Draft protocol PICO4 – v1.6; 7th March 2014
1
7
thMarch 2014
Systematic review of the effect of antibiotics and/or vaccination in preventing
subsequent disease among household contacts of cases of meningococcal disease
WHO protocol to inform the revision of meningitis outbreak response guidelines
L. Telisinghe1, 2, T. Waite1, 3, M. Gobin1, O. Ronveaux6, K. Fernandez6, J. Stuart2, 5, 6, R. J. P. M. Scholten41. Field Epidemiology Services, Public Health England, Bristol, BS1 6EH, UK 2. University of Bristol, Bristol, BS8 2PS, UK
3. Field Epidemiology Training Programme, Public Health England, London NW9 5EQ, UK 4. Dutch Cochrane Centre, 1100 DE Amsterdam, The Netherlands
5. London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK 6. World Health Organization, 1211 Geneva 27, Switzerland
Introduction
For over the last 100 years, the African meningitis belt has seen periodic large epidemics of meningococcal meningitis, with associated morbidity and mortality (1, 2). The majority of epidemics were caused by serogroup A (Nm A) Neisseria meningitides (2). In response, a serogroup A polysaccharide-tetanus toxoid conjugated vaccine (PsA-TT, MenAfrVac) was developed and is being rolled out across the region since December 2010, with good effect (2). This is likely to change the epidemiology of meningococcal disease in the region. In view of this, the World Health Organization (WHO) is revising the outbreak response guidelines for meningococcal meningitis in sub-Saharan Africa.
Current guidance for the meningitis belt recommends the use of chemoprophylaxis for close contacts of meningococcal disease, outside of epidemics (3, 4). Mass vaccination is recommended to halt the progression of meningitis outbreaks, when possible. However during a recent serogroup W135 Neisseria meningitides outbreak in the Gambia in 2012, ciprofloxacin was administered as chemoprophylaxis to close contacts of cases of meningococcal disease (5). In Europe no distinction is made for the use of prophylaxis (both antibiotics and vaccination where appropriate); which is recommended during outbreaks and for contacts of sporadic cases. In addition, with the use of PsA-TT, the scale and frequency of Nm A meningococcal disease outbreaks in the meningitis belt is likely to decrease. Given these, the WHO recommendation for the use of chemoprophylaxis
Draft protocol PICO4 – v1.6; 7th March 2014
2
and vaccination for the region needs to be reviewed and updated, based on an up to date and comprehensive review of the literature.
A previous systematic review of observational studies conducted in Europe and the USA in 2004, showed chemoprophylaxis to be effective in preventing subsequent cases of meningococcal disease among household contacts (6). A more recent systematic review of randomised controlled trials did not identify any suitable trials to determine the effect of chemoprophylaxis (7). A systematic review investigating the role of vaccination in addition to chemoprophylaxis conducted in 2008 of six observational studies supported the use of vaccination (8). A single randomised controlled trial in Nigeria conducted in 1978 also showed a beneficial effect of vaccination in the absence of chemoprophylaxis (9).
To enable evidence based recommendations to be made for the region, it is important to evaluate all literature, both interventional and observational on the use of prophylaxis, both antibiotics as chemoprophylaxis and appropriate vaccination. This systematic review, commissioned by the WHO will support the development of the new WHO guidelines.
Aims
To determine the effect of antibiotics and/or vaccination, in preventing subsequent meningococcal disease in household contacts of cases of meningococcal meningitis, in epidemic and non-epidemic settings.
Objectives
1) Conduct a systematic review of the literature using an appropriate search strategy.
2) Determine a combined estimate of the effect of appropriate antibiotics on the risk of subsequent meningococcal disease among household contacts of cases of meningococcal disease at 30 days and 1 year following the index case.
3) Determine a combined estimate of the effect of appropriate vaccination on the risk of subsequent meningococcal disease among household contacts of cases of meningococcal disease at 30 days and 1 year following the index case.
4) Determine a combined estimate of the effect of appropriate antibiotics and vaccination on the risk of subsequent meningococcal disease among household contacts of cases of meningococcal disease at 30 days and 1 year following the index case.
Draft protocol PICO4 – v1.6; 7th March 2014
3
5) Determine the number needed to treat with antibiotics, vaccination, and, antibiotics and vaccination to prevent one subsequent case of meningococcal disease among household contacts of cases of meningococcal disease at 30 days and 1 year.
6) Explore drug resistance in isolates of Neisseria meningitidis from subsequent cases of meningococcal disease given chemoprophylaxis.
