Clinical Science (1983) 64,307-3 14 307
Binding of testosterone and oestradiol to sex hormone binding
globulin, human serum albumin and other plasma proteins:
evidence for non-specific binding
of
oestradiol to sex hormone
binding globulin
M. J A W E D I Q B A L , M A U R E E N D A L T O N * A N D R O B E R T S . S A W E R S t Deparrriieiir of Biochemical Endocrinology, Chelsea Hospitalfor Women, London, *Department of Endocrinology, The Royal
Free Hospital, London. and TDepartment of Obstetrics and Gynaecologv, Jessop Hospital f o r Women, Shefield, U X .
(Received 22 April123 September 1982; accepted 25 October 1982)
Summary
1. The percentage binding of testosterone (T) and oestradiol (E,) to sex hormone binding globulin (SHBG) and human serum albumin (HSA) was determined over a range of SHBG concentrations of 16-250 nmol of dihydro- testosterone (DHT) boundll. It was found that the binding of both T and E, to HSA was a function of their binding to SHBG and bore an inverse relationship to it. After removal of both SHBG and HSA from plasma by affinity chroma- tography a ‘residual’ binding of about 11% for T and 12% for E, was still apparent. In addition to the specific high-aflinity, low capacity binding of E, to SHBG, non-specific low-affinity binding of 7-12% was demonstrated after selective denaturation of the specific binding site of the latter.
2. Competition studies indicated that although at the relatively higher levels of SHBG found in the normal female the physiological concentrations of E,, T and DHT need not be taken into account in estimating the unbound fractions of steroids, at the relatively lower levels of SHBG found in normal men and hirsute women, the physiological concentrations of T and DHT are effective in causing statistically significant displacement of E, from the common, specific binding site on SHBG.
3. A simple computerized technique is described for the determination of fractions of E,
Correspondence: Dr M. Jawed Iqbal, The Liver Unit, King’s College Hospital and Medical School, Denmark Hill, London SE5 9RS.
and T respectively, that are unbound to SHBG, unbound to SHBG and HSA, and unbound to all
plasma proteins, when the total plasma levels of E,, T, DHT and SHBG are known.
Key words: albumin, globulin, oestradiol, sex hormones, testosterone.
Abbreviations: DHT, dihydrotestosterone; E,, oestradiol; HSA, human serum albumin; SHBG, sex hormone binding globulin; T, testosterone.
In t r o d u c t i o n
Although it is widely accepted that protein-bound steroid is biologically inert 11, 21, the extent of biological activity of specifically bound and non-specifically bound steroid is not clear. In human plasma the bulk of T and E, is bound specifically to SHBG and non-specifically to HSA. Proteins like corticosteroid binding globulin and a,-acid glycoprotein also play a small part in the binding of these steroids. The binding of steroid to these proteins is dependent on the respective concentrations and af€inities of the latter for the steroid. Studies in vitro have adequately demonstrated the biological inactivity of protein-bound T [3, 41. The inverse relation- ship between the metabolic clearance rate of steroids and SHBG is a further example of the importance of SHBG in steroid physiology. The sex difference in metabolic clearance rate ob- served for DHT, T and E, results from women having about twice the SHBG levels found in men 151 and does not exist for androstenedione, which
does not bind to SHBG [61. The metabolic clearance rate also reflects the strength of binding of a steroid for SHBG, being much lower for DHT than for the relatively weakly bound E,. Binding of a steroid to HSA appears not to be of any significance in controlling the metabolic clearance rate. Although 68-85% of oestrone and 16-25% of oestriol is bound to HSA in plasma and neither oestrogen exhibits any appreciable binding to SHBG 171, there is no significant difference in the metabolic clearance rate of the two steroids [81.
