Therapy
European recommendations on the use of oral
antibiotics for acne
Brigitte DRÉNO1 Vincenzo BETTOLI2 Falk OCHSENDORF3 Alison LAYTON4 Håkan MOBACKEN5 Hugo DEGREEF6 1Department of Dermatology, Hotel Dieu, Place Alexis Ricordeau, 44093 Nantes Cedex 01, France 2Clinica Dermatologica, Azienda Ospedaliera Arispedale S. Anna, Università degli Studi di Ferrara, Corso Giovecca 189, 44100 Ferrara, Italy 3Klinikum der Johann Wolfgang Goethe-Universitat, Zentrum
der Dermatologie und Venerologie (ZDV) Theodor-Stern-Kai 7, D-60590 Frankfurt am Main, Germany
4Harrogate District Hospital, Lancaster Park Rd, Harrogate, North Yorks, HG2 7SX, UK 5Department of Dermatology, Sahlgrenska University Hospital, S-413 45 Göteborg, Sweden 6
University Hospital St Rafaël, Department of Dermatology, Kaucijnenvoer, 33, B3000 Leuven, Belgium Reprints: B. Dréno. Fax: (+ 33) 240083117 brigitte.dreno@wanadoo.fr Article accepted on 16/7/2004
Non-rational prescribing of oral antibiotics in acne is common, and there
is currently an unmet need for up-to-date guidelines that specifically
address these issues. Presented here is a set of recommendations on the
use of oral antibiotics in acne, developed by a group of European acne
specialists, designed to be considered by dermatologists and general
practitioners in their daily practice throughout Europe.
Recommenda-tions cover optimal choice of antibiotic, drug doses, duration of
treat-ment, combination treattreat-ment, and maintenance therapy.
Key words: Acne vulgaris, oral antibiotics, treatment
recommenda-tions.
T
here are many different drugs available for the treatment of acne vulgaris, including oral and topi-cal antibiotics, topitopi-cal benzoyl peroxide, topitopi-cal retinoids, oral isotretinoin, oral anti-androgens, and zinc salts. Many guidelines on how these different agents can be optimally used have been published over the years. These include the ‘Ad hoccommittee report’ on the use of syste-mic antibiotics for treatment of acne vulgaris (1975) [1]; the American Academy of Dermatology ‘Guidelines for care of acne vulgaris’ (1990) [2]; ‘Treatment of acne vul-garis: guidelines for primary care physicians’ (1991) [3]; ‘Oral treatment of acne’ in France (1999) [4]; and most recently the ‘Global alliance to improve outcomes in acne’ consensus recommendations (2003) [5]. Figure 1 shows the acne treatment algorithm proposed by the ‘Global alliance’, in which oral antibiotics, used in combination with topical retinoids, are recommended for the treatment of moderate to severe inflammatory acne.Other than the 1975ad hoccommittee report [1], the other guidelines listed above provide general recommendations on acne management, but fall short of providing detailed guidance on the use of oral antibiotics. This is probably due
to the fact that much of the clinical evidence derives from trials that vary greatly in acne definition, end-points, doses used, duration of treatments etc., making direct compari-sons difficult. Perhaps as a consequence of this lack of guidance, there is considerable variation in the way in which antibiotics are used to treat acne across Europe, and their inappropriate use is alarmingly common (patients are often treated for excessively long periods of time, and/or there is much variation in antibiotic dosage given). The preferred choice of antibiotic varies from country to country, with first generation cyclines (tetracycline HCl, and oxytetracycline) very popular in the UK, minocycline preferred in Belgium, and lymecycline particularly popular among dermatologists in France, Italy, and Nordic coun-tries. Some of these differences may be accounted for by climate (for example, doxycycline, known to have the po-tential to cause dose-dependent photosensitivity, is less commonly prescribed in Southern Europe during the sum-mer), and by various pharmacoeconomic considerations. Thus, despite the problems in forming truly evidence-based guidelines on the use of oral antibiotics in acne, there is clearly an unmet need for recommendations on the use of
Disclaimer
These recommendations have been prepared for dermatologists and general practitioners on behalf of the ‘European expert group on oral antibiotics in acne’ and reflect the best data available at the time the report was prepared. Caution should be exercised in interpreting the data: the results of future studies may require alteration of the conclusions or recommendations of this report. It may be necessary or even desirable to depart from the recommendations in the interests of specific patients or special circumstances. Just as adherence to these recommendations may not constitute a defence against a claim of negligence, so deviation from them should not necessarily be deemed negligent.
oral antibiotics that can be used in the daily practice of physicians treating acne across Europe. This paper there-fore presents the available clinical data and expert opinion, followed by a set of detailed and user-friendly recommen-dations on many aspects of the use of oral antibiotics in acne.
Methodology
These recommendations were developed over a series of three meetings in 2002 and 2003. During the first two meetings, a core of six independent European acne specia-lists reviewed current practices around Europe; conducted a systematic literature review (using Medline) covering the years 1992 to 2003; and discussed personal experiences. In a final workshop, the findings of this core group were presented to the wider group of 23 acne specialists, mainly from Europe, but also Brazil and Morocco(Appendix 1)for
discussion and review. Recommendations are based on efficacy, practical applicability in daily practice, safety/tolerability, antimicrobial resistance, and pharmaco-economic considerations.
I. Literature review
The pathophysiology of acne and rationale for using antibiotics
There are a number of pathophysiologic components to acne, including sebaceous gland hyperplasia with sebor-rhoea; altered follicular growth and differentiation; micro-bial colonization; and inflammation and other immune res-ponses [5]. The precursor lesion in all acne is the microcomedone, which features altered follicular growth and differentiation, and sebaceous gland hyperplasia with seborrhoea. Microcomedones can then enlarge to form non-inflammatory closed or open comedones, and
Mild Moderate Severe
Comedonal Papular/pustular Papular/pustular Nodular1 Nodular/ conglobate
1st3 Topical retinoid Topical retinoid + topical antimicrobial Oral antibiotic + topical retinoid +/– BP Oral antibiotic + topical retinoid + BP Oral isotretinoin2
Alternative3 Azelaic acid or salicylic acid Alternative topical antimicrobial + alternative topical retinoid or azelaic acid4 Alternative oral antibiotic + alternative topical retinoid +/– BP Oral isotretinoin or alternative oral antibiotic + alternative topical retinoid +/– BP/azelaic acid4
High dose oral antibiotic + topical retinoid + BP Alternatives for females3 See 1st choice See 1st
choice Oral antiandrogen + topical retinoid/azelaic acid4 +/– BP Oral antiandrogen + topical retinoid +/– oral antibiotic +/– alternative antimicrobial
High dose oral antiandrogen + topical retinoid +/– alternative topical antimicrobial Maintenance: Topical retinoid +/– BP
1
Papulopustular acne with some nodular lesions; 2Second course in case of relapse; 3Consider physical removal of comedones. 4There was no consensus on this alternative recommendation. However, in some countries, azelaic acid
prescribing is appropriate practice. BP: benzoyl peroxide.
