ABSTRACT
ADEWALE, HEATHER LEIGH BATEMAN. Neuroendocrine Effects of Neonatal or Perinatal Exposure to the Endocrine Disrupting Compounds Bisphenol-A and Soy Phytoestrogens in Females. (Under the direction of Dr. Heather Patisaul).
It is well established that exposure to estrogen during neonatal development can alter
pubertal timing and reproductive development in female rodents. Recent research suggests
that exposure to endocrine disrupting compounds (EDCs) can alter these endpoints as well
and may be the reason behind recent decreases in fertility and the advancement of pubertal
onset seen in western populations. EDCs are compounds found within the environment that
can interfere with the effects of endogenous hormones in both humans and animals. Such
compounds can be either synthetic or naturally occurring and exposure occurs through a
variety of sources. The research presented here focuses on the plastics component
bisphenol-a (BPA) bisphenol-and the soy phytoestrogen genistein, both of which hbisphenol-ave been shown to bind to
endogenous estrogen receptors. Here I demonstrate that exposure to these EDCs is
particularly detrimental when occurring during the neonatal or perinatal stages of
development and can result in effects that may not become apparent until adulthood. Such
exposure can have adverse effects on the development of the hypothalamic-pituitary-gonadal
axis (HPG). These effects include advanced pubertal onset, altered estrous cyclicity,
premature reproductive senescence, abnormal ovarian development and can interfere with
HPG organization and signaling pathways. Hypothalamic endpoints measured include, but
are not limited to, the quantification and activation of gonadotropin-releasing hormone,
Neuroendocrine Effects of Neonatal or Perinatal Exposure to the Endocrine Disrupting Compounds Bisphenol-A and Soy Phytoestrogens in Females
by
Heather Leigh Bateman Adewale
A dissertation submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the degree of
Doctor of Philosophy
Zoology
Raleigh, North Carolina
2011
APPROVED BY:
_______________________________ ______________________________
Dr. Heather Patisaul Dr. John Godwin
Committee Co-Chair Committee Co-Chair
_______________________________ ________________________________
ii DEDICATION
This dissertation is dedicated to my husband Zeke who has been there to encourage and
support me from the beginning, I love you. To my parents, Bill and Debby Bateman who
instilled in me the belief that I can accomplish anything I put my mind to and who have been
there to support me through their prayers and encouragement and to my puppy Maya who
iii BIOGRAPHY
My interest in pursuing my PhD began during my time at the University of Virginia in
the labs of Dr. Emilie Rissman and Dr. Marty Mayo. It was there that I narrowed down my
field of interest to the areas of behavior and neuroendocrinology. These interests expanded to
include the field of reproduction during my time at North Carolina State University (NCSU)
and the final product of my research is presented within this dissertation. My interest in
research however was not the only motivation to pursue my degree. I am also interested in
teaching at the university level. During my time at NCSU I participated in teaching
programs and activities outside of my research program. These included the Certificate of
Accomplishment in Teaching (CoAT) and the Preparing the Professoriate (PTP) programs. I
used my acceptance into the PTP program as an opportunity to create and teach a course
entitled Captive Animal Reproduction. More information on these programs and my course can be found on my online teaching portfolio
iv ACKNOWLEDGMENTS
I would like to thank my mentor Dr. Heather Patisaul for believing in my abilities as a
student and a researcher, and my committee members for guiding me through my graduate
career. Members of the Patisaul lab for both their help and their friendship: Sandra Losa and
Alana Sullivan my fellow graduate students, our technicians Karina Todd, Jillian Mickens
and Kelly McCaffrey and undergraduates Natalie Mabrey and Meghan Radford. I thank Dr.
Jane Lubischer and Dr. Jenny Campbell for their advice and guidance regarding my desire to
expand on my interests in teaching. All of the staff at the Biological Resource Facility for all
their help in animal care and maintenance, in particular Barbara Welker, Linda Hester and
Kay Coole.
Additionally I would like to thank members outside of the NCSU community. Thank you
to Dr. Emilie Rissman for helping me get started in the field of neuroendocrinology and for
your continued support along the way. Dr. Marty Mayo and Dr. Norman Reichenbach for all
the reference letters I asked them to write along the way and for their encouragement
throughout this very long process of deciding what to do with my life. Thank you also to all
of my family and friends for their support, with special thanks to my brothers Jeff and Jason,
v TABLE OF CONTENTS
LIST OF TABLES . . . .vii
LIST OF FIGURES. . . viii
LIST OF ABBREVIATIONS. . . .xii
CHAPTER 1: Introduction . . . 1
References. . . 15
CHAPTER 2: Experiment 1, Aim 1: Hypothesis and abstract. . . 21
Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density. (published manuscript) . . . .22
References. . . 29
CHAPTER 3: Experiment 2, Aim 2: Hypothesis and abstract . . . .32
3.1: Neonatal Bisphenol-A Exposure Alters Rat Reproductive Development and Ovarian Morphology Without Impairing Activation of Gonadotropin-releasing Hormone Neurons. (published manuscript) . . . 34
References. . . 42
3.2: Impact Of Neonatal Exposure to the ERα Agonist PPT, Bisphenol-A or Phytoestrogens on Hypothalamic Kisspeptin Fiber Density in Male and Female Rats. (published manuscript) . . . . . . .45
References. . . 52
vi References. . . 64
CHAPTER 4: Experiment 3, Aim 3: Hypothesis and abstract . . . .67
Neonatal Genistein Exposure Induces Abnormal Estrous Cycles in Female Rats but Fails
to Affect Pubertal Timing . . . .68
References. . . 89
CHAPTER 5: Experiment 4, Aim 4: Hypothesis and abstract. . . 100
Bisphenol-A and Genistein: Do the Reproductive Effects from Exposure to these EDCs
Change When Female Rats are Exposed to Both Compounds Simultaneously? . . . .102
Conclusion. . . .133
vii LIST OF TABLES
CHAPTER 3.1
Table 1. Summary of ovarian morphology. . . 38
CHAPTER 5
Table 1. Exposure groups for breeding cohorts 1-4. . . .112
Table 2. Characterization of each of the 4 main stages of the rodent estrous cycle . .113
Table 3. Average amount of daily water consumption in dams . . . 115
Table 4. Average amount of daily water consumption for pups between PND 21
and 40. . . .116
Table 5. Average amount of EDCs GEN or BPA found in dam plasma. . . .117
Table 6. Average amount of EDCs GEN or BPA found in pup plasma at PND
12. . . 117
Table 7. Average amount of EDCs GEN or BPA found in pup plasma at PND
34. . . 118