An accurate and meaningful definition of unbound or biologically active steroid is made more difficult as the protective effect of protein binding on steroids varies at various sites of metabolism [ 21 and in different physiological conditions. Attempts to identify the biologically active fraction, without having to measure the unbound steroid directly, have resulted in indices with elaborate equations such as the ‘apparent free testosterone concentration’ 151 or simply the ratio of steroid to SHBG has been employed as an index of the unbound fraction [l]. We have shown previously [7, 91 that steroid bound to SHBG and HSA respectively may be measured simultaneously and directly with reasonable accuracy in the same assay. In this report we present a computerized technique for the estima- tion of unbound fractions of T and E, which may be useful in simplifying these measurements.
Materials and methods
General
Tritiated DHT (1,2,4,5,6,7-’H), specific radio- activity 122 Ci/mmol; tritiated T (1,2,6,7-’H), specific radioactivity 109 Ci/mmol and tritiated E, (2,4,6,7-’H), specific radioactivity 106 Ci/ mmol were purchased from The Radiochemical Centre Amersham Ltd, U.K. All radio-inert steroids were purchased from Steraloids Ltd, U.K. HSA was purchased from Sigma Chemical Co. Ltd, U.K. Sepharose 4B and Sephadex LH-20 were purchased from Pharmacia (G.B.) Ltd. All reagents were analytical grade. The buffer used throughout was Tris (50 mmol/ l)/CaCl, (5 mmol/l)/HCl, pH 7.5. Radioactivity was determined in a Packard liquid scintillation counter (efficiency, 40%).
Determination of percentage T and E , binding to
SHBG and HSA
Plasma samples were pooled to give SHBG binding capacity values of 16, 22, 28, 42, 66, 81,
97, 145 and 250 nmol of DHT bound/l; these were determined as described previously [91. These undiluted plasma pools were charcoal- treated to remove endogenous steroids [ 101. Portions (400 pl) of these whole plasma pools were incubated with [’HIE, (20 000 c.p.m./ml) and [’HIT (20 000 c.p.m./ml) and the percentage binding to SHBG or HSA was determined by the two-tier column method [7,91.
Effect of the removal of HSA on the binding of T
and E , to SHBG
Samples of pooled plasma, of SHBG binding capacities of 18, 60 and 190 nmol of DHT bound/l respectively, were dealbuminized as described 191 and concentrated to their original volumes in A25 Minicon cells (Amicon, U.K.). Percentages of T and E, bound to SHBG were determined in these pools as described above. Percentage binding of T and E, was determined in the same plasma pools in the presence of HSA. To verify the absence of HSA in dealbuminized plasma, binding of [WIT and [’HIE, respectively was determined in the Cibacron Blue 3GA/ Sepharose 4B (Blue Sepharose), which forms the upper tier of the two-tier column [91. Analytical polyacrylamide gel electrophoresis was per- formed on dealbuminized plasma with pure HSA used as control [ 111, this being a further means of testing for the absence of HSA.
Determination of plasma binding
of
T and E , toproteins other than SHBG and HSA
All the plasma pools, representing SHBG values of 16-250 nmol of DHT bound/l, were dealbuminized and SHBG was removed from these by affinity chromatography [ l l l . The resultant supernatant was concentrated in Minicon cells to give 1 : 10 dilutions of the original plasma pools. Equilibrium dialysis was then performed [121 on these diluted plasma pools to determine the percentage T and E, binding respectively, in the absence of both HSA and SHBG. Buffer blanks were dialysed at the same time for both E, and T. Successful removal of SHBG by affinity chromatography was verified by binding studies using the two-tier column method, analytical polyacrylamide gel electro- phoresis using purified SHBG as control [ 1 11 and the double immunodiffusion technique 1131.
Determination of percentage binding of T and E ,
in whole plasma by equilibrium diahsis
Percentage binding of [3H]T and [’HIE, respectively was determined in whole plasma
Binding of sex steroids in human plasma 309 samples over the entire range of SHBG
concentrations by equilibrium dialysis [ 121. Effect
of
varying HSA concentrationPlasma pools representing SHBG levels of 18, 60 and 190 nmol of D H T bound/l were deal- buminized and the resultant supernatants were concentrated to the original volume. Amounts of HSA in powder form representing 1, 2-5 and 5 g/100 ml of plasma were added to these plasma pools. Binding of PHIE, and [3HlT to HSA and SHBG was determined by the two-tier column method for each series of plasma pools represent- ing known levels of SHBG, and added HSA concentrations.