Figure 1. Acne treatment algorithm suggested by Gollnick et al. (2003) [5]. Reprinted from the Journal of the American Academy of Dertatology,Vol. 49 (I suppl.); Gollnicket al.Management of acne: a report from a global alliance to improve outcomes in acne, pages S1-S38, © 2003, American Academy of Dermatology, Inc, with permission from Elsevier.
bial colonization can result in the formation of inflamma-tory lesions (papules, pustules, or nodules). By using diffe-rent agents in combination, acne pathophysiology can be targeted from a number of different angles simultaneously, improving therapeutic outcome [5]. For example, topical retinoids (some of which have proven anti-inflammatory properties) target the microcomedone, and are therefore suitable for use in combination with other drugs that target Propionibacterium acnes and inflammation, and may be suitable for maintaining remission following successful treatment [6].
Microorganisms that are commonly present on the skin of patients with inflammatory acne include the yeasts Pityrosporum spp., coagulase-negative staphylococci, and P. acnes[7]. Eradication ofPityrosporum spp., using anti-mycotic therapy, does not have any effect on acne [8], and use of antibiotics induces resistance in coagulase-negative staphylococci well in advance of observed responses to treatment [9]. Studies have shown that infection with the gram-positive, pleomorphic, anaerobic rod,P. acnescauses inflammation of sterile cysts [10], but that deadP. acnesor livingStaphylococcus epidermidisdo not cause inflamma-tion [11]. ThereforeP. acnes, has been implicated in in-flammatory acne lesions.
The concentration of P. acnes generally correlates with patient’s sebum production [12], but not with the degree of inflammation or the severity of acne [7, 13, 14]. However, the humoral and cellular immune responses to P. acnes correlate with acne severity. Colonization with P. acnes results in secretion of extracellular enzymes, cytokines such as IL-1a, and heat-shock proteins, all of which have mitogenic effects on T-cells [15-19]. Thus P. acnes is associated with inflammatory acne not in a concentration-dependent manner, but in an inflammation-concentration-dependent man-ner. Antibiotics that can both reduce the number ofP. acnes and reduce inflammation by different mechanisms are the-refore of utility in moderate to severe acne associated with papules, pustules, and nodules, and in acne conglobata (Figure 1). A recent study has shown that inflammation features in the very earliest stages of acne lesion develop-ment [20]. Follicles without microcomedonal features had elevated CD3 T-cells, CD4 T-cells, and macrophages in the perifollicular and papillary dermis, as well as changes and activation of vascular intercellular adhesion molecules [20].
Oral antibiotics used in acne
Cyclines, macrolides, clindamycin, trimethoprim, co-trimoxazole, and quinolones all have efficacy in acne [21, 22]. Notably, penicillins, cephalosporins, aminoglycosides, and chloramphenicol have very limited effects in inflam-matory acne [1, 23, 24].
Cyclines
Cyclines (tetracycline HCl, oxytetracycline, lymecycline, doxycycline, and minocycline) have very good efficacy in acne and generally have a good safety profile. Side effects include gastrointestinal disturbance, and some drug-specific effects discussed later in this paper. There is cross-resistance within the class, but no cross-cross-resistance to other antibiotic classes. Cyclines are contraindicated in children under 8-12 years (varying according to national licenses) and in pregnancy due to their effect on growing bone tissue (causing inhibition of skeletal growth in the foetus and discoloration of growing teeth). Cyclines form the
corner-stone of oral antibiotic therapy in acne, and are discussed in detail later in this paper.
Macrolides
The utility of oral macrolides (mainly erythromycin) in acne is increasingly limited due to the increasing problem of microbial resistance to these agents [23, 25]. Eadyet al. (1989) [26] demonstrated a clear correlation between car-riage of erythromycin-resistantP. acnesand poor clinical efficacy. There is frequently cross-resistance between erythromycin and clindamycin. Macrolides are therefore reserved for cases where cyclines are not tolerated, or are contraindicated (e.g. pregnancy and breastfeeding).
Clindamycin
Although effective, oral clindamycin is rarely used in acne because of potentially serious adverse effects [27]. Distur-bance of gastrointestinal flora by this agent can cause overgrowth ofClostridium diffıcile and result in pseudo-membranous colitis. Diarrhoea is seen in 5-20% of patients using this agent [28].
Co-trimoxazole and trimethoprim
The use of co-trimoxazole (trimethoprim plus sulpha-methoxazole) or trimethoprim alone is limited, and these agents are not licensed for use in acne. This is because of the potential for development of serious allergic reactions to the sulphamethoxazole component of co-trimoxazole, which may be seen in up to 3% of patients. Therefore, these agents are limited to situations where there is proven resis-tance to other agents or as a third-line treatment [22, 29].
Quinolones
Although one Japanese study has shown efficacy of levo-floxacin in acne [30], oral quinolones are not used in acne because of the small amount of compelling efficacy data, the problems of adverse events (seen in 3-6% of patients), potential for antibiotic resistance (particularly the develop-ment of quinolone resistance in commensal bacteria [31]), high price, and unsuitability of these agents for adolescents (due to potential effects on articular cartilage) [32]. Side effects include agitation, headache, hallucination, gastro-intestinal disturbance, arthralgia, tendinitis, and photosen-sitivity.
Mechanisms of action of antibiotics in acne Antibacterial actions
As already discussed, the density ofP. acneson the skin of acne patients does not correlate well with the degree of inflammation or the severity of acne. Similarly, the magni-tude of the reduction inP. acnescounts following antibiotic therapy does not correlate well with clinical efficacy [33, 34]. However, the fact that the presence ofP. acnesappears to be associated with and apparently required for the for-mation of inflammatory lesions; that successful antibiotic treatment of acne is associated with a reduction in theP.
acnes population; and that acne associated with
erythromycin- or tetracycline-resistant P. acnes does not always respond as well to treatment with those agents [26, 35, 36], may suggest an important role for antibacterial activity in the efficacy of antibiotics in acne. The different classes of agents exert their antibacterial effects in different ways. Cyclines, macrolides and clindamycin inhibit bacte-rial protein synthesis (by different mechanisms); trimetho-prim and sulphamethoxazole interfere with bacterial folate metabolism; whilst quinolones inhibit bacterial DNA gy-rase.