viii LIST OF FIGURES
CHAPTER 1: Introduction
Figure 1. EDC Structures . . . .2
Figure 2A- B. Non-monotonic dose curves. . . 8
Figure 3. Sexual differentiation of the hypothalamus . . . 10
CHAPTER 2: Experiment 1
Figure 1A. Day of vaginal opening in females neonatally exposed to
phytoestrogens or ER agonists . . . .24
Figure 1B. Percentage of females displaying normal estrous cycles with
age following neonatal exposure to phytoestrogens or ER
agonists. . . 24
Figure 2A- B. Single (A) and double (B) immunolabeled GnRH neurons. . . .26
Figure 2C. Percentage of double labeled GnRH/FOS-ir neurons in females
neonatally exposed to phytoestrogens or ER agonists. . . .26
Figure 3A-D. Kisspeptin-ir fibers in the AVPV of female rats . . . .27
Figure 3E-F. AVPV kisspeptin-ir fiber density in females exposed
neonatally to phytoestrogens (E) and ER agonists (F) . . . 27
Figure 4. ARC kisspeptin-ir fiber density in females exposed neonatally
to phytoestrogens and ER agonists. . . 27
CHAPTER 3: Experiment 2: Subsection 3.1
Figure 1. Day of vaginal opening in females exposed neonatally to BPA
ix Figure 2. Percentage of females displaying normal estrous cycles with
age following neonatal exposure to BPA or PPT agonists. . . 38
Figure 3. Sexual receptivity in females exposed neonatally to BPA or
PPT. . . .38
Figure 4. Adult ovarian morphology. . . .39
Figure 5A. Representative immunolabeled GnRH neurons in the OVLT. . . .40
Figure 5B. Percentage of double labeled GnRH/FOS-ir cells in females
exposed neonatally to BPA or PPT. . . 40
CHAPTER 3: Experiment 2: Subsection 3.2
Figure 1A. AVPV kisspeptin-ir fiber density in females exposed
neonatally to BPA or PPT . . . .49
Figure 1B. ARC kisspeptin-ir fiber density in females exposed neonatally
to BPA or PPT. . . .49
Figure 2A-B. NiDAB labeled kisspeptin neurons in AVPV of ovary intact
control (A) and OVX (B) females. . . .50
Figure 2C. AVPV kisspeptin-ir fiber density in neonatally EDC exposed
females. . . .50
Figure 2D-E. Immunolabeled GnRH neurons in the OVLT of a male (D) and
OVX female (E). . . .50
Figure 2F. ARC kisspeptin-ir fiber density in neonatally EDC exposed
females. . . .50
x Figure 1A. Body weight of PND 99 females neonatally exposed to BPA or
PPT. . . .58
Figure 1B. Proceptive behavior in females neonatally exposed to BPA or
PPT. . . .58
Figure 2A. Representative NiDAB labeled OT and OT/FOS neurons in
female PVN. . . 59
Figure 2B. Average number of OT-ir cells in PVN of females exposed
neonatally to BPA. . . 59
Figure 3A-B. Representative immunolabeled 5-HT fibers in VMN of control
(A) and EB (B) female. . . .60
Figure 3C. VMNvl 5-HT-ir fiber density of females exposed neonatally to
BPA or PPT. . . .60
Figure 4A-B. Representative immunolabeled ERα cells in MPOA of control
(A) and EB (B) female. . . .61
Figure 4C. Average number of ERα-ir cells in MPOA of females exposed neonatally to BPA or PPT . . . .61
CHAPTER 4: Experiment 3
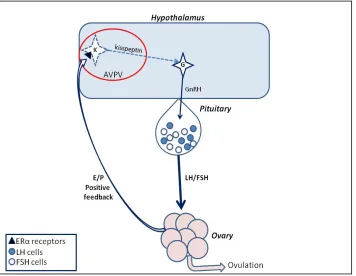
Figure 1. The hypothalamic-pituitary-ovarian signaling pathway. . . 72
Figure 2. Day of vaginal opening in GEN exposed females. . . .77
Figure 3. The percentage of females showing normal estrous cycles with
xi Figure 4A-C. Serum levels of LH in females at baseline (A), after EB/P (B)
and kisspeptin (C) administration. . . 80
CHAPTER 5: Experiment 4
Figure 1. Assessment of pubertal onset in females exposed to BPA, GEN
or both. . . 119
Figure 2A-C. Percentage of females displaying normal (A), prolonged (B), or
xii LIST OF ABBREVIATIONS
5-HT - serotonin
ANOVA – one-way analysis of variance
ARC – arcuate nucleus
AVPV – anterior ventral periventricular nucleus
BPA – bisphenol-a
bw – body weight
CL – corpora lutea
DA - dopamine
DOV – day of vaginal opening
DPN – diarylpropionitrile
Ds - diestrus
E - estrogen
EB – estradiol benzoate
EDC – endocrine disrupting compounds
EE – ethinyl estradiol
EPA – Environmental Protection Agency
EQ - equol
ER/ESR – estrogen receptor
Es - estrus
FDA – Food and Drug Administration
xiii GEN – genistein
GnRH – Gonadotropin Releasing Hormone
HPG axis – Hypothalamic-Pituitary-Gonadal axis
IHC – immunohistochemistry
-ir - immunoreactive
KiSS-1/Kiss-1 – kisspeptin gene (mRNA)
KISS – kisspeptin protein
LH – luteinizing hormone
LOAEL – lowest observed adverse effect level
LQ – lordosis quotient
MPOA – medial preoptic area
Ms - metestrus
NS – no soy diet
OT – oxytocin
OVLT – vascular organ of the lamina terminalis
OVX - ovariectomized
P - progesterone
PND – post-natal day
PPT – 1,3,5-tris(4-Hydroxyphenyl)-4-propyl-1H-pyrazole
Ps - proestrus
PS – plus soy
xiv s.c. (sc) – sub cutaneous
TH – tyrosine hydroxylase
VEH – vehicle control
1
CHAPTER 1: Introduction
Timing is everything. This phrase rings true within a number of different fields of study and even more so in the realm of developmental biology. In 1990 David Barker proposed that
the environment experienced by the developing fetus programs adult physiology, specifically
the susceptibility to metabolic diseases such diabetes, stroke, and coronary heart disease
(Barker 1990). This hypothesis suggests that if the environment experienced by the
developing fetus is one characterized by malnutrition, slow fetal growth or low birth weight
than the fetus is “programmed” to be more likely to develop certain diseases later on in life.
This concept can be further applied to now include the presence of external compounds in the
fetal environment that can affect fetal programming. One such class of compounds are
endocrine disrupting compounds (EDCs). The United States Environmental Protection
Agency (EPA) defines an EDC as “an exogenous chemical substance or mixture that alters
the structure or function(s) of the endocrine system and causes adverse effects….at the level
of the organism, its progeny, and populations or subpopulations of organisms.” This
definition includes disruption of lactation, sexual maturation, the ability to produce viable,
fertile offspring, sex specific behavior, and premature reproductive senescence. To date, the
EPA has identified hundreds of compounds that fit this definition (for more information visit
1996). The list includes compounds such as pesticides and herbicides which were initially
2 retardants and a number of other commercial products. Synthetic chemicals are not the only
compounds classified as EDCs. Naturally occurring compounds, such as the soy
phytoestrogens, can also fit this definition. These compounds can interfere with the normal
functioning of the endocrine system at multiple levels. Some mimic endogenous hormones,
most commonly androgens or estrogens, and can agonize or antagonize their receptors
(Zacharewski 1998). Others can alter the synthesis or degradation of hormones by acting
upon enzymes in the steroid synthesis pathways (Gore 2008). Included on this list are the two
compounds that are the subject of my research, bisphenol-a (BPA) and genistein (GEN).