Determination of non-specific binding of E , and T to SHBG
Plasma pools representing SHBG levels of 18, 60 and 190 nmol of DHT bound/l were treated with charcoal to remove endogenous steroids and dealbuminized. The resultant supernatants were concentrated to their original volumes as above and amounts of ethylenediaminetetra-acetic acid (EDTA) equivalent to 2 mmol/l were added to each plasma pool. The plasma pools were then heated in a water bath at 45OC for 25 min. Two different sets of experiments were performed on these plasma pools: (a) binding of ["HIE, and VHIT to SHBG and HSA was determined for each pool by the two-tier column method; (b) binding of PHIE, and PHIT were determined by equilibrium dialysis for each pool in the manner described except that aliquots of the pools were dialysed against charcoal-treated, HSA- and SHBG-free plasma which had been similarly heat-treated (25 min at 45OC) in the presence of EDTA.
In a further series of experiments samples with SHBG levels of 18, 60 and 190 nmol of DHT bound/l were charcoal-treated, dealbuminized and heated at 63OC for 45 min in the absence of EDTA. Binding of i3H]E, and 13H]T was determined as in (a) and (b) above. Buffer blanks were dialysed in each series of equilibrium dialysis experiments.
Investigation of the saturability of non-speciJc binding of E , to SHBG
Plasma pools with SHBG values of 18,60 and 190 nmol of DHT bound/l and treated as described in the first paragraph of the preceding section were dialysed as described in (b) of that section and binding was determined in the presence of increasing concentrations of radio-
inert E, (0, 0.18, 0.36, 0.73, 1-82, 3.65, 7.30 and 18.2 nmol/l) and 20000 c.p.m. of ["HIE,. Results determined at the end of dialysis were subjected to Scatchard analysis.
Study of the competition of E,, T and DHT for
the common binding site on SHBG
Portions (400 pl) of whole plasma pools representing SHBG binding capacities of 16-250 nmol of DHT bound/l were incubated with a constant amount of [3H]E, (86 pmol/l) and increasing amounts of radio-inert E, (93, 185, 460, 918 and 1835 pmol/l) and percentage binding to HSA and SHBG respectively was determined by the two-tier column method. Displacement of I3H1E, was studied for all plasma pools when increasing amounts of T 8.68, 17.36 and 43.4 nmol/l) were added. Similarly, displacement of [ 3H]T (83 pmol/l) by increasing amounts of radio-inert E, (185-1835 pmol/l), and displacement of I3H1T by increasing amounts of radio-inert T (0.043-43.4 nmol/l), were determined. Displacement of both [3HIE, and VHIT, as well as ['HIDHT (74-5 pmol/l), was investigated for all plasma pools when increasing amounts of radio-inert DHT (0.043, 0.086, 0.170, 0.430, 0.860 and 4.34 nmol/l) were added. Displacement of 13H]DHT by increasing amounts of E, and T respectively was also investigated. In parallel experiments, similar competition studies were carried out in whole plasma pools by using equilibrium dialysis. (0.043, 0.086, 0.170, 0.430, 0.860, 1.74, 4.34,
Use of a computer program for the estimation of unbound E , and T and comparison of these estimations with values obtained by direct measurement of the unbound fractions
From the data representing the respective unbound fractions of E, and T and the roles SHBG and HSA have in determining these fractions, together with the results of competition studies described above, a computer program was derived (Fortran 5 computer) as follows: owing
to
the alteration in the binding of E, and T by physiological concentrations of T, the concentration of the latter was one variable: the other variable was the level of SHBG. For the computer analysis of unbound E, fractions there was a further variable, the DHT concentration, since variations over the physiological range of this steroid caused substantial displacement of PHIE,. The computer program employed inter- polation between the various known values of unbound T and E, fractions respectively, the LeGrange formula being used for three-point interpolation between the first two values, Gaus- sian four-point interpolation for the middle values and linear interpolation for the highest value. Each point has interpolation in the ‘T’ and ‘SHBG’ direction for the analysis of unbound fractions of T; for analysing similar fractions of unbound E, each point has interpolations in the ‘T’, ‘DHT’ and SHBG direction. Interpolation by a computer program could be calculated to a high degree of accuracy so the results are limited by experimental accuracy alone. The same degree of accuracy cannot be obtained if extrapolation (especially downwards) is incorporated in the computer program owing to complications aris- ing from binding and displacement data at low SHBG values. For this reason, the Fortran 5
program for analysing unbound fractions of T and E, is used only for SHBG values of between
16 and 250 nmol of DHT bound/l.