Non-antibacterial actions
Non-antibacterial actions include bacterial lipase inhibition [37-39], and anti-inflammatory/immunomodulatory effects [40-43].P. acnessecretes lipases, which convert diglyceri-des and triglyceridiglyceri-des into free fatty acids. Free fatty acids in turn cause follicular hyperkeratinisation and contribute to the clinical picture of inflammatory acne. It has been shown that cyclines and macrolides inhibit bacterial lipases, inde-pendent of their antibacterial effects [37-39].
Macrolides and cyclines also interact with the immune response in a complex way, and many different effects have been observed [40-43]. These include direct, dose-dependent inhibition of lymphocyte mitosis; inhibition of phagocytosis; decrease in the secretion of the pro-inflammatory cytokines TNF-a, IL-1 and IL-6; increase in the secretion of the anti-inflammatory cytokine IL-10; inhi-bition of leukotaxis; decreased activation of complement protein C3 (only noted with cyclines); modulation of a-MSH (demonstrated with minocycline); and inhibition of reactive oxygen species generation [44-47].
One of the main questions today concerning the efficacy of antibiotics in acne is to determine whether the main activity of antibiotics in acne is antibacterial or antiinflammatory. The observed efficacy of antibiotics used at low (sub-MIC) doses suggests that the non-antibacterial effects play an important role [34].
Antibiotic resistance inP. acnes
Antibiotic resistance inP. acneswas first described in 1979, when erythromycin resistance was found in a single isolate in the USA [48]. Since then, the incidence of resistance has risen, and a recent survey, conducted throughout Europe, showed that at least 50% of acne patients are colonized by erythromycin- and clindamycin-resistant strains ofP. ac-nes, and as many as 20% are colonized with cycline-resistant strains [25]. Resistance emerges through either selection of pre-existing resistant bacterial strains, or throughde novoacquisition of a resistant phenotype. Emer-ging antibiotic resistance in acne has been shown to deve-lop in response to antibiotic prescribing [25]. The duration of treatment required before resistance emerges varies greatly between patients, but the longer the duration of treatment, the more likely antibiotic-resistantP. acneswill emerge, and courses of 6 months are highly likely to result in resistance.
There is a recognized correlation between the presence of antibiotic-resistantP. acnesand clinical response to treat-ment with erythromycin and tetracyclines (although this is less well established) [26, 35, 36]. Antibiotic resistance should therefore be considered as a possible contributory factor to, or possible cause of, therapeutic failure. High dose antibiotics will reduce sensitive strains and allow overgrowth of resistant strainsin situ. However, depending on the mechanism of resistance, low dose antibiotics may also encourage overgrowth of resistant strains. In addition, low dose antibiotics may inducede novoantibiotic resis-tance in other commensal bacteria present (e.g. staphylo-cocci), which often develop resistance much more quickly than resistance inP. acnes. For these reasons, the common practice of using low dose antibiotics for prolonged periods of time should not be recommended.
Antibiotic-resistant strains can be transmitted between in-dividuals, and studies have shown that 41-85.7% of untrea-ted close contacts of acne patients under long-term antibio-tic treatment harbor erythromycin-resistant strains of P.
acnes[25]. Furthermore, 25 out of 39 acne specialists tes-ted were colonized by resistant strains, compared with 0 out of 27 non-dermatologist physicians [25]. Resistant strains can be reduced by using the topical antibacterial agent benzoyl peroxide at the site of application. However no single available agent will fully eradicate antibiotic-resistantP. acnes.
Other ways of preventing the emergence of resistant strains include:
1. Do not use antibiotics where other acne treatments can be expected to bring about the same degree of benefit. 2. Use antibiotics according to clinical need.
3. Do not use antibiotics as a monotherapy [5].
4. Stop antibiotic therapy when you and the patient agree there is no further improvement or the improvement is only slight (one UK-based study suggested that 6-8 weeks into treatment might be one appropriate time-point at which to assess response to antibiotics [36]).
5. Try to avoid continuing antibiotics beyond six months. 6. Use benzoyl peroxide either concomitantly or pulsed as an anti-resistance agent.
7. Do not switch antibiotics without adequate justification (i.e. re-use the same antibiotic for subsequent courses if patients relapse).
Risk factors for developing or acquiring antibiotic-resistant P. acnesinclude prolonged duration of antibiotic therapy, multiple courses of antibiotics, close contact with acne patients being treated with antibiotics, and poor compliance with treatment [25].
Topical benzoyl peroxide has been shown to be active against fully sensitive and resistant strains ofP. acnes.This agent therefore reduces the likelihood of antibiotic-resistant P. acnes emerging and reduces the number of resistant bacteriain situ[49]. Other ‘anti-resistance’ agents include topical zinc acetate [50] and oral isotretinoin [51]. The development of antibiotic resistance can also be redu-ced by ensuring that antibiotics are not used unnecessarily, treatment duration is not excessively long, and patients comply well with treatment [25]. Combining therapy with topical retinoids will also expediate improvement while targeting the microcomedone [5].
In cases where antibiotic resistance is suspected, these drugs are often simply discontinued. Ideally, however, such cases should be managed by first swabbing and culturing to verify the presence of resistant strains, and then using non-antibiotic therapies such as topical benzoyl peroxide, topical or systemic retinoids, hormonal therapies, or syste-mic zinc salts. Raising antibiotic doses can also be consi-dered.
