THE PROPERTIES, ORIGIN, AND MECHANISM O F
CONVERSION-TYPE INHERITANCE A T THE B LOCUS IN MAIZE1
E. H. COE, JR.2
U . S. Department of Agriculture and Department of Field Crops, University of Missouri, Columbia, Missouri
Received November 15, 1965
EGULAR, heritable changes in gene function directed by a n allele were first demonstrated by BRINK (1956a, b, c) at the R locus in maize. Changes of this type have been designated paramutation (BRINK 1958a), a convenient ge- neric term emphasizing both the heritable nature of the changes and their origina- nation through heterozygous association of a sensitive (paramutable) allele with an inducing (paramutagenic) allele. Similar behavior (continuing conversion- type inheritance, an extreme form of paramutation) has been observed at the B locus in maize (COE 1958, 1959a), and somewhat similar behavior (somatic con- version) with the sulf alleles in tomato (HAGEMANN 1958a, b)
.
In each case, an inducing allele ( R S t , Rmb, and certain Rst derivatives; B’ and certain paramutants;su1fPsra and sulfvag) causes heritable alteration in a sensitive allele (R‘ and certain alleles; B and certain alleles; s u l f f ) in the direction of reduced function.
Earlier reports (COE 1958, 1959a, b, 1960, 1961a, b) describe the basic B’ phe- nomenon. B B plants (intense anthocyanin color) give rise occasionally to weak- colored mutants (B’)
.
Progeny from these B’ mutants, self-pollinated or crossed to B or b, are all weak in color. Repeated backcrosses of B’ to B yield only B’ plants without segregation, even though markers on either side of B segregate and re- combine normally. Segregation is found, however, in progeny from B’ b hybrids, demonstrating association of the phenomenon with the B locus: the cross of B‘ b XB B gives half B’ plants (genetically B’ B’ in the succeeding generation) and half
B plants (genetically B b )
.
The phenomenon has been considered broadly to in- dicate conversion of B to B’ during a life cycle of exposure to B’ in heterozygous individuals, with b intractable to conversion. A gene-dependent plasmid model has been considered, but does not appear to be involved ( COE 1961 b) .The present paper reports experiments designed to examine in detail the prop- erties and mechanism of the B’ phenomenon. A model is developed that is com- patible with the controlling element ( MCCLINTOCK 1951, 1961 )
,
parachromatinBRINK
1960) or episome (JACOB and WOLLMAN 1961) concepts. An abstract of the general conclusions has been published ( COE 1963).Contribution from Crops Research Division, Agricultural Research Service, U.S. Department of Agriculture, and the
Geneticist, Agricultural Research Service, U.S. Department of Agriculture. Address. Curtis Hall, University of
l\.Iissouri Agricultural Experiment Station. Journal Series No. 2992.
Missouri, Columbia, Missouri, 65202.
1035 E . H . COE, J R . TABLE 1
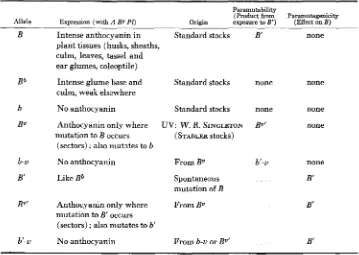
Ezpression, paramutability and paramutagenicity of B alleles
Paramutability
(Product f” Paramutagenicity Allele Expression ( w i d A R g PI) Origin exposure to E’) (Effect on E )
B
Bb
b
B O
b-u
B’
BV’
b‘-u
Intense anthocyanin i n Standard stocks plant tissues (husks, sheaths,
culm, leaves, tassel and ear glumes, coleoptile)
Intense glume base and culm, weak elsewhere
No anthocyanin Standard stocks
Anthocyanin only where mutation to B occurs
(sectors) ; also mutates to b
No anthocyanin From Bv
Like Bb Spontaneous
Standard stocks
UV: W. R. SINGLETON
( STADLER stocks)
mutation of B
Anthocyanin only where From Bv mutation to B’ occurs
(sectors) ; also mutates to b’
No anthocyanin From b-u or Bv‘
B‘ none
none none
none none
Bz” none
b’-u none
B‘
B’
. . . B‘
MATERIALS A N D METHODS
Table 1 lists the symbols, origins, and properties of the B alleles used in this study. Among the non-paramutagenic members ( B , Bb, b, Bv, b - U ) , the allele with the higher level of pigmen- tation is dominant. All paramutagenic alleles show “dominance” of weak color (B’ B, B’ b, B V ’ B, and b’-u B are all weakly pigmented).