With the two-tier column method and equilib- rium dialysis, a comparison of the results obtained by direct measurement and by the computer program was carried out for six plasma samples (not treated with charcoal) of known SHBG binding capacity, total T, E, and DHT levels.
Results
The percentage binding of T and E, respectively to SHBG and HSA is illustrated in Figs. 1 and 2. Percentages of T and E, not bound to any plasma component, as determined by equilibrium di- alysis, are illustrated in Fig. 3. Linear regression analyses showed negative correlations between T bound (%)to SHBG and that bound to HSA (r =
-0.997, JJ = - 0 . 7 9 8 ~
+
82.4,P
<
0.0001);100 r
0 5 0 1 0 0 150 200 2 5 0
SHBG (nmol of DHT bound/l)
FIG. 1. Means SD of seven determinations of percen- tages of testosterone bound to SHBG (0) and HSA
(0) respectively at SHBG concentrations between 16 and 250 nmol of DHT bound/l.
between SHBG levels and T bound (%) to HSA
(r = -0.914, y = - 0 . 1 7 4 ~
+
43.1,P
<
0.001);and between percentages of E, bound to SHBG and that bound to HSA (r = -0.999, y =
- 0 . 8 4 ~
+
83.1,P
<
0.0001). Successful removal of HSA, which was verified by lack of binding in the Blue gel of the two-tier column and by analytical polyacrylamide gel electrophoresis, did not alter percentage binding of either T or E, toloo
I
I
0 5 0 100 150 200 250
SHBG (nmol of DHT bound/l)
FIG. 2. Means
a
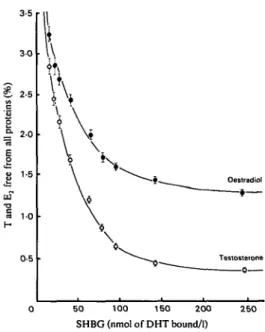
SD of seven determinations of percen- tages of oestradiol bound to SHBG (0) and HSA (0) respectively at SHBG concentrations between 16 and 250 nmol of DHT bound/l. ‘ € , Oeotradid €- \ I 0 50 100 150 200 2 5 0 SHBG (nmol of DHT bound/l)FIG. 3. Means f SD of seven determinations of percen- tages of testosterone (0) and oestradiol (0) free from all plasma proteins, as established by equilibrium dialysis, at SHBG concentrations between 16 and 250 nmol of DHT bound/l.
Binding of sex steroids in human plasma 311 SHBG (Table 1). When both HSA and SHBG
were removed by affinity chromatography (the successful removal of the latter was again verified by lack of binding by using the two-tier column, polyacrylamide gel electrophoresis and double immunodiffusion 131) the mean percentage T binding was 10.56 f SD 0.515
(n
= 9) and was not significantly different for any one pool. Similarly the mean percentage E, binding in the absence of SHBG and HSA was 11.77 f 0.278(n = 9) and was not significantly different for any one pool. Varying HSA concentration from 1 to 5 g/100 ml of plasma did not result in any significant change in percentage binding of T or
E, to SHBG at any of the SHBG levels investigated (Table 2).