The use of oral cyclines in acne Pharmacokinetics
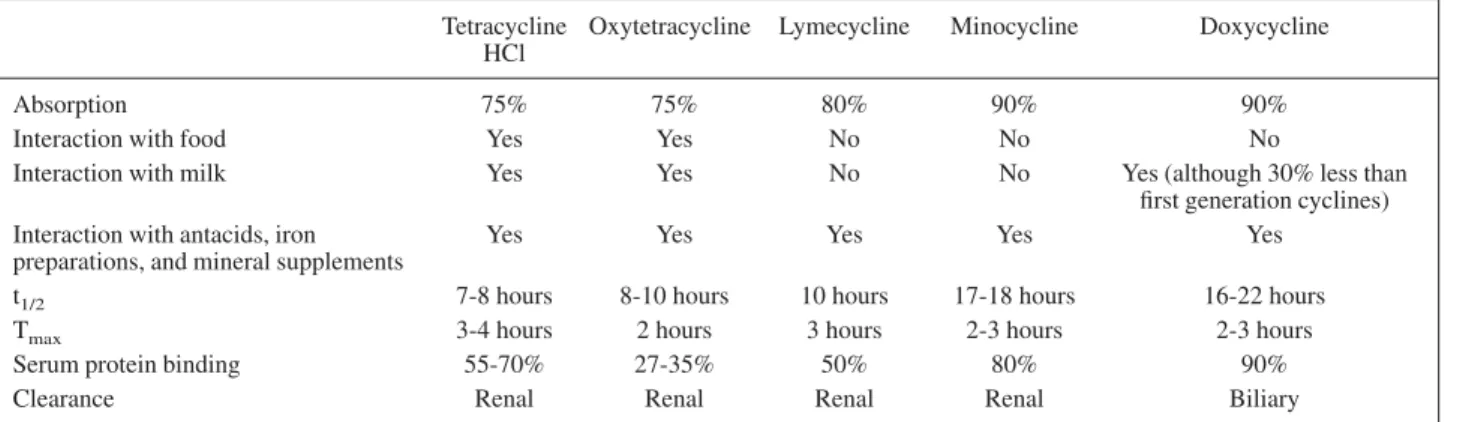
Cyclines can be classified as ‘first generation’ (tetracycline HCl and oxytetracycline) and ‘second generation’ (lymecy-cline, doxycy(lymecy-cline, and minocycline). The key difference between first and second generation cyclines is their phar-macokinetic profiles (Table I). First generation cyclines must be taken at least twice daily and their absorption is impaired by food and milk, whereas second generation cyclines can be taken once or twice daily with their absorp-tion unaffected by food. These differences may adversely affect compliance with first generation cyclines (particu-larly among adolescents), which may lead to therapeutic failure and the development of resistance. There are few pharmacokinetic differences between individual second
generation cyclines, although it is noteworthy that only doxycycline is cleared by the liver, allowing this agent to be used in patients with renal impairment.
Efficacy
While the efficacies of first and second generation cyclines have not been compared in large randomized clinical trials, second generation cyclines have been compared in a num-ber of clinical trials(Table II)[52-57]. It can be seen that there is little to choose between these molecules in terms of efficacy, with reductions noted in the number of inflamma-tory lesions of 51-77% following 3-6 months of treatment. Efficacy and speed of response to treatment can be signifi-cantly improved by using topical retinoids concurrently (to target microcomedones, comedones and inflammation). In a study of 242 acne patients, a three month course of lymecycline (300 mg daily) was compared with lymecy-cline (300 mg daily) plus topical adapalene (0.1%) applied daily [58]. Lymecycline and combined therapy reduced the
total number of lesions by a mean of 47.9% and 58.7%, respectively (P = 0.0033); inflammatory lesions were reduced by a mean of 45.6% vs 60.3%, respectively (P = 0.0001); and non-inflammatory lesions were reduced by a mean of 47.6% vs 56.6%, respectively (P = 0.01) [58]. Mobacken (1993) [54] and Campoet al.(2002) [55] exa-mined the effects of treatment for up to 6 months. After 1 month of treatment with lymecycline (600 mg daily), Mobacken (1993) [54] found that facial papules and pustu-les had reduced in number by 47.5%, compared with 76.5% and 76% following a further 5 months of treatment with lymecycline at 300 mg or 600 mg daily, respectively. Thus it can be seen that additional efficacy can be gained by extending treatment with oral antibiotics beyond 1 month. Campo et al.(2002) [55] compared the efficacy of treat-ment with lymecycline (300 mg daily for 2 weeks, fol-lowed by 150 mg daily) plus topical adapalene, with mino-cycline (100 mg daily) plus topical adapalene for up to Table I. Pharmacokinetics of cyclines compared
Tetracycline HCl
Oxytetracycline Lymecycline Minocycline Doxycycline
Absorption 75% 75% 80% 90% 90%
Interaction with food Yes Yes No No No
Interaction with milk Yes Yes No No Yes (although 30% less than
first generation cyclines) Interaction with antacids, iron
preparations, and mineral supplements
Yes Yes Yes Yes Yes
t1/2 7-8 hours 8-10 hours 10 hours 17-18 hours 16-22 hours
Tmax 3-4 hours 2 hours 3 hours 2-3 hours 2-3 hours
Serum protein binding 55-70% 27-35% 50% 80% 90%
Clearance Renal Renal Renal Renal Biliary
Table II. Effıcacies of second generation cyclines compared
Study Design Lymecycline
(mean % reduction in number of lesions) Minocycline (mean % reduction in number of lesions) Doxycycline (mean % reduction in number of lesions) 3 months 6 months 3 months 6 months 3 months Schollhammer and
Alirezai 1994 [52]1 Multicentre, randomized, parallel.N = 77 72.7 68.4 62.4 Bossuytet al.
2003 [53]2
Multicentre, randomized, parallel, investigator-blind. N = 134
58.7 64.5
Mobackenet al.
1994 [54]3 Open-label, parallel dose-rangingstudy. N = 221 76 (76.5) Campoet al.
2002 [55]4 Multicentre, randomized, parallel,investigator-blind. N = 152 67 77 62 67 Cunliffeet al.
1998 [56]5 Multicentre, randomized, double-blind, double-dummy. N = 144 50.6 52.2 Dubertretet al.
2003 [57]6 Multicentre, randomized, double-blind study. N = 270 62 (56.4)
1This study compared lymecycline (300 mg daily for 2 weeks, then 150 mg daily), minocycline (100 mg daily for 2 weeks, then 50 mg daily), and doxycycline
(100 mg daily for 2 weeks, then 100 mg every other day).
2This study compared lymecycline (300 mg daily) with modified-release minocycline (100 mg daily).
3In this study, patients received 600 mg lymecycline daily for 1 month, followed by either 600 mg lymecycline daily or 300 mg lymecycline daily. The value in
brackets refers to the population randomized to the lower dose.
4This study compared lymecycline (300 mg daily for 2 weeks then 150 mg daily) plus topical adapalene, with minocycline (100 mg daily) plus adapalene. 5This study compared lymecycline (300 mg daily for 2 weeks, then 150 mg daily) with minocycline (100 mg daily for 2 weeks, then 100 mg every other day). 6This study compared lymecycline (300 mg OD), lymecycline (150 mg BD), and placebo. The value in brackets refers to the population randomized to the BD
6 months, measuring efficacy every 2 months. After 3 months, the number of acne lesions had reduced by 67% and 62% in the lymecycline and minocycline groups, res-pectively, compared with 77% and 67%, resres-pectively, after 6 months of treatment. These two studies show that patients may benefit from treatment with oral antibiotics for > 1 month, but that there is little advantage in using these agents for > 3 months. In addition, the risk of deve-loping antibiotic resistance is known to increase when treatment is continued beyond 3 months. These issues should be taken into account when considering extending treatment beyond 3 months.