Both of these EDCs mimic endogenous estrogens and can bind to endogenous nuclear and/or
membrane estrogen receptors (ERs) to exert their effects (Figure 1).
Figure 1.EDC Structures. Schematic representation of the chemical structures of estradiol, BPA and GEN. BPA is a component of plastic production while GEN is a phytoestrogen found in soy based products. The presence of a phenol ring (box) allows BPA and GEN to bind to endogenous ERs
3 An introduction to bisphenol-a (BPA)
BPA is a chemical component of plastic products, originally developed as a synthetic
estrogen in 1891 and later entering the plastics manufacturing process (Dodds and others
1938; Vandenberg and others 2009). BPA has since been found in polycarbonate plastics
including baby and water bottles, food containers, the epoxy resin lining the insides of metal
cans, flame retardants, medical equipment, plastic tubing and thermal paper among other
products (Biedermann and others 2010; Geens and others 2011; Vandenberg and others
2007). Human exposure occurs when BPA leaches from these products into the food,
beverage or medication and is consumed. Leaching is a byproduct of incomplete
polymerization of BPA to the plastic or resin and stress such as extreme heat or ultra-violate
(UV) light can cause BPA to be released and contaminate stored foods or beverages (Brede
and others 2003; Calafat and others 2009; Carwile and others 2010; Lakind and Naiman
2010; Markey and others 2002; Vandenberg and others 2007; Vandenberg and others 2009;
vom Saal and others 2007). Despite the fact that BPA was created initially as an estrogen
mimic, concerns over exposure were minimal due to its weak affinity for nuclear ERs
compared to endogenous estradiol. BPA has a 10,000 fold lower binding affinity for ERs α and β than estradiol and was therefore initially thought not to be a threat (Kuiper and others 1998). Concern over BPA exposure increased when in vitro studies showed that BPA can stimulate cellular responses at doses below the EPA’s proclaimed “safe” or reference dose of
50µg/kg per day and that, in some tissues, BPA exhibited equal potency to that of estradiol
(Welshons and others 2006; Welshons and others 2003). These concerns were raised even
4 human urine, with levels in infants and children higher than those found in adults (Calafat
and others 2005; Calafat and others 2009). Reasons for this are multifold. Exposure of infants
can occur through the use of plastic baby bottles (Cao and others 2009a; Cao and others
2008), through lactation from the nursing mother (Tsutsumi 2005)or through putting plastic
toys or materials treated with flame retardants in their mouths (Talsness and others 2009).
Importantly, studies have shown that fetuses and newborns have reduced ability to conjugate,
and thus deactivate, BPA (Welshons and others 2006) and that exposure to EDCs during
early periods of development can have detrimental effects later in life (Bourguignon and
Parent).
An introduction to the soy phytoestrogen genistein
Synthetic chemicals like BPA are not the only compounds in our environment that are
endocrine disrupting. Naturally occurring compounds can also be classified as EDCs and the
soy phytoestrogens are a prime example. Phytoestrogens are naturally occurring, steroid-like,
compounds found in plants that “mimic” endogenous estrogens either structurally or
functionally (Kurzer and Xia 1997). Isoflavones are a specific class of phytoestrogen found
in members of the legume family, most notably soybeans. Soybeans and soy based products
have recently gained popularity due to their cardiac protective effects and other health
benefits, and consumption has increased among all age groups, especially infants and
children (Cao and others 2009b; Setchell 2001; Setchell and others 1984; Setchell and others
1998). They are used as staple ingredients in many foods including soy milk, soy infant
formula, tofu, energy and granola bars, cereals and are also taken in concentrated forms as
5 2001; Setchell and Cole 2003). One of the main isoflavones found in soy-based products that
is suspected of having estrogenic properties is GEN. Concern over GEN arises from its
ability to cross the placental barrier during gestation, its presence in the breast milk of
nursing mothers who consume soy products and the use of soy in infant foods and formulas
(Franke and others 1998; Todaka and others 2005). Public awareness about the sensitivity of
fetuses, infants and children to phytoestrogen exposure and the potential risks of consuming
excess phytoestrogens is lacking. Additionally, many studies looking at the health impacts of
soy consumption fail to consider that its phytoestrogen components can act as EDCs and
therefore might pose health risks, specifically in infants and children.
Human health effects and EDCs
Concern over EDC effects is growing because the reproductive health of women and men
in westernized countries appears to be declining (Brannian and Hansen 2006; Frey and Patel
2004; Joensen and others 2008; Nyboe Andersen and Erb 2006). Two notable trends are a
rise in infertility and increased numbers of girls experiencing precocious puberty. Precocious
puberty is characterized by the decreasing age at which girls in today’s society are
experiencing breast development (thelarche) or first menstruation (menarche) (Aksglaede
and others 2009; Herman-Giddens and others 1997; Parent and others 2003; Partsch and
Sippell 2001; Proos and others 1991). While the negative implications for decreased fertility
are obvious, those associated with early puberty are less so. Girls entering puberty as early as
8 years of age are not only at risk for early menopause but also experience increased
anti-social behaviors and sexual precocity (Phinney, et al. Adolescence, 1990; Ge, et al. Child
6 it more difficult for them to fit in with their peers (Celio and others 2006; Ge and others
1996; Phinney and others 1990). My research tested the hypothesis that exposure to GEN,
BPA or both during early life might interfere with developmental programming of the
hypothalamic-pituitary-gonadal (HPG) axis. Specifically, exposure will result in the
advancement of pubertal onset, premature reproductive senescence (indicated by abnormal
estrous cycles in rodents and premature entrance into a state of persistent estrus, where
females fail to progress through the normal stages of the estrous cycle) and interfere with
sexually dimorphic organization of brain nuclei within the hypothalamus.
Timing is everything
The developing organism undergoes well defined critical time periods of cellular and
molecular programming during which it is highly sensitive to changes in the hormonal
environment, both internal and external. In contrast, the adult organism has lost this degree of
plasticity and is therefore not as sensitive to the organizational effects of hormones and other
environmental components (Arnold and others 1996). (A timeline of critical developmental
periods for each system of the body can be found at the Endocrine Disruption Exchange
by increased cell growth, division and differentiation making them particularly sensitive to
compounds that interfere with these processes (Nicoletto and Rinaldi 2011; Nijland and
others 2008). This concept has been termed developmental programming (Barker 1990).
Developmental programming can be defined as “the response by the developing mammalian
organism to a specific challenge during a critical time window that alters the trajectory of
7 phenotype” (Nijland and others 2008). It occurs during the embryonic stage and/or
immediately after birth during the neonatal stage, depending on the organism, and is
comprised of a number of biological milestones that determine the fate of the adult organism.