No binding of either [3HlT or [3H]E, was detected either to SHBG or HSA with the two-tier column method for any of the three steroid-free, dealbuminized plasma pools heat- treated at 45OC for 25 min in the presence of EDTA. Equilibrium dialysis of these pools gave percentage mean binding -CSD of l3H1E,, for plasma pools of SHBG levels 18, 60 and 190 nmol of DHT bound/!, of 7 -C 0.580% (n = 7),
10
i-
0.438% (n = 6) and 12 f 0.387% (n = 7); increasing the amounts of radio-inert E, showed no significant difference in binding for anyTABLE 1. Effect of removal of HSA on percentage binding of T and E , to SHBG in undilutedplasma
Mean values SD for seven determinations are shown. Student’s r-test showed no signillcant difference after dealbuminization.
~~ ~~ ~ ~~ ~
SHBG Binding to SHBG (%)
(nmol of DHT
boundh) Before removal of albumin Afler removal o f albumin
T E l T E l 18 45.09 13.71 45.31 13.69 f 1.456 f 0.503 f 1.467 f 0.441 60 65.56 22.61 65.42 22.48 f 2.132 f 0.727 f 2.010 f 0.742 190 90.41 47.82 90.69 47.64 f 2.765 f 1.386 f 2.981 f 1.523
TABLE 2. Effect of varying HSA concentrations on percentage binding of T and E , to SHBG and HSA in undilutedplasma
Mean values
-+
SD of seven determinations are shown. Student’s t-test showed no significant difference between values at any HSA concentration.HSA added Binding of T (%) Binding of El (%)
(g/ 100 ml
of plasma) To SHBG To HSA To SHBG To HSA
~~~~ ~~ SHBG = 18 nmol of DHT bound/I 1 .o 45.40 f 1.496 2.5 45.12f 1.438 5.0 45.26 f 1-442 SHBG = 60 nmol of DHT boundh 1 .o 65.38 f 1.950 2.5 65.81 f 1.973 5.0 65.73 f 1.980 SHBG = 190 nmol of DHT boundll 1.0 91.18 f 2.731 2.5 91.31 f 2.987 5.0 90 *84 f 2.860 45.51 f 1.743 45.72 2 1.806 45.90 f 1.730 31.27f 1.402 31.62 f 1.310 31.45 f 1.380 8.58 f 0.31 1 8.72 f 0.334 8.80 f 2.309 13.76 f 0.418 13.58 f 0.430 13.82 f 0.427 22.46 f 0.830 22.82 f 0.740 22.74 f 0.787 47.73 C.I.490 47.68 f 1.356 47.53 f 1.512 71.16 f 1.540 71.51 f 1.468 71.47f 1.530 65.39 f 1.410 65.80 f 1.438 65.46 f 1.470 43.37 f 1.445 42.81 f 1.390 42.72 f 1.361
TABLE 3. Competition studies: mirlirtiurn concentrations of T and DHT at which sigtr~carrt displacement of ['HIE, and I'HlToccurred at each SHBG concentration
Figures in parentheses are the percentages of tritiated steroid displaced. The maximum concentrations of radio-inert T and DHT added were 43.4 and 4.34 nrnol/l respectively (range 0.043,0.086, 0.17,0*43,0.86,
1.74,4.34,8.68, 17.36 and 43.4 nmol/l). N.S., No significant displacement.
SHBG (nmol of DHT bound/l) ... 16 22 28 42 66 81 97 145 250 ~~~~ ~ T(nmol/l) 0.86 4.34 8.68 17.36 17.36 17.36 17.36 43.4 N.S. vs ['HIE, (4.0%) (4.0%) (4.4%) (13.3%) (10.0%) (7.1%) (4.0%) (4.2%) DHT(nmol/l) 0.86 0.86 1.74 4.34 N.S. N.S. N.S. N.S. N.S. T(nmol/l) 4.34 8.68 17.36 43.4 43.4 N.S. N.S. N.S. N.S. vs ['HIE, (6.9%) (4.5%) (3.9%) (3.7%) vs I'HIT (4.4%) (3.9%) (6.1%) (8.7%) (6.0%)
plasma pool. No plasma pool showed any measurable binding for L3H1T by equilibrium dialysis under these conditions. The same plasma pools heat-treated at 63OC for 45 min in the absence of EDTA again showed no binding of either steroid to SHBG or HSA, as determined by the two-tier column method or equilibrium dialysis.