Stocks used in the experiments were generally similar in background genotype; most had been derived by recovery from first or second backcrosses to a single uniform line, stock 3, homozygous
Rg b PI ( R g , colored aleurone, no seedling o r anther pigment; b green plant; PI, full-purple
plant pigmentation in presence of B ) . In certain experiments, a uniform RO B pZ line (in which B produces intense, sun-red pigmentation) was employed. Several important combinations of mark- ers in chromosome 2 were provided by E. B. PATTERSON from the Maize Cooperative stocks; these were also recovered from backcrosses to stock 3. The markers lg (liguleless leaf, position l l ) , gl,
(glossy seedling, 3 0 ) , B (plant color booster, 49), sk (silkless ear, 56), and u4 (virescent seedling, 83) were used; the centromere is between sk and u4. Where necessary, external contamination markers (wz, C’, y, su) were included.
E X P E R I M E N T S A N D OBSERVATIONS
SeZection: Among 51 plants in one B’ family, the two most lightly and two most darkly pigmented individuals were selected and crossed by B
PI
(PI series) and B p l (pZ series) pollen parents. For two additional generations, among 30 to 50P A R A M U T A T I O N M E C H A N I S M 1037 were then coded, planted in random order in two replications, and graded without identification on a scale of increasing intensity. The arbitrary grading scale used in this experiment was not quantitative; in pl backgrounds, distinctions were minor in the range from 0 to 3, great from 3 to 5 , and minor from 5 to 6; in
P1
backgrounds, they were great from 0 to 3, minor from 3 to 5, great from 5 to 6, and minor from 6 to 9. Differences in color level were thus numerically exag- gerated by spreading grades within the range of B‘ expression.The grade distributions and averages, presented in Table 2, showed small dif- ferences in the direction of selection but no striking changes either to very low or very high levels of pigmentation. No important effect of selection is indicated; no quantitative variation in the degree of effect can be suggested.
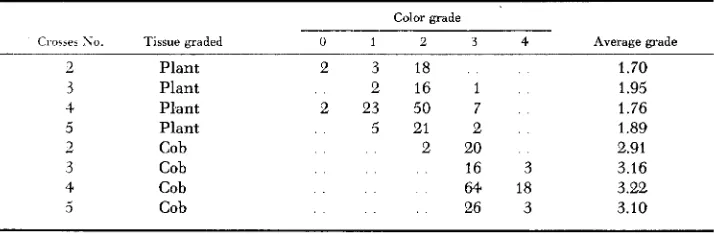
Repeated induction: To determine whether the effectiveness of B‘ is diminished by repeated exposure to B or by repeated transfer of its properties, all available ears from a repetitive series of backcrosses of B’ to a uniform B Pl parent were sampled. five seeds per ear. The progenies were graded without reference to pedi- gree on a n arbitrary scale somewhat more quantitative visually than that used in the selection experiment. Both plants and cobs were graded for the same group of individuals (Table 3 ) . There is no regular pattern of effect from repeated back- crossing, and no suggestion of segregation for “old” and “new” paramutants.