Interference with these processes can lead to alterations in development that may not be
apparent until adulthood. Numerous studies have shown that, both large and seemingly
insignificant, alterations in the fetal environment during these periods can have adverse
effects on a number of biological systems including disease susceptibility, metabolic
syndromes, cancers, reproductive disorders, behavior and cognitive processes (Daniel and
Bohacek 2010; Gore 2008; Nijland and others 2008; Thornton and others 2009; Vandenberg
and others 2009; Welshons and others 2006). Importantly, periods of developmental
programming differ between species. In rodents sexual differentiation of the brain and
gonads occurs immediately prior to and following birth, while in more precocial mammals
this window occurs entirely in utero (Gorski 1985; Robinson 2006; Simerly 2002). For example, in sheep these systems develop prenatally, between gestational days 30-90 (Clarke
and others 1976a; Clarke and others 1976b)while in many primates, including humans,
sexual differentiation occurs largely during the first trimester (Herman and others 2000;
Wallen 2009). Sensitivity to hormones, and thus EDCs, is heightened during these periods
and even small changes in hormone presence or concentrations have been shown to have
greater effects during development than at other times (Aksglaede and others 2006). Thus,
the hypothesis that low dose exposures to BPA and other EDCs can induce adverse outcomes
on neuroendocrine development, that persist into adulthood, has so far been underexplored
8 studies addressing dose effects utilize a non-linear dose curve and the “safe” level for human
consumption is set 1000x below the lowest observed adverse effect level (LOAEL) (Erler
and Novak). Many EDCs, BPA included, have failed to display a linear dose curve and
instead are characterized by non-linear, U or inverted U-shaped, dose curves (Erler and
Novak; Vandenberg and others 2007; Vandenberg and others 2009; Welshons and others
2006; Welshons and others 2003). U-shaped dose curves can be interpreted two ways. First,
compounds that exhibit this type of curve can cause effects at both low and high doses but
not at doses falling in between. This is considered to be a normal “U” dose curve.
Conversely, a compound with an inverted “U” dose curve shows no activity at the low and
high end of the curve, but can cause effects at doses that fall in between (Figure 2). This can
lead to erroneous conclusions when using a linear model to set “safe” or reference doses for
9 the case of BPA, recent studies have shown that BPA can affect reproductive physiology and
behavior at doses below the “safe” dose of 50µg/kg per day set by the EPA (Honma and
others 2002; Kubo and others 2003; Vandenberg and others 2009; Welshons and others
2006).
An Introduction to Sexual Differentiation
The developing reproductive system is complex and intricate. Its interconnectivity often
means that compounds that affect one area are likely to have direct or indirect effects
elsewhere within the reproductive axis. For example, if a particular EDC affects fertility the
underlying problem could be located in the brain. Reproduction is governed by the HPG axis
and requires interaction and communication between the brain and the gonads. An EDC that
reduces fertility could therefore act directly at the level of the ovary or testis but could also
interfere with the signaling pathways that connect the ovary or testis to the hypothalamus.
Like reproductive development, the areas of the brain governing reproduction are highly
sensitive to changes in the hormonal environment during critical periods making them
candidates for endocrine disruption. Both the gonads and the brain depend on genotypic sex
and gonadal steroids to develop sex specific phenotypes, a concept known as sexual
differentiation (Simerly 1998; Simerly 2002). There is evidence that BPA, GEN and other
EDCs can affect the organization and development of these sexually dimorphic circuits,
specifically those that govern ovulation and reproductive behavior. Under normal
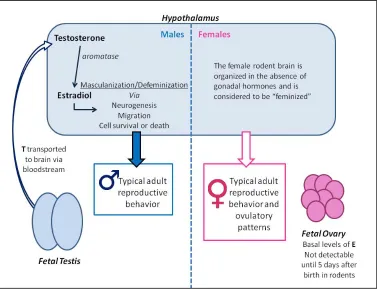
circumstances the fetal testis secretes testosterone into the blood stream where it travels to
10 estrogen which then induces the male-pattern of brain development (Arnold and Breedlove
1985; Simerly 2002) The female brain, however, develops largely in the absence of gonadal
hormones. . Classic studies demonstrating the importance of hormones in sexual
differentiation of the brain and behavior show that neonatally castrated male rodents who
lack both estrogens and androgens will display de-masculinized, female-like, behavior and
brain patterns of development as adults. Conversely, females neonatally ovariectomized and
11 If gonadectomized and treated as adults however, these changes in the brain, and thus
behavior, do not occur. Additional studies further revealed that the timing of exposure is
important and hormone treatment outside the perinatal windows of sexual differentiation will
fail to alter organization of the brain (Barraclough and Gorski 1961; Harris and Levine 1965;
Romeo 2003; Sisk and Zehr 2005; Whalen and Edwards 1966; Whalen and Edwards 1967;
Whalen and Nadler 1963). If alterations in gonadal steroids have the ability to affect sexually
dimorphic organization of the brain, then it follows that the presence of EDCs that mimic
gonadal steroids may also alter hormone dependent organization of sexually dimorphic areas.
The studies presented here will address this possibility by looking at whether exposure to
BPA, GEN or both alters hypothalamic organization or neuronal activity. Specifically, I will
look at the circuits involved in the regulation of the estrous cycle, ovulation and pubertal
onset in female rats to assess whether exposure to BPA, GEN or both alters the development
or activity of these nuclei.
Summary
My goal with the research presented here is to address current concerns over the
potentially hazardous effects of the EDCs BPA and GEN, with a focus on how exposure to
these compounds during development can affect reproductive endpoints and their potential to
compromise fertility. My research adds to a body of literature aimed at increasing public
awareness about the presence of EDCs within the environment and, how early life exposures
to these compounds might impair reproductive fecundity later in life. Specifically, I look at
environmentally relevant doses of GEN and low dose effects of BPA when administered
12 comparing the effects of GEN and BPA to those exhibited by ERα and ERβ specific agonists. Animals in the experiments presented here were exposed to levels of BPA that span the
LOAEL (50mg/kg body weight (bw) /day), falling below the EPA’s recommended “safe”
dose (50µg/kg bw/day), and are within estimated levels of human exposure. Doses of GEN
used in this study mimic those ingested by infants on a soy based infant formula or through
maternal transfer during gestation or lactation.