Competition studies showed that concentra- tions of E, over the physiological range are ineffective in causing the displacement of ['HIE,,
[)HIT or ['HIDHT from SHBG. Results of competition studies involving T and DHT are summarized in Table 3. No displacement of L3HIDHT from SHBG by DHT was shown over the entire range of DHT concentrations added. Results also indicate that when significant displacement of steroid from SHBG is caused, e.g. of ['HIE, at low SHBG levels by high physiological concentrations of T or DHT, the steroid displaced from the specific protein is principally bound by HSA. This was confirmed when competition studies were carried out by using equilibrium dialysis, as no displacement of any tritiated steroid by any radio-inert steroid could be demonstrated over the physiological concentration range of these steroids.
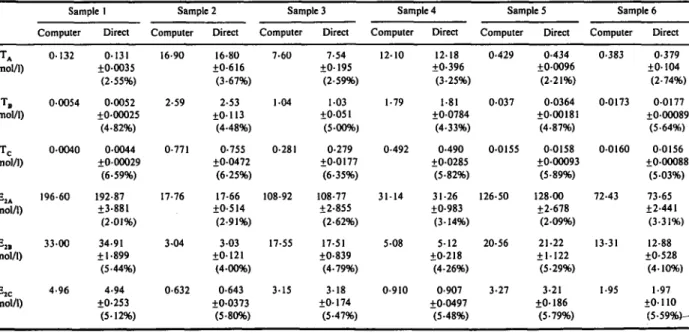
Comparison of results in six plasma samples of T and E, unbound to SHBG (designated A), unbound to SHBG and HSA (designated B) and those unbound to all plasma proteins (designated C), estimated by computer analysis and deter- mined directly by the two-tier column method and equilibrium dialysis, are presented in Table 4. These agreed to within 5%.
Discussion
Any discussion of unbound steroid must first
define that entity. We find three fractions of unbound steroid in plasma. Which of these fractions should be regarded as biologically active? The answer is not a simple one; the relative activity of the different unbound fractions will depend on the strength of binding at the site of steroid consumption and will vary from site to site. Identification of the biologically active fraction cannot therefore be absolute and studies in uitro provide only an indirect assessment.
In
this report we are able at least to discount some previous misconceptions.
In the absence of the two most important binding proteins for T and E,, a significant percentage of the two steroids is still bound in the plasma; it is unlikely that this percentage will still be as high as 1 1 or 12% in the presence of SHBG and HSA, but this residual binding may be of importance at low SHBG levels.
With regard to E, binding the situation is more complex, since E, exhibits weak non-specific binding to SHBG over and above the residual binding of 12% it shows in the absence of HSA and SHBG. Although heat treatment at 6OoC for periods of around 45 min results in total loss of SHBG binding activity, it has been demonstrated
[141 that SHBG loses half its specific binding activity in 6 min by heat treatment at 5OoC in the presence of EDTA at 1 mmol/l; no attempt was made, however, to measure non-specific binding of SHBG in that study. Our results show that at the relatively lower temperature of 45OC heat inactivation for a shorter period (25 min) in the presence of EDTA at 2 mmol/l selectively denatures the specific binding site of SHBG, whereas some non-specific binding still remains, at least for E,. This non-specific binding is distinct from the specific binding to SHBG, for which there is only one saturable binding site
TABLE 4. Unbound E , and Testimated from total E,, Tand SHBG by computer analysis compared with results by direct measurement
Direct measurement of A and B fractions of T and E, was by the two-tier column method; C fractions were measured by equilibrium dialysis. Means & SD and coefficient of variation (%)of seven different determinations are given.