Safety and tolerability
Tetracycline HCl and oxytetracycline are generally well tolerated, but can produce gastrointestinal disturbances. Like all cyclines, first generation molecules can inhibit skeletal growth in the developing foetus and cause discolo-ration of growing teeth (particularly with tetracycline HCl). Cyclines are therefore contraindicated during pregnancy and in young children (under 8-12 years, depending on national licenses).
Lymecycline has a good safety profile and is generally well tolerated, occasionally causing, like other cyclines, tran-sient mild gastrointestinal disturbances, and rarely allergic reactions.
Doxycycline is noted particularly for causing photosensiti-vity. This effect is dependent on doxycycline dose, UVA intensity, and skin type. Photosensitivity also greatly in-creases the potential for phototoxicity (burning effects and photo-onycholysis). Bjellerup and Ljunggren (1994) [59] compared the phototoxicity of doxycycline (200 mg daily) and lymecycline (1200 mg daily) using UVA light. In this study, lymecycline induced a slight increase in erythema compared with placebo at 75 J.cm–2. In contrast, doxycy-cline caused a highly significant increase in erythema com-pared with placebo at 50, 75, and 100 J.cm–2. Patients and doctors should therefore be conscious of the dangers of UVA solaria when using doxycycline, and this drug should be used with caution in hot climates during the summer. Non-comedogenic sun-screens that protect against both UVB and UVA should be considered when taking doxycy-cline (depending on dose and climate).
Minocycline causes a number of rare but severe side effects, including autoimmune disorders (lupus-like syndrome, autoimmune hepatitis, arthritis, thyroiditis, polyarteritis nodosa) [60-68]; and hypersensitivity reactions (pneumo-nitis, eosinophilia, serum-sickness-like syndrome, DRESS [Drug Reactions with Eosinophilia and Systemic
Symp-toms] syndrome, arthritis, vasculitis, and hepatitis) [68]. It has been postulated that minocycline may generate a spe-cific reactive species that could be responsable for such reactions [68]. Other effects include skin hyperpigmenta-tion (particularly in areas exposed to the sun); single organ dysfunction (early onset, dose-related); Sweet syndrome; pseudotumour cerebri [69]; and vestibular disturbances [70]. In a review of minocycline efficacy and safety in acne, Garneret al.(2002) [64] suggested that because of a lack of proven efficacy advantages over other agents, and an uncer-tain safety profile, there could be no justification in conti-nuing to use minocycline as a first line therapy in acne, and in France, the national regulatory authority (AFSSAPS) now recommends that oral minocycline should be reserved as a second choice oral antibiotic in acne.
Pharmacoeconomic considerations
One of the problems with attempting to include a cost component in developing pan-European recommendations on antibiotic use is the very large variation in drug prices across Europe (Table III.e.g. minocycline is almost three times more expensive in the UK than in Italy). For this reason, it is difficult to extrapolate data from one country to another. However, pharmacoeconomic analyses can be use-ful at a national level [71].
One recent study by Bossuytet al.(2003) [53] compared the cost-effectiveness of minocycline (as Minocin MR®) with lymecycline (as Tetralysal 300®) for the treatment of acne in the UK, France and Belgium. In this study, ‘treat-ment success’ was defined as the percentage of patients in which acne was ‘cleared’, ‘much improved’, or ‘improved’. Treatment success was similar for both agents (98.4 and 91.5% for lymecycline and minocycline, respectively, over all countries). However, ‘cost effectiveness’ (calculated as treatment cost divided by treatment success) was 65.89, 42.48, and 49.30 Euros per treatment success for lymecy-cline in Belgium, France, and UK, respectively, compared with 118.81, 80.12, and 125.33 Euros per treatment success for minocycline in Belgium, France, and UK, respectively. This study shows that lymecycline is a more cost-effective acne treatment than minocycline in Belgium, France and the UK, but particularly in the UK.
Cyclines and oral contraceptives: an interaction?
Acne patients are often concerned about the potential inter-action between cyclines and oral contraceptives. Cyclines, and other broad-spectrum antibiotics, could theoretically alter the gut flora, reducing the bacterially-induced hydro-lysis of steroid conjugates formed in the liver and gut wall, Table III. Prices (W) of second generation cyclines per treatment course across Europe at the time of writing
Country Lymecycline
(300 mg daily for 12 weeks)
Minocycline (100 mg daily for 12 weeks)
Doxycycline (100 mg daily for 12 weeks)
Belgium 64.84 108.71 71.22
France 41.80 73.31 33.60
Germany Not currently available 73.96 32.35
Italy 103.201 37.44 34.69
Sweden 64.662 Not currently available 82.423
UK 47.97 114.68 54.08
1300 mg daily for 2 weeks, then 150 mg daily for 10 weeks. 2600 mg daily for 4 weeks, then 300 mg daily for 8 weeks. 3200 mg daily for 4 weeks, then 100 mg daily for 8 weeks.
resulting in a decreased quantity of free steroid for reab-sorption, and hence reduced plasma levels of active steroid. There have been occasional anecdotal reports of oral contraceptive failure in patients taking cyclines, and two uncontrolled retrospective studies have claimed failure ra-tes of 1.2 and 1.4 per 100 years of therapy in patients taking cyclines [72, 73]. One controlled retrospective study exa-mined the failure rate of oral contraceptives in 356 patients who had taken oral contraceptives and antibiotics concur-rently, compared with control patients [74]. This study showed no statistically significant difference between the two groups. A recent large review concluded that available scientific and pharmacokinetic evidence does not support the hypothesis that antibiotics (with the exception of rifam-picin) lower the contraceptive efficacy of oral contracepti-ves [75]. In addition, the American College of Obstetrics and Gynaecologists states that “tetracycline, doxycycline, ampicillin and metronidazole do not affect oral contracep-tive steroid levels” [76]. The weight of this evidence sug-gests that oral cyclines do not interfere with oral contracep-tives and that alternative forms of contraception are not necessary whilst using these agents.