My research begins with a look at two phytoestrogens, GEN and equol (EQ), and their
effects on pubertal timing and HPG axis signaling necessary for ovulation and reproduction
(experiment 1, Ch. 2). To begin to understand the potential mechanisms/receptors through
which these phytoestrogens act, I included two additional controls that selectively agonized
one of the two main forms of nuclear estrogen receptors (ERα and ERβ). GEN is known to
have a relatively high binding affinity for endogenous ERs, particularly ERβ (Kuiper and others 1998), leading me to hypothesize that the effects of GEN would more closely
resemble those seen with the ERβ agonist. I next sought to determine if a synthetic EDC with a relatively weak binding affinity for endogenous ERs could induce similar effects
(experiment 2, Ch. 3). Until recently the estrogenic properties of BPA have been overlooked
due to its weak affinity for ERs (1000 fold weaker binding affinity than estradiol) (Kuiper
and others 1998) despite being tested for use as a synthetic estrogen (Vandenberg and others
2009). To investigate the low dose effects of BPA I chose two doses: the LOAEL (50mg/kg
bw/day) and the EPA’s “safe” dose (50µg/kg bw/day). I hypothesized that neonatal BPA
exposure would advance puberty and induce abnormal estrous cycles similar to the results
13 problems within the ovary (Ch. 3.1) and hypothalamic organization (Ch 3.2 and 3.3) and that
they would be dose specific. Results from this experiment indicated that BPA can have
differential effects on reproductive development dependent on the dose administered. This
led me to re-evaluate whether or not GEN also displayed dose specific effects. For
experiment 3 I chose 2 doses of GEN: the high dose (10mg/kg bw/day) simulates the amount
of GEN infants on soy based formula are exposed to, while the low dose (1mg/kg bw/day)
approximates the lower levels of exposure seen through placental or lactational transfer from
mother’s on a high soy diet (2008; Setchell and others 1998; Setchell and others 1997;
Whitten and Patisaul 2001). Results from experiments 1, 2 and 3 demonstrated that neonatal
exposure to phytoestrogens and BPA had similar, dose dependent, effects on pubertal
advancement and premature reproductive senescence but not on hypothalamic organization.
Both GEN and EQ decreased hypothalamic kisspeptin fiber density, in the anterior ventral
periventricular nucleus (AVPV), and gonadotropin releasing hormone (GnRH) activation.
Neonatal GEN dose dependently reduced the ability of females to respond to steroid positive
feedback further demonstrating its ability to impair HPG signaling. In contrast, BPA failed to
decrease either of these endpoints indicating that GEN and BPA may utilize different
mechanisms to affect the development and organization of the HPG axis. Further results
concluded that BPA exposure dose dependently altered ovarian histology. Females exposed
to the high dose of BPA displayed less corpora lutea (CL, a marker of successful ovulation)
than vehicle controls while the low dose females showed increased CL compared to vehicle
controls. Together these results support my hypothesis that GEN and BPA can exhibit
14 For my initial work (experiments 1,2 and 3) EDCs were given by injection and their
effects were explored individually. However, to better model human exposure I chose to
orally administer BPA, GEN or a mixture of both in my last experiment. In reality, exposures
to EDCs are rarely limited to a single compound, making research addressing the effects of
mixtures essential. Many studies focus on only one EDC and those that have looked at
mixture effects usually look at compounds that are within the same class (all synthetic, or all
pesticidal components, etc) (Bourguignon and Parent 2010). For this reason I explored
mixture effects of BPA (a synthetic EDC) and soy phytoestrogens (naturally occurring EDCs) both common elements found and ingested in our environment. Additionally, because
many of the sexually dimorphic patterns set during neonatal development are further
developed during puberty (Ahmed and others 2008; Schulz and others 2004; Sisk 2004; Sisk
and Zehr 2005) the exposure window for experiment 4 was extended to include gestation
through puberty. Together my dissertation covers the effects of gestational, neonatal and
pubertal exposure to the EDCs BPA and soy phytoestrogens, alone and in combination. I
assess the effects of BPA, soy phytoestrogens or both on multiple reproductive endpoints
within the HPG axis, address potential mechanisms of action and provide evidence that
exposure to these compounds can advance pubertal timing, adversely affect estrous cycles,
induce premature reproductive senescence, interfere with HPG signaling and can alter
15 REFERENCES
2008. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. U.S. Department of Agriculture, Agricultural Research Service.
Adlercreutz H, Mazur W. 1997. Phyto-oestrogens and western diseases. Ann Med 29:95-120.
Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. 2008. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 11(9):995-7.
Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson A-M. 2006. The sensitivity of the child to sex steroids: possible impact of exogenous estrogens. Human Reproduction Update 12(4):341-349.
Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. 2009. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics 123(5):e932-9.
Arnold AP, Breedlove SM. 1985. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19(4):469-98.
Arnold SF, Collins BM, Robinson MK, Guillette LJ, Jr., McLachlan JA. 1996. Differential interaction of natural and synthetic estrogens with extracellular binding proteins in a yeast estrogen screen. Steroids 61:642-646.
Barker DJ. 1990. The fetal and infant origins of adult disease. Bmj 301(6761):1111.
Barraclough CA, Gorski RA. 1961. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology 68:68-79.
Biedermann S, Tschudin P, Grob K. 2010. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem 398(1):571-6.
Bourguignon JP, Parent AS. 2010. Early homeostatic disturbances of human growth and maturation by endocrine disrupters. Curr Opin Pediatr 22(4):470-7.
Brannian J, Hansen K. 2006. Assisted reproductive technologies in South Dakota: the first ten years. S D Med 59(7):291-3.
Brede C, Fjeldal P, Skjevrak I, Herikstad H. 2003. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam 20(7):684-9.
16 Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. 2009. Exposure to
bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 117(4):639-44.
Cao XL, Corriveau J, Popovic S. 2009a. Migration of bisphenol A from can coatings to liquid infant formula during storage at room temperature. J Food Prot 72(12):2571-4.
Cao XL, Dufresne G, Belisle S, Clement G, Falicki M, Beraldin F, Rulibikiye A. 2008. Levels of bisphenol A in canned liquid infant formula products in Canada and dietary intake estimates. J Agric Food Chem 56(17):7919-24.
Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. 2009b. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. Journal of exposure science & environmental epidemiology 19(2):223-34.
Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY. 2010. Use of polycarbonate bottles and urinary bisphenol a concentrations. Environmental Health Perspectives.
Celio MM, Karnik NNS, Steiner HH. 2006. Early maturation as a risk factor for aggression and delinquency in adolescent girls: A review. International journal of clinical practice 60(10):1254-62.
Clarke IJ, Scaramuzzi RJ, Short RV. 1976a. Effects of testosterone implants in pregnant ewes on their female offspring. J Embryol Exp Morphol 36(1):87-99.
Clarke IJ, Scaramuzzi RJ, Short RV. 1976b. Sexual differentiation of the brain: endocrine and behavioural responses of androgenized ewes to oestrogen. J Endocrinol 71(1):175-6.
Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE and others. 1998. Environmental endocrine disruption: an effects assessment and analysis. Environmental Health Perspectives 106(Suppl 1):11-56.
Daniel JM, Bohacek J. 2010. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta 1800(10):1068-76.
Dodds EC, Goldberg L, Larson W, Robinson R. 1938. Estrogenic activity of certain synthetic compounds. Nature 141:247.
Erler C, Novak J. 2010. Bisphenol a exposure: human risk and health policy. J Pediatr Nurs 25(5):400-7.
17 Franke AA, Custer LJ, Tanaka Y. 1998. Isoflavones in human breast milk and other biological fluids.
Am J Clin Nutr 68(6 Suppl):1466S-1473S.
Frey KA, Patel KS. 2004. Initial evaluation and management of infertility by the primary care physician. Mayo Clin Proc 79(11):1439-43; quiz 1443.
Ge X, Conger RD, Elder GH, Jr. 1996. Coming of age too early: pubertal influences on girls' vulnerability to psychological distress. Child Dev 67(6):3386-400.
