
—— —— —— —— ——
1) waterproofing and sealing (if needed)
aim: to prevent the components from being adversely affected by moisture; one or more coats; shellac , zein , or a polymer
as cellulose acetate phthalate
2) Subcoating aim: to bond the sugar coating to the tablet and provide rounding
b) When the tablets are partially dry they are sprinkled with a dusting powder, usually a mixture of powdered sugar and starch but sometimes talc, acacia, or precipitated chalk as well.
3) smoothing and final rounding
aim: to complete the rounding and smooth the coatings
5 to 10 additional coatings of a thick syrup; This syrup is sucrose-based with or without additional components as starch and calcium carbonate.
4) finishing and coloring
5) imprinting
aim: to impart identification codes and other distinctive symbols to the product
The imprint may be debossed, embossed, engraved, or printed on the surface with ink.
6) polishing
aim: to render the tablets the desired sheen/gloss/luster
a) pans lined with canvas cloth impregnated with carnauba waxand/or beeswax
b) Pieces of wax may be placed in a polishing pan
Tablet coating
——
film-coating tablets
1) The disadvantages of sugarcoating process
a) time-consuming
b) requiring the expertise of highly skilled technicians
c) doubling the size and weight of the original uncoated tablets
d) may vary in size from batch to batch and within a batch
2) The advantages of film-coating process
a) coated tablets having essentially the same weight, shape, and size as the originally compressed tablet
b) The coating is thin enough to reveal any identifying monograms.
c) far more resistant to destruction by abrasion than are sugar-coated tablets
3) The components of nonaqueous film-coating solutions: a) film former: e.g. CAP
b) alloying substance: to provide water solubility or permeability to the film e.g. PEG
c) plasticizer: to render flexibility and elasticity to the coating e.g. castor oil
d) surfactant: to enhance spreadability of the film e.g. polyoxyethylene sorbitan derivatives
e) opaquants and colorants: e.g. titanium dioxide, FD&C or D&C dyes
4) The components of a typical aqueous film-coating solutions:
a) film-forming polymer (7-18%): e.g. cellulose ether polymers as HPMC, HPC and MC
b) plasticizer (0.5-2.0%): e.g. glycerin, propylene glycol, PEG, diethyl phthalate, and dibutyl subacetate
c) colorant and opacifier (2.5-8%): FD&C or D&C lakes and iron oxide pigments
5) Some problems with aqueous film-coating
a) picking and peeling the appearance of small amounts or large am ounts of film fragments flaking from the tablet surface
b) orange peel effect roughness of the tablet surface due to failure of spray droplets to coalesce
c) mottling an uneven distribution of color on the tablet surface d) bridging
filling-in of the score-line or indented logo on the tablet by the film e) tablet erosion
5) Some problems with aqueous film-coating
a) picking and peeling the appearance of small amounts or large am ounts of film fragments flaking from the tablet surface
b) orange peel effect roughness of the tablet surface due to failure of spray droplets to coalesce
c) mottling an uneven distribution of color on the tablet surface d) bridging
filling-in of the score-line or indented logo on the tablet by the film e) tablet erosion
The reasons for capping,
splitting or laminating of
tablets
1) air entrapment
2) not immaculately cleaned or not perfectly smoothed punches
3) too great a proportion of fine powder
quality standards and compendial
requirements
The apparent physical features of compressed tablets:
1) shape: round, oblong, unique 2) thickness: thick or thin 3) diameter: large or small 4) flat or convex
5) unscored or scored in halves, thirds and quadrants
6) engraved or imprinted with an identifying symbol and/or code number 7) coated or uncoated 8)colored or uncolored 9) number of layer.
quality standards and compendial
requirements
Other physical specifications and quality standards:
tablet weight weight variation
content uniformity tablet thickness
tablet hardness tablet disintegration
quality standards and compendial requirements
—— tablet weight and Chp weight variation
Chp weight variation:
sample amount 20 tablets
Tablets should comply with the following
requirements stated in the table below.
Average
weight
Weight
variation
limit
Less than
0.3 g
± 7.5%
0.3 g or
more
quality standards and compendial requirements
—— tablet weight and Chp weight variation
The procedure of weight variation determination in Chp:
quality standards and compendial requirements
—— tablet hardness and friability
Tablet hardness
1)The greater the pressure applied, the harder the tablets.
2) The hardness required by different tablets
a) lozenges and buccal tablets: hard (dissolve slowly)
b) the tablets for immediate drug release: soft
quality standards and compendial requirements
—— content uniformity
applys to potent drug of low dose.
USP method, 10 tablets are individually assayed for their content.
quality standards and compendial requirements
—— tablet hardness and friability
(continued)
Friability
1) It is used to determine a tablet’s durability
2) Method: allowing the tablets to roll and fall within the rotating apparatus (friabilator); determine the loss in weight;
quality standards and compendial requirements
—— tablet dissolution
1) The importance of in vitro dissolution test
a) to guide the formulation and product development process toward product optimization
b) to monitor the performance of manufacturing process
c) to assure bioequivalence from batch to batch
2) The goal of in vitro dissolution is to provide a
reasonable prediction of the product’s in vivo
bioavailability.
Basis: The combinations of a drug’s solubility and
its intestinal permeability are supposed as a
Considered are drugs determined to have:
a) high solubility and high permeability (IVIVC
may be expected.)
b) low solubility and high permeability (IVIVC
may be expected.)
3) The formulation and manufacturing factors
affecting the dissolution of a tablet
a) the particle size of the drug substance
b) the solubility and hygroscopicity of the formulation
c) the type and concentration of the disintegrant,
binder, and lubricant used
d) the manufacturing method, particularly, the
compactness of the granulation and the
compression force
4) Test method
a) A volume of the dissolution medium is placed in the ves sel and allowed to come to 37℃±0.5℃.
b) The stirrer is rotate at the specified speed.
c) At stated intervals, samples of the medium are withdraw n for chemical analysis
5) Requirement for rate of dissolution
6) Inconsistencies in dissolution
occur not between dosage units from the same production batch, but rather between batches or between products f rom different manufacturers.