Prescribing cyclines for patients with hyperseborrhoea
In a study of 255 acne patients treated for 6 months, Layton et al.(1992) [77] found a correlation between sebum excre-tion rate and degree of improvement in acne during treat-ment with erythromycin, minocycline and oxytetracycline, with higher sebum excretion rates being associated with a poorer clinical response to treatment (r = – 0.529). The authors suggest that this could be due to a reduced concen-tration of drug within the follicles as a result of drug dilution with sebum. Laytonet al.(1992) [77] suggest that when sebum excretion rates are > 2.5 µg.cm–2.min–1, hi-gher doses of cyclines may be required (e.g. 600 mg ly-mecycline daily, or up to 200 mg minocycline or doxycy-cline daily). When using higher doses, patients and physicians should be conscious of the increased risk of side effects (particularly with minocycline).
Conclusions
Oral antibiotics are a mainstay of treatment for moderate to severe inflammatory acne (including acne on the trunk) and, while a number of questions remain unanswered (e.g. regarding the optimal dose of antibiotic, the optimal dura-tion of treatment and the length of maintenance therapy), there is clearly an unmet need for practical, user-friendly treatment guidelines for these drugs based on our current knowledge. The recommendations set out below provide guidance on some of the key questions regarding oral antibiotic therapy in acne (choice of oral antibiotic, doses, and duration of therapy), as well as combination therapy and maintenance therapy. By providing clear guidance that can be used in daily practice, these recommendations should improve the care of acne patients.
II. Recommendations for the use of oral
antibiotics
Due to wide differences between published acne studies with respect to disease definitions, end-points, doses used, duration of treatments etc., as well as the many unanswered questions that require further investigation, it is not cur-rently possible to derive definitive treatment guidelines
backed up by high quality evidence. The recommendations below should therefore be viewed as guidance based on expert opinion of the available data.
These recommendations are intended to provide improved patient benefit, and are based on analyses of efficacy, resis-tance, safety, cost effectiveness, expert opinion, and the needs and limitations of daily practice.
First choice of antibiotic
1. Based on advantages in efficacy, safety, and antibiotic resistance, cyclines should be used in preference to other classes of antibiotics in the treatment of acne.
2. Based on pharmacokinetic advantages, second genera-tion cyclines should be used in preference to first generagenera-tion cyclines.
3. Based on side effect profiles1; lymecycline and doxycy-cline should be used in preference to minocydoxycy-cline. The choice of agent will depend on the patient characteristics, season, UV exposure and country.
Cycline doses
1. Lymecycline should be used at a dose of 300-600 mg daily.
2. Minocycline and doxycycline should be used at a dose of 100-200 mg daily2.
3. Tetracycline HCl and oxytetracycline should be used at a dose of 1 g daily.
Duration of treatment
1. According to the available literature, oral antibiotics should be used for 3 months. In clinical practice, they may be continued longer until clinical improvement is achieved. 2. If oral antibiotics are used for prolonged periods3, they should only be continued where further clinical benefit is likely, and should always be used in combination with an agent that reduces the likelihood of propionibacterial resis-tance emerging (e.g. benzoyl peroxide).
3. Compliance should be checked in patients who do not respond well to therapy.
Combination therapy
1. Oral antibiotics should not be used alone (as this will target only two pathophysiologic factors of acne).
2. Oral antibiotics should be combined from the start of treatment with a topical retinoid (this will target three pathophysiologic factors of acne, as well as the microcome-done [the precursor of all acne lesions]).
3. Benzoyl peroxide can be used in combination with topi-cal retinoids and oral antibiotics, and should always be considered for patients receiving oral antibiotics for more than 3 months.
4. Oral antibiotics should not normally be combined with topical antibiotics (this may increase the risk of P. acnes resistance and provides no additive benefit).
1Lymecycline: rare side effects; doxycycline: rare side effects, but
dose-dependent phototoxicity; Minocycline: rare but severe side effects.
2200 mg should be reserved for special cases such as hyperseborrhoea,
and patients and physicians should be aware of potential side effects at this dose.
3Studies have shown most improvement occurs within the first 4 months
Maintenance therapy
1. Maintenance therapy should be considered in order to limit relapse. Optimal duration and dosing remains unde-termined.
2. Topical retinoids are the treatment of choice for mainte-nance therapy.
3. Maintenance therapy should be continued for as long as individually needed (this recommendation is not currently evidence-based).
4. Benzoyl peroxide can be added to topical retinoids if necessary (to decrease the number of antibiotic-resistantP. acnes). j
Acknowledgements. These recommendations were deve-loped at workshops supported financially by Galderma International. The authors have no financial interest in any of the products related to this work.
References
1.Ad HocCommittee report: systemic antibiotics for treatment of acne vulgaris: efficacy and safety. Arch Dermatol 1975; 111: 1630-6.
2. Drake LA. Guidelines for care of acne vulgaris. J Am Acad Dermatol1990; 22: 676-80.
3. Taylor MB. Treatment of acne vulgaris. Guidelines for primary care physicians.Postgrad Med1991; 89: 40-2, 45-7.
4. Oral treatment of acne.Presse Med1999; 28: 2044-5. 5. Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ,et al. Management of acne: a report from a global alliance to improve outcomes in acne.J Am Acad Dermatol2003; 49 (1 Suppl): S1-S38. 6. Thielitz A, Helmdach M, Ropke EM, Gollnick H. Lipid analysis of follicular casts from cyanoacrylate strips as a new method for studying therapeutic effects of antiacne agents.Br J Dermatol2001; 145: 19-27.
7. Leeming JP, Holland KT, Cunliffe WJ. The microbial colonization of inflamed acne vulgaris lesions.Br J Dermatol1988; 18: 205-8. 8. Jappe U, Ingham E, Henwood J, Holland KT. Propionibacterium acnes and inflammation in acne; P. acnes has T-cell mitogenic activity.Br J Dermatol2002; 146: 202-9.
9. Marples RR, McGinley KJ. Corynebacterium acnes and other anaerobic diphtheroids from human skin.J Med Microbiol1974; 7: 349-57.
10. De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM. Intradermal injection of Propionibacterium acnes: a model of inflam-mation relevant to acne.J Invest Dermatol1984; 83: 394-8. 11. Kirschbaum JD, Kligman AM. The pathogenic role of Coryne-bacterium acnes in acne vulgaris.Arch Dermatol1963; 88: 832-3. 12. McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol1978; 12: 672-5.
13. Cove JH, Cunliffe WJ, Holland KT. Acne vulgaris: is the bacterial population size significant?Br J Dermatol1980; 102: 277-80. 14. Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Age-related changes in the resident bacterial flora of the human face.J Invest Dermatol1975; 65: 379-81.