Geens T, Goeyens L, Covaci A. 2011. Are potential sources for human exposure to bisphenol-A overlooked? Int J Hyg Environ Health.
Gore AC. 2008. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol 29(3):358-74.
Gorski RA. 1985. Sexual dimorphisms of the brain. Journal of Animal Science 61 Suppl 3:38-61.
Harris GW, Levine S. 1965. Sexual differentiation of the brain and its experimental control. J Physiol 181(2):379-400.
Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. 1997. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99(4):505-12.
Herman RA, Jones B, Mann DR, Wallen K. 2000. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav 38(1):52-66.
Honma SS, Suzuki AA, Buchanan DLDL, Katsu YY, Watanabe HH, Iguchi TT. 2002. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse
reproduction. Reproductive Toxicology 16(2):117-22.
Joensen UN, Jorgensen N, Rajpert-De Meyts E, Skakkebaek NE. 2008. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol 102(2):155-61.
Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. 2003. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res 45(3):345-56.
Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, Van Der Berg B, Gustafsson JA. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor b. Endocrinology 139:4252-4263.
Kurzer MS, Xia X. 1997. Dietary Phytoestrogens. Annual Review of Nutrition 17:353-381.
18 Markey CM, Rubin BS, Soto AM, Sonnenschein C. 2002. Endocrine disruptors: from Wingspread to
environmental developmental biology. J Steroid Biochem Mol Biol 83(1-5):235-44.
Nicoletto SF, Rinaldi A. 2011. In the womb's shadow. EMBO Rep 12(1):30-34.
Nijland MJ, Ford SP, Nathanielsz PW. 2008. Prenatal origins of adult disease. Curr Opin Obstet Gynecol 20(2):132-8.
Nyboe Andersen A, Erb K. 2006. Register data on Assisted Reproductive Technology (ART) in Europe including a detailed description of ART in Denmark. International Journal of Andrology 29(1):12-6.
Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. 2003. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine Reviews 24(5):668-93.
Partsch CJ, Sippell WG. 2001. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Human Reproduction Update 7(3):292-302.
Phinney VG, Jensen LC, Olsen JA, Cundick B. 1990. The relationship between early development and psychosexual behaviors in adolescent females. Adolescence 25(98):321-32.
Proos LA, Hofvander Y, Tuvemo T. 1991. Menarcheal age and growth pattern of Indian girls adopted in Sweden. I. Menarcheal age. Acta paediatrica Scandinavica 80(8-9):852-8.
Robinson J. 2006. Prenatal programming of the female reproductive neuroendocrine system by androgens. Reproduction 132(4):539-47.
Romeo RD. 2003. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol 15(12):1185-92.
Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. 2004. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45(4):242-9.
Setchell KD. 2001. Soy isoflavones--benefits and risks from nature's selective estrogen receptor modulators (SERMs). J Am Coll Nutr 20(5 Suppl):354S-362S; discussion 381S-383S.
Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. 1984. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr 40(3):569-78.
19 Setchell KD, Cole SJ. 2003. Variations in isoflavone levels in soy foods and soy protein isolates and
issues related to isoflavone databases and food labeling. J Agric Food Chem 51(14):4146-55.
Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. 1998. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. American Journal of Clinical
Nutrition 68:1453S.
Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. 1997. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 350:23-27.
Simerly RB. 1998. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92(2):195-203.
Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507-36.
Sisk C. 2004. The neural basis of puberty and adolescence. Nature Neuroscience 7(10):1040-1047.
Sisk CL, Zehr JL. 2005. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26(3-4):163-74.
Talsness CE, Andrade AJM, Kuriyama SN, Taylor JA, vom Saal FS. 2009. Components of plastic: experimental studies in animals and relevance for human health. p 2079-2096.
Thornton J, Zehr JL, Loose MD. 2009. Effects of prenatal androgens on rhesus monkeys: A model system to explore the organizational hypothesis in primates. Hormones and Behavior 55(5):633-644.
Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T and others. 2005. Fetal exposure to phytoestrogens--the difference in phytoestrogen status between mother and fetus. Environmental research 99(2):195-203.
Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr., Jégou B, Jensen TK, Jouannet P, Keiding N and others. 1996. Male reproductive health and environmental xenoestrogens. Environmental Health Perspectives 104(Suppl 4):741-803.
Tsutsumi O. 2005. Assessment of human contamination of estrogenic endocrine-disrupting chemicals and their risk for human reproduction. The Journal of Steroid Biochemistry and Molecular Biology 93(2-5):325-30.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24(2):139-77.
20 vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F,
Guillette LJ, Hauser R, Heindel JJ and others. 2007. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive Toxicology 24(2):131-8.
Wallen K. 2009. The Organizational Hypothesis: Reflections on the 50th anniversary of the
publication of Phoenix, Goy, Gerall, and Young (1959). Hormones and Behavior 55(5):561-565.
Welshons WV, Nagel SC, vom Saal FS. 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147(6 Suppl):69.
Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111(8):994-1006.
Whalen RE, Edwards DA. 1966. Sexual reversibility in neonatally castrated male rats. J Comp Physiol Psychol 62(2):307-10.
Whalen RE, Edwards DA. 1967. Hormonal determinants of the development of masculine and feminine behavior in male and female rats. Anat Rec 157(2):173-80.
Whalen RE, Nadler RD. 1963. Suppression of the development of female mating behavior by estrogen administered in infancy. Science 141:273-4.
Whitten PL, Patisaul HB. 2001. Cross-species and interassay comparisons of phytoestrogen action. Environmental Health Perspectives 109(Suppl 1):5-23.
21
CHAPTER 2: Experiment 1
Aim 1: Determine the effects of neonatal exposure to EDCs on reproductive development.
Hypothesis: Neonatal exposure to the soy phytoestrogens GEN and EQ will advance pubertal onset, induce abnormal estrous cycles and alter sexually dimorphic organization of the hypothalamus in female rats.
My first aim investigates how neonatal exposure to the soy phytoestrogens GEN and EQ
alters reproductive development in female rats. Additionally I wanted to begin to parse out
potential mechanisms of action underlying these effects. I therefore added two ER selective
agonists, diarylpriopionitrile (DPN), an ERβ selective agonist, and
1,3,5-tris(4-Hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), an ERα selective agonist. Estradiol benzoate
(EB) was used as a positive control due to its ability to bind both ERα and ERβ. Results from this study supported my hypothesis that neonatal exposure to GEN or EQ can disrupt female
reproductive development through the advancement of pubertal onset and induction of
premature reproductive senescence. Furthermore, GEN and PPT decreased GnRH activation
and kisspeptin fiber density in the hypothalamus, suggesting that this may be the underlying
cause behind these effects. The ability of PPT, but not DPN, to decrease GnRH activation
32
CHAPTER 3: Experiment 2
Aim 2: Determine the effects of BPA exposure, at both high and low doses, on reproductive development. In addition to pubertal onset and estrous cyclicity I will assess ovarian
histology, sexually dimorphic endpoints in the hypothalamus and will further explore the role
of ERα in the mechanisms underlying these effects.