7) Explore the proportion of household contacts given prophylaxis (both antibiotics and vaccination), who develop side effects due to prophylaxis.
Methods
Study designs: Only comparative studies will be considered including interventional studies (both randomised controlled trials and quasi-randomised trials) and comparative observations studies.
Study populations: Household contacts, defined as persons living and/or sleeping in the same household as a case of meningococcal disease. Adults and children, both healthy and with underlying disease conditions will be included.
Interventions: The main interventions of interest are antibiotics recommended as chemoprophylaxis for Neisseria meningitidis and vaccines effective against Neisseria meningitidis. Only studies with clear exposure groups (antibiotics and comparator group; vaccine and comparator group; antibiotics and vaccine and comparator group) will be included.
Antibiotics: recommended as chemoprophylaxis for contacts of cases of meningococcal disease (ciprofloxacin, rifampicin, ceftriaxone, azithromycin and cefixime) (10, 11); Comparator group: placebo, no antibiotics or any other interventions.
Vaccine: any effective vaccine against Neisseria meningitidis (all serogroups, both polysaccharide and conjugated); Comparator group: placebo, no vaccine or any other intervention.
Outcomes: Primary - Occurrence of subsequent meningococcal disease among household contacts in the 30 days and 1 year following disease onset in the index case. Secondary – 1) The proportion of isolates of Neisseria meningitidis from subsequent cases of meningococcal disease which were resistant to the prophylactic antibiotics used; 2) Proportion of household contacts with side effects attributed to antibiotics and/or vaccination given as prophylaxis.
Search strategy
Draft protocol PICO4 – v1.6; 7th March 2014
4
The Cochrane Library (including DARE)
Medline
Embase
CAB Health
TRIP database
If an appropriate systematic review is identified, a search for primary study articles since the end date of the review will be undertaken. If a systematic review is not identified, the literature search will be undertaken without a date limit. The databases that will be searched for primary study articles are:
Medline
Embase
African Index Medicus
WHO regional databases (Global Index Medicus)*
Google with country filters – for grey literature
*unless a previous systematic review explicitly states that developing country databases were searched, Global Index Medicus will be searched without a date limit to supplement any identified systematic reviews.
The reference list of all retrieved primary study articles will be searched for literature to supplement the search. Experts involved in the WHO guideline development will be contacted to identify any additional sources of data. Search terms:Appropriate text words and subject headings will be used to search databases. Table 1 details the proposed search terms for Medline Ovid. Search strings will be adapted as needed for each database. There will be no language restrictions.
Table 1: The proposed search strings in Medline Ovid Intervention 1) exp Chemoprevention/
2) exp Post-Exposure Prophylaxis/
3) chemoprophyl* or chemoprevent* or prophyla* 4) 1 or 2 or 3
5) exp Anti-Bacterial Agents/ 6) exp Drug Therapy/
7) antibiotic* or antimicrobial* or antibacterial* or ((drug*) adj3 treatment*) or ((drug*) adj3 therap*) 8) azithromycin* or azithro* or ciprofloxacin* or cipro* or quinol* or fluoroquinol* or
fluoro-quinolon* or ceftriaxon* or cefix* or rifampi* or cephalospori*
9) 5 or 6 or 7 or 8
10) 4 and 9
Draft protocol PICO4 – v1.6; 7th March 2014
5
12) exp Vaccination/
13) exp Meningococcal Vaccines/ 14) vaccin* or immun*
15) 11 or 12 or 13 or 14
16) 10 or 15
Outcome 17) exp Neisseria meningitidis/ 18) exp Meningitis, Meningococcal/
19) "N. meningitid#s" or meningococ* or ((neisseria or epidemic) adj3 mening*) or ((neisseria) adj3 disease*) or ((neisseria) adj3 infect*)
20) 17 or 18 or 19
Setting 21) exp Contact Tracing/
22) exp Disease Transmission, Infectious/ 23) exp Disease Outbreaks/
24) contact* or household* or cluster* or transmi* or outbreak* or ((subsequent or associated) adj2 case*)
25) 21 or 22 or 23 or 24
Overall 26) 16 and 20 and 25
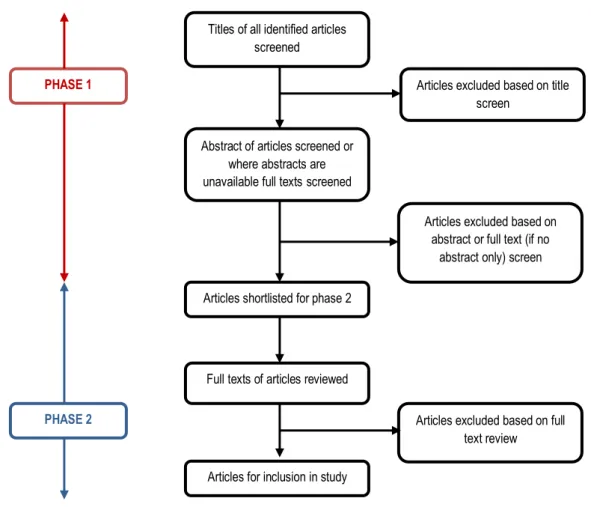