15. Holland KT, Holland DB, Cunliffe WJ, Cutcliffe AG. Detection of Propionibacterium acnes polypeptides which have stimulated an immune response in acne patients but not in normal individuals.Exp Dermatol1993; 2: 12-16.
16. Holland KT, Aldana O, Bojar RA, Cunliffe WJ, Eady EA, Holland DB,et al. Propionibacterium acnes and acne.Dermatology1998; 196: 67-8.
17. Jappe U. Akne und Propionibakterien.Deutscher Dermatologe 2002: 583-9.
18. Ashbee HR, Muir SR, Cunliffe WJ, Ingham E. IgG subclasses specific to Staphylococcus epidermidis and Propionibacterium acnes in patients with acne vulgaris.Br J Dermatol1997; 136: 730-3.
19. Eady EA, Cove JH. Is acne an infection of blocked piloseba-ceous follicles ? Implications for antimicrobial treatment.Am J Clin Dermatol2000; 1: 201-9.
20. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation.J Invest Dermatol2003; 121: 20-7.
21. Leyden JJ. Current issues in antimicrobial therapy for the treat-ment of acne. J Eur Acad Dermatol Venereol2001; 15 Suppl 3: 51-5.
22. Meynadier J, Alirezai M. Systemic antibiotics for acne. Derma-tology1998; 196: 135-9.
23. Plewig G, Kligman AM.Acne and rosacea. 3rded. New York:
Springer-Verlag. 2000.
24. Technote report 2001. www.ahrg.gov/clinic/evrptfiles.htm#acne. 25. Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V,et al. Antibiotic-resistant acne: lessons from Europe.Br J Dermatol 2003; 148: 467-78.
26. Eady EA, Cove JH, Holland KT, Cunliffe WJ. Erythromycin resistant propionibacteria in antibiotic treated acne patients: asso-ciation with therapeutic failure.Br J Dermatol1989; 121: 51-7. 27. Ray AJ, Donskey CJ. Clostridium difficile infection and concur-rent vancomycin-resistant Enterococcus: colonization in a health care worker: case report and review of the literature.Am J Infect Control 2003; 31: 54-56.
28. Carrasco DA, Vander Straten M, Tyring SK. A review of antibio-tics in dermatology.J Cut Med Surg2002; 6: 128-50.
29. Cunliffe WJ, Aldana OL, Goulden V. Oral trimethoprim: a relatively safe and successful third-line treatment for acne vulgaris.Br J Dermatol1999; 141: 757-8.
30. Kawada A, Aragane Y, Tezuka T. Levofloxacin is effective for inflammatory acne and achieves high levels in the lesions: an open study.Dermatology2002; 204: 301-2.
31. Drlica K, Malik M. Fluoroquinolones: action and resistance. Curr Top Med Chem2003; 3: 249-82.
32. Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett2002; 127: 269-77.
33. Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Propionibacte-rium levels in patients with and without acne vulgaris. J Invest Dermatol1975; 65: 382-4.
34. Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J,et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne.Arch Dermatol2003; 139: 459-64. 35. Leyden JJ, McGinley KJ, Cavalieri S, Webster GF, Mills OH, Kligman AM. Propionibacterium acnes resistance to antibiotics in acne patients.J Am Acad Dermatol1983; 8: 41-5.
36. Ozolins M, Eady EA, Avery A, Cunliffe WJ, Li Wan Po A, O’Neill C,et al. A randomised controlled multiple treatment compa-rison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne. Report for NHS study 94/48, January 2002.
37. Hassing GS. Inhibition of Corynebacterium acnes lipase by tetracycline.J Invest Dermatol1971; 56: 189-92.
38. Webster GF, McGinley KJ, Leyden JJ. Inhibition of lipase produc-tion in Propionibacterium acnes by sub-minimal-inhibitory concentra-tions of tetracycline and erythromycin. Br J Dermatol1981; 104: 453-7.
39. Akamatsu H, Tomita T, Horio T. Effects of roxithromycin on the production of lipase and neutrophil chemotactic factor by Propioni-bacterium acnes.Dermatology2002; 204: 277-80.
40. Hamilton-Miller JM. Immunopharmacology of antibiotics: direct and indirect immunomodulation of defence mechanisms.J Chemo-ther2001; 13: 107-11.
41. Brinkmeier T, Frosch PJ. Oral antibiotics with antiinflammatory/ immunomodulatory effects in the treatment of various dermatoses. Hautarzt2002; 53: 456-65.
42. Tamaoki J, Kondo M, Kohri K, Aoshiba K, Tagaya E, Nagai A. Macrolide antibiotics protect against immune complex-induced lung injury in rats: role of nitric oxide from alveolar macrophages. J Immunol1999; 163: 2909-15.
43. Wales D, Woodhead M. The anti-inflammatory effects of macro-lides.Thorax1999; 54 (Suppl 2): S58-S62.
44. Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review.Infection 1996; 24: 275-91.
45. Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R,et al. Anti-inflammatory activity of macrolide antibiotics.J Pharma-col Exp Ther2000; 292: 156-63.
46. Jain A, Sangal L, Basal E, Kaushal GP, Agarwal SK. Anti-inflammatory effects of erythromycin and tetracycline on Propioni-bacterium acnes induced production of chemotactic factors and reactive oxygen species by human neutrophils. Dermatol Online J 2002; 8: 2.
47. Sainte-Marie I, Tenaud I, Jumbou O, Dreno B. Minocycline modulation of alpha-MSH production by keratinocytes in vitro.Acta Derm Venereol1999; 79: 265-7.
48. Crawford WW, Crawford IP, Stoughton RB, Cornell RC. Labo-ratory induction and clinical occurence of combined clindamycin and erythromycin resistance in corynebacterium acnes. J Invest Dermatol1979; 72: 187-190.
49. Eady EA, Bojar RA, Jones CE. The effects of acne treatment with combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin resistant propionibacteria. Br J Dermatol 1996; 134: 107-113.
50. Bojar RA, Eady EA, Jones CE, Cunliffe WJ, Holland KT. Inhibi-tion of erythromycin-resistant propionibacteria on the skin of acne patients by topical erythromycin with and without zinc.Br J Dermatol 1994; 130: 329-336.
51. Coates P, Adams CA, Cunliffe WJ, McGinley KT, Eady EA, Leyden JJ,et al. Does oral isotretinoin prevent Propionibacterium acnes resistance?Dermatology1997; 195 Suppl 1: 4-9; discussion 38-40.