Hypothesis: Neonatal exposure to BPA will advance pubertal onset and induce
premature reproductive senescence. I further hypothesize that abnormal ovarian histology and decreased kisspeptin expression and GnRH activation will be the underlying cause of these effects.
Results from aim 1 indicated that neonatal exposure to compounds that mimic estrogen,
in this case soy phytoestrogens, can adversely impact hypothalamic signaling and alter
estrous cyclicity. The soy phytoestrogen GEN is known to have a relatively high binding
affinity for endogenous ERs. My next step was to look at another EDC with weak binding
affinity for endogenous ERs to see if similar effects could be produced. I chose to focus on
the plastics component BPA. BPA has a 10,000 fold weaker affinity for endogenous ERs
compared to estradiol and was therefore, initially, considered not be pose a threat to human
health. However, the abundance of BPA in commercial products has lead to rising concern
33 institutions, may have adverse effects on physiological systems. For this reason aim 2
focused on the effects of neonatal exposure to BPA on hypothalamic and ovarian
development. Results from this aim were published as three different manuscripts and
together show that neonatal exposure to BPA can adversely affect reproductive development
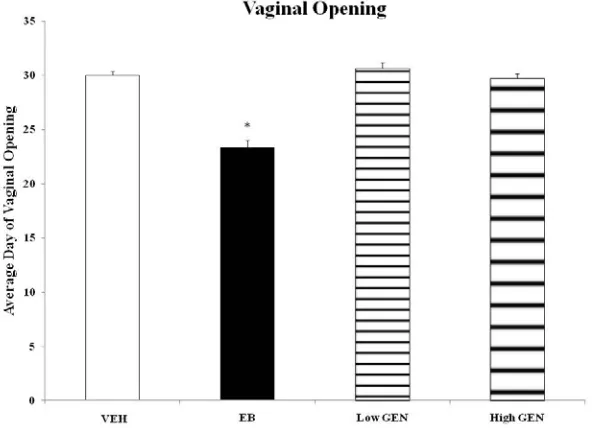
in female rats and that its effects were dose specific. Females exposed to the low, but not the
high, dose of BPA exhibited advanced pubertal onset, while both doses induced abnormal
estrous cycles. Neither of these effects were associated with decreased reproductive
behaviors following ovariectomy. Compared to controls, ovarian histology in both groups
was abnormal, with the high dose group showing decreased CL and increased numbers of
antral follicles, indicating the absence of normal ovulation. Neither of these effects was
associated with decreased GnRH activation as hypothesized, but the high dose of BPA
diminished kisspeptin fiber density in the arcuate nucleus. Additional sexual dimorphic
endpoints assessed include oxytocin (OT) and ERα cell density and 5-HT fiber density in hypothalamic nuclei critical for female reproductive physiology and behavior. Selective
agonism of ERα during the neonatal period also advanced pubertal onset, induced premature reproductive senescence and abnormal ovarian histology. However, unlike BPA, PPT
reduced GnRH activity and kisspeptin fiber density, suggesting that BPA is not utilizing ERα
34
3.1
: Neonatal Bisphenol-A Exposure Alters Rat Reproductive
Development and Ovarian Morphology Without Impairing Activation
45
3.2
:
Impact of Neonatal Exposure to the ERα agonist PPT, Bisphenol
-A or Phytoestrogens on Hypothalamic Kisspeptin Fiber Density in
54
3.3:
The Impact of Neonatal Bisphenol-A Exposure on Sexually
67
CHAPTER 4: Experiment
3Aim 3: Determine the effects of neonatal exposure to the soy phytoestrogen GEN on reproductive potential and steroid positive feedback.
Hypothesis: Neonatal exposure to genistein will alter pubertal timing and will interfere with normal estrous cyclicity and the ability of females to release luteinizing hormone in response to estrogen positive feedback on the hypothalamus.
The naturally occurring phytoestrogen GEN is a component of soy and soy-based
products with the potential to exert endocrine disrupting effects on the developing
reproductive system. Here I present data describing the effects of neonatal exposure to
genistein on pubertal timing and estrous cyclicity in female rats. Two separate doses of GEN
were administered, a high dose of 10mg/kg bw and a low dose of 1mg/kg bw. Neither dose
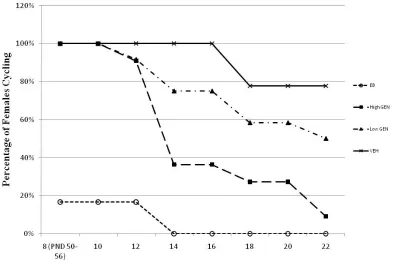
advanced pubertal timing however both doses induced premature reproductive senescence.
By 14weeks of age, females exposed to GEN started to display abnormal estrous cycles with
over 50% entering a state of persistent estrus by the end of the study. Following the
cessation of normal estrous cycles the ability of females produce a luteinizing hormone (LH)
surge in response to estrogen/progesterone (E/P) and kisspeptin administration was assessed.
Females exposed to the high dose of GEN failed to show elevated levels of LH compared to
controls following administration of E/P as well as kisspeptin. Females exposed to the low
68 not significantly different from controls when administered E/P. The data presented here
suggest that neonatal GEN administration can interfere with the ability of females to display
normal estrous cycles and may hasten the onset of reproductive senescence. It is possible
that neonatal exposure to GEN reduced the ability of females to respond to steroid positive
feedback and generate a pre-ovulatory LH surge and, that this underlies the inability of GEN
69
Neonatal Genistein Exposure Induces Abnormal Estrous Cycles
in Female Rats but Fails to Affect Pubertal Timing.
Heather B. Adewale and Heather B. Patisaul
Although there is concern over exposure to synthetic EDCs, such as BPA, phthalates or
other commercially produced chemicals, there is less concern over naturally occurring EDCs
like the soy phytoestrogens. Soy phytoestrogens have been touted for their positive effects
on cardiovascular health, carcinogenesis, osteoporosis and as a replacement for hormone
therapy in post-menopausal women (Baber 2010; Cassidy and Hooper 2006; Kim 2008;
Messina and others 2002). Recent studies, however, have begun to reveal that not all effects
of phytoestrogens are beneficial and that, similar to other EDCs, these compounds can have
adverse effects on the developing reproductive system (reviewed in (Bondesson and
Gustafsson 2010; Patisaul and Jefferson 2010; Setchell 2001). My previous study (Bateman
and Patisaul 2008) looked at the effects of two isoflavones found in soy thought to have the
most estrogenic potency, GEN and EQ. For this study I chose to focus specifically on GEN
for a few reasons. First, EQ is the metabolite of the phytoestrogen daidzein and is produced
exclusively by flora in the gut that are not present in all humans (Lampe 2009). The ability
to convert genistin to its active form GEN however is present in everybody, even infants,
thus anyone who consumes soy based products is exposed to GEN. Second, GEN has both
the ability to cross the placenta and, has been found in breast milk (Doerge and others 2001).