Literature search: Identifying articles for inclusion in the study will be undertaken independently by two researchers (LT and TW; see Figure 1 and Appendix 1). Phase 1 will consist of a title and abstract screen. Initially titles of articles identified will be screened. The abstracts of articles considered suitable by title screen will then be reviewed. Where abstracts are unavailable, full texts will be reviewed. This will generate a shortlist of articles for consideration in phase 2. During phase 2 full texts of all short listed articles will be reviewed to determine suitability for inclusion in the study. Any disagreements on articles selected between researchers will be resolved through discussion or if required, consultation with a third researcher (JS). This process will be carried out to identify both prior systematic reviews and primary study articles.
Draft protocol PICO4 – v1.6; 7th March 2014
6
Figure 1: Identifying articles for inclusion in the systematic review
Data handling: All data required will be abstracted and entered onto standardised case report forms, independently by two researchers (LT and TW; see Appendix 2). All data will be double entered into a relational Epidata database (Epidata Association), with range and consistency checks.
Assessing the risk of bias
Two researchers (LT and TW) will assess the risk of bias in all prior systematic reviews and primary study articles identified for inclusion in the study. Researchers assessing the risk of bias will not be blinded to authors, affiliated institutions, journal of publication or results. Disagreements will be resolved by discussion or if required consultation with a third researcher (RS).
Abstract of articles screened or where abstracts are unavailable full texts screened
Titles of all identified articles screened
Articles excluded based on title screen
Articles excluded based on full text review Articles shortlisted for phase 2
Full texts of articles reviewed
Articles for inclusion in study
Articles excluded based on abstract or full text (if no
abstract only) screen PHASE 1
Draft protocol PICO4 – v1.6; 7th March 2014
7
Systematic review: An adapted version of the AMSTAR tool will be used to assess the methodological quality of all systematic reviews identified (see Appendix 3, (12)). If more than one systematic review is identified for a particular research question, we will focus on the most complete systematic review. The quality of the review will be assessed and if high, this review will be processed further. If not high, we will assess the next most complete review for inclusion. If a reproducible risk of bias assessment is not available, the most complete systematic review will be used as the starting point and worked up further as elaborated in the Summary of Findings Table and quality of evidence (GRADE) section of the protocol.
Randomised controlled trials (RCT): All randomised controlled trials will be assessed for risk of bias using the Cochrane collaboration’s tool ((13), see Appendix 4).
Observational studies: Risk of bias in observational studies will be determined using the tool described in Appendix 5. To the Cochrane guidance for assessing the risk of bias in non-randomised studies, questions from the Newcastle-Ottawa quality assessment tool ((13, 14)); was added to assess 1) the representativeness of the exposed group; 2) selection of the non-exposed group; 3) ascertainment of exposure; 4) demonstrating that the outcome was not present at the start; 5) assessing comparability of groups; 6) outcome assessment and 7) the length and adequacy of follow-up. In addition to this a question on controlling for confounding was also added.
Statistical analysis
All statistical analysis will be undertaken using Stata version 12 (Stata Corporation, Texas, USA). All categorical data will be summarized as frequencies and percentages, and, continuous data as means and standard deviations or medians and interquartile ranges. Risk of bias assessments and evidence tables will be generated using Review Manager V5.2 software (Review Manager 2012) (15)
Data handling: In primary studies with no cases in either the intervention or comparator group, 0.5 will be added to all four cells. In primary studies with no cases in the intervention and comparator group risk ratios will not calculated. For all randomised controlled trials, intention to treat analyses will be undertaken.