52. Schollhammer M, Alirezai M. Étude comparative de la lymécy-cline (Tetralysal®), de la minocycline (Mynocine®) et de la
doxycy-cline (Tolexine®) dans le traitement de l’acné vulgaire.Réal
Théra-peut Dermato-Vénérol1994; 42: 24-6.
53. Bossuyt L, Bosschaert J, Richert B, Cromphaut P, Mitchell T, Al Abadie M,et al. Lymecycline in the treatment of acne: an efficacious, safe and cost-effective alternative to minocycline. Eur J Dermatol 2003; 13: 130-5.
54. Mobacken H. Oral tetracycline-treatment of acne. Rapid facial improvement, but back lesions are more difficult to treat. Lakartidnin-gen1993; 90: 2755-7.
55. Campo M, Zuluaga A, Escobar P, Motta A, Argote A, Jaramillo C, Rueda MJ, et al. A comparative study on the effectiveness of lymecicline and adapalene versus minocicline and adapalene in the treatment of acne vulgaris.Proceedings 20th World Congress of Dermatology. Paris, France, 1-5 July2002: P0005.
56. Cunliffe WJ, Grosshans E, Belaich S, Meynadier J, Alirezai M, Thomas L. A comparison of the efficacy and safety of lymecycline and minocycline in patients with moderately severe acne vulgaris.Eur J Dermatol1998; 8: 161-6.
57. Dubertret L, Alirezai M, Rostain G, Lahfa M, Forsea D, Niculae BD,et al. The use of lymecycline in the treatment of moderate to severe acne vulgaris: a comparison of the efficacy and safety of two dosing regimens.Eur J Dermatol2003; 13: 44-48.
58. Cunliffe WJ, Meynadier J, Alirezai M, George SA, Coutts I, Roseeuw DI,et al. Is combined oral and topical therapy better than oral therapy alone in patients with moderate to moderately severe acne vulgaris? A comparison of the efficacy and safety of lymecy-cline plus adapalene gel 0.1%, versus lymecylymecy-cline plus gel vehicle.J Am Acad Dermatol2003; 49: S218-26.
59. Bjellerup M, Ljunggren B. Differences in phototoxic potency should be considered when tetracyclines are prescribed during summer-time. A study on doxycycline and lymecycline in human volunteers, using an objective method for recording erythema.Br J Dermatol1994; 130: 356-60.
60. Bernier C, Dréno B. Minocycline.Ann Dermatol Venereol2001; 128: 627-37.
61. Eichenfield AH. Minocycline and autoimmunity.Curr Opin Pe-diatr1999; 11: 447-56.
62. Sturkenboom MC, Meier CR, Jick H, Stricker BH. Minocycline and lupus-like syndrome in acne patients.Arch Intern Med 1999; 159: 493-7.
63. Schlienger RG, Bircher AJ, Meier CR. Minocycline-induced lupus. A systematic review.Dermatology2000; 200: 223-31. 64. Garner SE, Eady EA, Popescu C, Newton J, Li wan po A. Minocycline for acne vulgaris: efficacy and safety.Cochrane Data-base Syst Rev2002; 2: CD002086.
65. Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol1996; 134: 693-5.
66. Bachmeyer C, Cadranel JF. Minocycline-induced lupus and autoimmune hepatitis: family autoimmune disorders as possible risk factors.Dermatology2002; 205: 185-6.
67. Teitelbaum JE, Perez-Atayde AR, Cohen M, Bousvaros A, Jonas MM. Minocycline-related autoimmune hepatitis: case series and literature review.Arch Pediatr Adolesc Med1998; 152: 1132-6. 68. Knowles SR, Shapiro L, Shear NH. Serious adverse reactions induced by minocycline.Arch Dermatol1996; 132: 934-9. 69. Chiu AM, Chuenkongkaew WL, Cornblath WT, Trobe JD, Digre KB, Dotan SA,et al. Minocycline treatment and pseudotumor cerebri syndrome.Am J Ophthalmol1998; 126: 116-21.
70. Corona R. Minocycline in acne is still an issue.Arch Dermatol 2000; 136: 1143-5.
71. Chen SC. Cost-effectiveness analyses: a basic overview for dermatologists.J Cutan Med Surg2001; 5: 217-22.
72. Hughes BR, Cunliffe WJ. Interactions between the oral contra-ceptive pill and antibiotics.Br J Dermatol1990; 122: 717-8. 73. London BM, Lookingbill DP. Frequency of pregnancy in acne patients taking oral antibiotics and oral contraceptives.Arch Derma-tol1994; 130: 392-3.
74. Helms SE, Bredle DL, Zajic J, Jarjoura D, Brodell RT, Krishnarao I. Oral contraceptive failure rates and oral antibiotics.J Am Acad Dermatol1997; 36: 705-10.
75. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: a myth debunked. J Am Acad Dermatol 2002; 46: 917-23.
76. ACOG: The use of hormonal contraception in women with coexisting medical conditions.ACOG Prac Bull2000; 18: 1-13. 77. Layton AM, Hughes BR, Hull SM, Eady EA, Cunliffe WJ. Sebor-rhoea – an indicator for poor clinical response in acne patients treated with antibiotics.Clin Exp Dermatol1992; 17: 173-5.
Appendix 1. Attendees at meetings of the European expert panel on the use of oral antibiotics in the management of acne
Elena Aravijskaya, Russia; Nicole Auffret, France; Rui Bello, Portugal; Vincenzo Bettoli, Italy; Olivier Chosidow, France; Brigitte Dréno, France; Daniele Innocenzi, Italy; Francois Jordaan, South Africa; Martin Kaegi, Switzerland; Jean Marie Lachapelle, Belgium; Hakima Lakhdar, Morocco; Julien Lambert, Belgium; Andrzej Langner, Poland; Alison Layton, UK; Håkan Mobacken, Sweden; Falk Ochsendorf, Germany; Pier Paulo Pedrazetti, Switzerland; Marie Manuela Pronesti, Italy; Franco Rogioletti, Italy; Werner Sinclair, South Africa; Alexandre Jose de Souza Sittart, Brazil; Dente Valentina, Italy; Sarah Wakelin, UK.
![Figure 1. Acne treatment algorithm suggested by Gollnick et al. (2003) [5]. Reprinted from the Journal of the American Academy of Dertatology, Vol](https://thumb-us.123doks.com/thumbv2/123dok_us/1111295.2647892/2.892.86.806.98.701/treatment-algorithm-suggested-gollnick-reprinted-journal-american-dertatology.webp)