Sample I Sample 2 Sample 3 Sample 4 Sample 5 Sample 6
Computer Direct Computer Direct Computer Direct Computer Direct Computer Direct Computer Direct T A (nmolll) TC (nmolll) E2.4 (pmolll) E*, (pmolll) EX (pmol/l) ~ ~ 0.132 0.131 f0.0035 (2.55%) 0.0054 04052 20.00025 (4.82%) 04CHO 04044 20.00029 (6.59%) 196.60 192.87 23.881 (2.01%) f I .899 (5 '44%) f0.253 33.00 34.91 4.96 4.94 (5.12%) ~~ 16.90 16.80 f0.6 16 (3.67%) 2.59 2.53 f0. I 13 (4.48%) 0.771 0.755 20.0472 (6.25%) 17.76 17.66 20.514 (2.91%) f0.121 (4.00%) f0.0373 (5.80%) 3.04 3.03 0.632 0.643 7.60 7.54 20. 195 (2.59%) I .04 1.03 f0.05 1 (540%) 20.0177 (6.35%) 0.281 0.279 108.92 108.77 22.855 (2.62%) 17.55 17.51 f0.839 (4.79%) 3.15 3.18 f0. I74 (5.47%) 12.10 12.18 f0.396 (3.25%) 1.79 1.81 20.0784 (4.33%) 0.492 0.490 f 0.0 2 8 5 (5.82%) 31.14 31.26 20.983 (3.14%) 5.08 5.12 20.218 (4.26%) 0.910 0.907 f0.0497 (5.48%) ~~~ ~ 0.429 0.434 20.0096 (2.21%) 0.037 0.0364 f0.00181 (4.87%) 0.0155 0.0158 f0.00093 (5.89%) 126.50 128.00 f2.678 (2.09%) f1.122 (5.29%) 20.56 21.22 3.27 3.21 f0. 186 (5.79%) ~ 0.383 0.379 f0.104 (2.74%) 0.0173 0.0177 f040089 (5.64%) 0.0160 0.0156 f0.00088 (5.03%) 72.43 73.65 f2.441 (3.31%) 13.31 12.88 f0.528 (4.10%) 1.95 1.97 f0. I LO (5.59Wk- ta 5' f?-
09"'
3
x 3 a. r: 33
w w c.which is also shared by T and DHT. Our data suggest that non-specific binding between SHBG and E, is of relatively high capacity and low affinity. That the affinity constant of this non- specific binding of E, to SHBG is lower than that which E, has for HSA is demonstrated by the observation that unlike the non-specifically bound E2-HSA complex [91 the non-specifically bound E,-SHBG complex could not survive passage through the 1 ml of Sephadex LH-20 column which forms the lower tier of the two-tier column. It can be seen that binding of E, and T to HSA constitutes a major fraction of the total steroid concentration, but this non-specific binding is greatly influenced by SHBG in plasma. This fact has been ignored by Vermeulen [51 in calculation of the ‘apparent free testosterone concentration’. These authors derive the T bound to HSA by determining the concentration of this protein and assuming one binding site per mol of HSA [51, which is a questionable assumption as the indications are that HSA is a multivalent non- specific binder. Our results are in agreement with recent reports that HSA levels do not exert significant influence on percentage binding of T [151 or E, [161.
In view of the finding that SHBG has a single and common binding site for the steroids that it binds [ l l , 171, the sigmoid curve obtained on plotting percentage E, binding to SHBG against increasing SHBG concentrations is unexpected (Fig. 2). It is unlikely that SHBG has more than one binding site per mol for E, and it seems more likely that at the higher concentrations of SHBG (66 nmol of DHT bound/l and above), non- specific binding of E, to SHBG demonstrated in this paper becomes relatively more important, and as the specific binding of E, is relatively weak as compared with that of T, the resultant curve has the shape for more than one set of non- homogeneous binding sites. Such is not the case for T (Fig. l), where the specific binding is relatively strong and overshadows any non- specific binding to the SHBG molecule. Elaborate studies of the kind reported previously [3,41 may help to clarify the relative importance of the three unbound fractions of steroid in different metabolic states. Use of the present methodology should make such studies more meaningful.
References
I I I ANDERSON, D.C. (1974) Sex hormone binding globulin.