Meta-analysis: Meta-analyses of RCTs will be performed according to the guidelines described in the Cochrane Handbook (16). Results of studies that are sufficiently clinically homogeneous, i.e. sufficiently similar with respect to the participants, interventions, outcomes and timing of the follow-up measurements will be combined by the use of a fixed-effect model (Mantel-Haenszel), unless the studies are statistically heterogeneous. If so, a random-effects model will be used and if sufficient studies are available, heterogeneity will be explored by subgroup analyses. Statistical heterogeneity will be assessed by a combination of visual inspection of the forest plots, the Chi-square test for homogeneity (p-value set at 0.1 to increase the power of this test) and the I2 statistic. The latter two statistics will be interpreted in the light of the size of the studies included in the meta-analysis (e.g. if many large studies are included that have clinically irrelevant different effect estimates, the
Chi-Draft protocol PICO4 – v1.6; 7th March 2014
8
square test will become significant [due to high power] and I2 will approach 100%; in that case the results of the visual inspection will dominate the judgment of heterogeneity).
For comparative observational studies the generic inverse variance (GIV) method will be used for meta-analysis (16). For each study the adjusted effect estimates (aORs) and their standard errors (SE) will be used. If SEs are not reported, they will be derived from the 95% confidence interval of the aOR or from the reported p-value (if at least two decimal places have been reported).
RCTs and comparative observational studies will be analysed separately for all outcomes (17). If RCTs and comparative observational studies are processed for the same outcome, the same measure of treatment effect will be estimated for both study types to enable easy comparison of the results. Meta-analyses will then be presented in one forest plot, with subgroups according to study type.
Sensitivity analysis: A sensitivity analysis will be undertaken comparing studies with a low overall risk of bias with all identified randomised controlled trials (including those of unclear and high risk of bias).
Reporting bias: If appropriate, funnel plots will be used to explore if reporting bias is likely.
Summary of Findings Tables and quality of evidence (GRADE)
For each research question the evidence will be summarized by comparison (intervention versus comparator) and, within each comparison, by outcome in a Summary of Findings (SoF) Table. The SoF Table will contain information on:
all important and critical outcomes (predefined by the GDG);
a measure of the typical burden of these outcomes (e.g. based on control group risk);
a measure of the risk in the intervention group and/or a measure of the difference between the risks with and without the intervention;
the relative magnitude of effect (for dichotomous outcomes);
number of participants and studies addressing these outcomes;
a rating of the overall confidence in effect estimates for each outcome (quality of evidence);
comments.
If the body of evidence consists of RCTs and comparative observational studies, the SoF Table will include summaries of both, but presented separately.
The quality of evidence for each outcome will be assessed using the GRADE approach and presented in a GRADE-profile (18). In short, GRADE specifies four quality categories (high, moderate, low, very low). When the
Draft protocol PICO4 – v1.6; 7th March 2014
9
quality of evidence is high, one can be very confident that the true effect lies close to the estimated effect. When the quality of evidence is low, there is very little confidence in the validity of the effect estimate.
For therapeutic research questions, RCTs are considered high quality evidence and observational studies as low quality evidence. Study limitations, inconsistency, imprecision, indirectness and publication bias decrease one’s confidence in the effect estimates, resulting in downgrading of the evidence quality. For observational studies, a large effect size, a dose-response gradient and the direction of plausible confounding can increase one’s confidence in the effect estimate and result in upgrading the quality level.
The GRADE assessment will be based on the series of papers on GRADE in the Journal of Clinical Epidemiology (19) and the WHO Process Book (20).
The level of evidence, that is the overall quality of evidence across outcomes, is (in most circumstances) determined by the lowest quality level of the critical outcomes.
To create the GRADE-profiles and SoF Tables, the GRADEpro software (21), or the new Guideline Development Tool (updated version of GRADEpro) will be used. The Guideline Development Tool is expected to become available in September 2013. For SoF Tables, the American College of Chest Physicians (ACCP) format will be used.
For updates of systematic reviews the profile presented in the review will be updated. If no GRADE-profile is presented in the review (which according to our experience is the rule rather than the exception), it will only be possible to GRADE the results when the systematic review includes an extensive description of the characteristics of included studies (to enable judgement of the domains of inconsistency and indirectness), b) an extensive report of a risk of bias assessment (to enable judgement of the domain study limitations), c) detailed report (or meta-analyses) of the outcomes of interest (to enable judgement of the domain imprecision) and d) an assessment of possible selective reporting (to enable judgement of the domain publication bias). If this data are unavailable in the review, GRADEing requires retrieval, risk of bias assessment and data-extraction of the primary studies that were included in the review. This may be feasible if the number of included studies is limited.