70 and infancy (Franke and others 1998). Exposure to GEN however is not limited to maternal
transfer. Many lactose intolerant infants are fed soy based infant formula exposing them to
even higher levels than those obtained through maternal transfer (Bhatia and Greer 2008;
Setchell and others 1998). Therefore the high dose of GEN (10mg/kg bw) used in this study
represented the estimated daily intake of phytoestrogens in infants fed soy based formula and
the low dose was set 10x below this at 1mg/kg bw and approximated that obtained through
placental or lactational transfer (2008; Franke and Custer 1996; Franke and others 1998;
Setchell and others 1998).
Soy protein is also currently being used to fortify a wide range of processed food
products including energy and granola bars, cereals, imitation dairy and meat products,
among others (2008). This prevalence, combined with the fact that GEN has a higher
binding affinity for endogenous ERs than many synthetic EDCs, including BPA (Kuiper and
others 1998; Nikov and others 2000), and can be transferred from mother to infant,
constitutes the rationale for concern about developmental exposure to soy. The ability of
GEN to agonize endogenous ERs and interfere with reproductive development (Jefferson
2010; Jefferson and others 2007; Kuiper and others 1998; Nagao and others 2001; Rozman
and others 2006; Strauss and others 1998) may be contributing to the recent decline in
reproductive health in western countries. This decline is evidenced by increased sub-fertility
rates in both men and women and the earlier ages at which girls are entering puberty
(Adlercreutz and Mazur 1997; Brannian and Hansen 2006; Carlsen and others 1992; Joensen
and others 2008; Partsch and Sippell 2001; Swan and others 2000; Swan 2006).
71 depending on dose and importantly, how it can affect reproductive development of infants
and children, is necessary if consumption of soy products continues to rise.
Importantly, adverse effects on ovarian development, estrous cyclicity and pubertal
timing have been demonstrated following neonatal exposure to GEN (Bateman and Patisaul
2008; Jefferson and others 2002; Jefferson and others 2005; Jefferson and others 2007;
Jefferson and Williams 2011; Losa and others 2011; Nikaido and others 2005; Nikaido and
others 2004; Rozman and others 2006). In aim 1 (Ch. 2, (Bateman and Patisaul 2008) I
found that GEN exposed females in persistent estrus also had impairments in HPG signaling,
specifically a decrease in GnRH activity and kisspeptin fiber density. Together, these results
imply that GEN’s ability to advance puberty, induce abnormal estrous cycles, early
reproductive senescence (Bateman and Patisaul 2008) and alter ovarian development
(Jefferson and others 2006; Jefferson and others 2002) may be due to its effects on the
signaling pathways of the HPG axis.
The HPG axis is responsible for regulating multiple aspects of reproduction including
ovulation, steroid hormone synthesis and behavior and, is sexually dimorphic in its
organization (Simerly 2002). Normal development of the HPG axis of females results in a
positive/negative feedback loop between the hypothalamus, the pituitary and the ovaries that
regulates the female estrous cycle in rats or menstrual cycle in humans. E positive feedback
from the ovaries is relayed to kisspeptin neurons in the AVPV of the hypothalamus.
Kisspeptin is released onto GnRH neurons leading to a surge in the release of GnRH to the
pituitary and the subsequent pre-ovulatory surge of LH from the pituitary (Figure 1)
72 the blood to determine whether the hypothalamus has responded to steroid positive feedback
(Mikkelsen and others 2009; Navarro and others 2005b).
Studies from our lab using rodent models, have shown evidence for the ability of GEN to
interfere with, or alter, the organization and activation of sexually dimorphic nuclei in the
hypothalamus (Bateman and Patisaul 2008; Losa and others 2011), providing a potential Figure 1. The hypothalamic-pituitary-ovarian signaling pathway. Ovulation in females is governed by the HPG axis. The ovaries produce E and P which feedback to the hypothalamic nucleus known as the AVPV in rats. Once per estrous cycle there is
73 mechanism for the interference of GEN with reproductive physiology. For example,
neonatal GEN exposure alters the sexually dimorphic expression of neurons containing
tyrosine hydroxylase (TH) in the male AVPV, increasing them to female-like levels (Patisaul
and others 2006). It has also been observed to decrease GnRH activation and kisspeptin fiber
density in females (Bateman and Patisaul 2008; Losa and others). Together, these studies
point to the ability of neonatal GEN exposure to affect the organization of neurons involved
in HPG signaling. Alterations in the sexually dimorphic organization of the hypothalamus
can have permanent effects on reproductive fecundity and sex-specific behaviors later on in
life and, can disrupt the function of the HPG axis (Arnold and Breedlove 1985; Foster and
others 2006; Gore 2008; Goy and others 1988; Goy and others 1967; Phoenix and others
1959; Robinson 2006; Schwartz and McCarthy 2008; Whalen and Edwards 1966; Whalen
and Nadler 1963). This study will expand on my previous study (aim 1, Ch. 2) by looking at
two separate doses of GEN to better understand its dose dependent effects on reproductive
physiology and HPG signaling. I hypothesized that female Long-Evans rats exposed
neonatally to GEN would not only display advanced pubertal timing and abnormal estrous
cycles but that disruption of HPG signaling, specifically the inability of anestrus females to
respond to steroid positive feedback, underlies these effects. To attempt to narrow down
where the potential disconnect in HPG signaling is I will administer kisspeptin peptide
injections. Previous studies have demonstrated the ability of peripheral administration of
kisspeptin to stimulate LH secretion (Matsui and others 2004; Navarro and others 2005a;
Navarro and others 2005b). Peripherally administered kisspeptin acts directly at the level of
74 kisspeptin neurons are refractory to steroid positive feedback, or, if kisspeptin
synthesis/release onto GnRH neurons is impaired, then females who failed to respond to E/P
feedback should respond to kisspeptin administration (Figure 1). To determine if females are
able to respond I will measure LH levels in the blood between 15-30min post kisspeptin
stimulation as previous research has shown the LH surge to be highest at this point
(Mikkelsen and others 2009; Navarro and others 2005b).
Materials and Methods
Animals and neonatal treatment
Female pups were obtained from cross-fostered litters born to timed pregnant Long Evans
rats (n = 15, Charles River, NC). All dams were individually housed in a humidity and
temperature controlled room with a 12h light cycle (lights on from 7:00 to 19:00) at 23°C
and 50% average relative humidity at the Biological Resource Facility at North Carolina
State University (NCSU) and maintained on a semi-purified, phytoestrogen-free diet ad
libitum for the duration of the experiment (AIN-93G, Test Diet, Richmond, IN).
Beginning on the day of birth, the pups were subcutaneously (sc) injected with vehicle
(VEH, 0.05 ml), EB, 10 µg, Sigma, St. Louis), 10 mg/kg body weight (bw) GEN (High dose
GEN, Sigma), 1 mg/kg bw GEN (Low dose GEN). My intent was to also include pups
exposed to the ERβ selective agonist diarylpropitionate (DPN) as GEN has been shown to have a 30-fold higher binding affinity for ERβ than for ERα (Jefferson and others 2002;
Kuiper and others 1997; Kuiper and others 1998) however the mom cannibalized her pups
following neonatal injections of DPN. This is an unfortunate but ethologically normal rat