Clinical Endocrinology, 3,69-96.
(21 HEYNS, W. (1976) The steroid binding pglobulin of human plasma. In: Advances in Steroid Biochemistry and Pharmacology, vol. 6, pp. 59-79. Ed. Briggs, M.H. & Christie, G.A. Academic Press, London.
131 LASNIIZKI, 1. & FRANKLIN, H.R. (1972) The influence of serum on uptake, conversion and action of testosterone in rat prostate glands in organ culture. Journal of Endocrinology, 54, 333-342.
141 HAMPh R. & S T ~ C A , L. (1975) Effect of testosterone- oestradiol binding globulin on the enzymic oxidoreduction of 17-oxygenated C,, steroids. Hormone Research, 6,57-64.
151 VERMEULEN, A. (1977) Transport and distribution of an- drogens at different ages. In: Androgens and Antiandrogens,
pp. 53-65. Ed. Martini, L. & Motta, M. Raven Press, New York.
161 SOUTHREN, A.L., CORDON. G.G. & TOCHIMOTO, S. (1968) Further study of factors affecting the metabolic clearance rate of testosterone in man. Journal of Clinical Endocrinology and Metabolism, 28, 1105-1 11 1.
171 WHITEHEAD, M.I., TOWNSEND. P.T., KITCHIN, Y., DYER, G.,
(1980) Plasma steroid and protein hormone profiles in post-menopausal women following topical administration of oestradioLl7P. In: Percutaneous Absorption of Steroids, pp. 23 1-248. Ed. Mauvais-Jarvis, P., Vickers, C.F.H & Wepierre, J. Academic Press, New York.
I81 LIPSEIT, M.B. (1978) Steroid hormones. In: Reproductive Endocrinology, Physiology, Pathophysiology and Clinical Management, pp. 80-90. Ed. Yen, S.S. & Jaffe, R.B. W. B. Saunders and Co. London.
191 IQEAL M.J. & JOHNSON, M.W. (1977) Study of steroid protein binding by a novel ‘two-tier’ column employing Cibacron Blue F3G-A-Sepharose 4B. I. Sex hormone binding globulin.
Journal of Steroid Biochemisiry, 8,977-983.
1101 HEYNS, W., VAN BAELEN, H. & DE MOOR, P. (1967) Study of steroid-protein binding by means of competitive adsorption: application to cortisol binding in plasma. Clinica Chimica Acta, 18, 361-370.
1 1 1 1 IQBAL, M.J. & JOHNSON, M.W. (1979) Purification and characterization of human sex hormone binding globulin.
Journal of Sieroid Biochemistry, 10,535-540.
1121 VERMEULEN, A. & VERDONCK, L. (1968) Studies on the binding of testosterone to human plasma. Steroids, 11,
609-635.
1131 GREENWAY, B.A., IQBAL M.J., JOHNSON, P.J. & WILLIAMS, R. (1981) Oestrogen receptor proteins in malignant and fetal pancreas. Briiish Medical Journal, 283,75 1-753.
1141 ROSNER, W., TOPPEL S. & SMITH, R.N. (1974) Testosterone- estradiol-binding globulin of human plasma: denaturation and protection. Biochimica et Biophysica A d a , 35 1.92-98.
1151 MOLL G.W. & ROSEWIELD, R.L. (1979) Testosterone
binding and free plasma androgen concentrations under physiological conditions: Characterization by flow dialysis technique. Journal of Clinical Endocrinology and Meiabolism.
49,730-736.
I161 VIGERSKY, R.A., KONO, S., SAUER, M., L I P S E ~ , M.B. &
LORIAUX, D.L. (1979) Relative binding of testosterone and estradiol to testosterone-estradiol binding globulin. Journal of
Clinical Endocrinology and Metabolism, 49,899-904. 1171 ROSNER, W. & SMITH, R. (1975) Isolation and characteriza-
tion of testosterone-estradiol binding globulin from human plasma. Use of a novel affinity column. Biochemistry, 14, 481 3-48 19.