Roles and responsibilities
1) LT: protocol design, develop study tools, literature review, assess risk of bias, data analysis, manuscript preparation
2) TW: literature review, assess risk of bias, manuscript preparation
3) MG: protocol design, oversee literature review, oversee data analysis, manuscript preparation 4) OR: oversee systematic review process
Draft protocol PICO4 – v1.6; 7th March 2014
10
5) KF: oversee systematic review process
6) JS: protocol design, oversee literature review, oversee data analysis, manuscript preparation
7) RS: protocol design, methodology input, oversee literature review, data analysis manuscript preparation
Proposed timelines
September – protocol design
October to November – literature review
December – February – data analysis and manuscript preparation
Dissemination of findings
A report of findings will be generated and presented to the WHO meningitis team, which will be used to inform the WHO guideline development process. In addition, manuscripts will be prepared for publication in appropriate peer reviewed journals.
Draft protocol PICO4 – v1.6; 7th March 2014
11
References
1. Lapeyssonnie L. [Cerebrospinal Meningitis in Africa]. Bulletin of the World Health Organization. 1963;28 Suppl:1-114. PubMed PMID: 14259333. Pubmed Central PMCID: 2554630. La m'eningite c'er'ebro-spinale en afrique.
2. Daugla D, Gami J, Gamougam K, Naibei N, Mbainadji L, Narbe M, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2013 Sep 12. PubMed PMID: 24035220.
3. World Health Organization. Control of epidemic meningococcal disease; WHO practical gudielines 1998. Available from:
http://www.who.int/csr/resources/publications/meningitis/WHO_EMC_BAC_98_3_EN/en/index.html.
4. World Health Organization. Managing meningitis epidemics in Africa: A quick reference guide for health authorities and health-care workers 2010. Available from:
http://www.who.int/csr/resources/publications/HSE_GAR_ERI_2010_4/en/index.html.
5. Hossain MJ, Roca A, Mackenzie GA, Jasseh M, Hossain MI, Muhammad S, et al. Serogroup W135 meningococcal disease, The Gambia, 2012. Emerging infectious diseases. 2013;19(9):1507-10. PubMed PMID: 23965435. Pubmed Central PMCID: 3810914.
6. Purcell B, Samuelsson S, Hahne SJ, Ehrhard I, Heuberger S, Camaroni I, et al. Effectiveness of antibiotics in preventing meningococcal disease after a case: systematic review. Bmj. 2004 Jun
5;328(7452):1339. PubMed PMID: 15178612. Pubmed Central PMCID: 420283.
7. Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. The Cochrane database of systematic reviews. 2011 (8):CD004785. PubMed PMID: 21833949.
8. Hoek MR, Christensen H, Hellenbrand W, Stefanoff P, Howitz M, Stuart JM. Effectiveness of vaccinating household contacts in addition to chemoprophylaxis after a case of meningococcal disease: a systematic review. Epidemiology and infection. 2008 Nov;136(11):1441-7. PubMed PMID: 18559124. Pubmed Central PMCID: 2870749.
9. Greenwood BM, Hassan-King M, Whittle HC. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. British medical journal. 1978 May 20;1(6123):1317-9. PubMed PMID: 417754. Pubmed Central PMCID: 1604678.
10. Bilukha OO, Rosenstein N, National Center for Infectious Diseases CfDC, Prevention. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2005 May 27;54(RR-7):1-21. PubMed PMID: 15917737.
11. European Centre for Disease Prevention and Control. Public health management of sporadic cases of invasive meningococcal disease and their contacts. 2010. Available from:
http://www.ecdc.europa.eu/en/publications/publications/1010_gui_meningococcal_guidance.pdf.
12. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC medical research
methodology. 2007;7:10. PubMed PMID: 17302989. Pubmed Central PMCID: 1810543.
13. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) 2011. Available from: www.cochrane-handbook.org.
14. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. . Available from:
http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
15. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2012.
16. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org. 17. Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from: www.cochrane-handbook.org.
Draft protocol PICO4 – v1.6; 7th March 2014
12
18. Schunemann H, Brozek J, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group 2009. Available from: http://ww.cc-ims.net/gradepro.
19. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology. 2011 Apr;64(4):383-94. PubMed PMID: 21195583.
20. World Health Organization. Handbook for guideline development. Geneva 2012.
21. GRADEpro. [Computer program]. Version 3.6 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann 2009.