Comparisons among plants with various constitutions for B, B’, and b: Func- tional capacity of B, B‘ and b in various combinations can provide evidence on
TABLE 2
Distributions and average color grades for B‘ plants after three generations of backcrossing to B and divergent soleetion for color level
Color grade
- Grade range of
Series and selection 0 1 2 1 4 5 Averagegrade recurrent parent
p l light 26 84 0.76 5-6
p l dark 15 110 0.88 5-6
P1 light 6 78 27 3.19 6 9
PI dark 52 55 10 3.64 6 9
TABLE 3
Plant and cob color distributions and average grades in a repeated series of backcrosses of B‘ to B
Color grade
Cios.ea S o . Tissue graded 0 1 2 3 4 Average grade
-
2 Plant 2 3 18 1.70
3 Plant 2 16 1 1.95
4 Plant 2 23 50 7 1.76
5 Plant 5 21 2 1.89
2 Cob 2 20 2.91
3 Cob 16 3 3.16
4 Cob 64 18 3.22
1033 E. 11. COE, JR.
TABLE 4
Plant and cob color grade distributions and average grades for different genotypes involving B, B’, and b
~ ~ ~ ~~ ~ ~
Color grade Genotype
$‘/d Tissue graded 0 1 2 3 4 5 6 7 8 9 Averagegrade
Plant 30 153 31 2.00
Plant 59 92 5 1.65
Plant 14 174 71 5 3.26
Plant 5 29 19 2 3.33
Plant 1 20 51 7.69
Plant 4 4 9 6 7.74
Cob 56 98 25 15 3 1 0 5
Cob 13 115 15 3 1.05
Cob 8 101 83 63 1.79
Cob 3 21 13 16 1.79
Cob 43 20 4 6.42
Cob 1 10 7.91
timing and degree of paramutational events, and on reciprocal effects that would be indicative of extranuclear systems. A series of closely comparable progenies from crosses among B’, B and b stocks of PI constitution was replicated, ran- domized, and blind-graded both at flowering (composite judgment on husks, sheaths, and tassels) and in the mature, dry cobs (Table 4 ) . In the B / B entry, two exceptional plants were conspicuous new B’ mutants; these are excluded from the table.
Since B’/B‘ and B ‘ / b are not greatly different, a single dose of B’ has nearly as much effect as two; B is also nearly completely dominant to b. The reciprocal hybrids B’/B and B/B’ (allele from egg parent written first) are not distinguish- able, indicating no maternal effects. Finally and most significantly, B’/B and B/B’ plants are clearly more strongly pigmented than B’/B’ and B ’ / b , demon- strating that the functional capacity of B is not completely changed or suppressed during the first life cycle of exposure to B’, even though repeated induction (Table
3) and selection (Table 2) show no inherited functional difference between ‘‘old” B’ and newly induced B’. These data alone do not permit distinction among: ( 1 ) suppression of B function by B’, with B unchanged until very late; (2) partial change in B early, completed late; or ( 3 ) confounding of suppression and partial change. However, B clearly is not changed to the functional equivalent of B’ until very late in the life cycle-specifically, as late as the stage of terminal maturation and pigment synthesis. The change in germinal tissues might possibly be as late as meiosis, inasmuch as the graded somatic tissues (including tassels and cobs) derive from the same advanced meristematic cells as the germinal tissues out of which completely changed B (to B’) arises. Deletion analyses presented in a subse- quent section demonstrate that B actually is not changed during most of the life cycle, but that it is suppressed in function when B’ is present in the same cell.
PARAMUTATION MECHANISM 1039 heterozygote proves B-locus control of the B’ phenomenon (COE 1959a). How- ever, only limited numbers of segregants were reported previously, so occasional exceptions derived from crossing over or other infrequent events might have been undetected. Accordingly, a somewhat larger family of plants from B’ b X B was progeny-tested by self-pollination or backcrossing to b. Progeny families (20 to 45 plants each) from 44 B’ individuals were entirely B’ (i.e., b was absent), while those from 48 B individuals were all segregating B-b (i.e., B’ was absent). A few new B’ exceptions (approximately 0.5%) appeared in the latter group; this is not a n unusual frequency of new exceptions. Segregation of B’ from b is quite regular.
Cytology and recombination
in
the B region: In a previous report ( COE 1961b), markers flanking B in chromosome 2 were shown to segregate, recombine and transmit normally in hybrids involving B’. Cytological examinations and more extensive recombination data have been obtained. A series of hybrids was pre- pared as follows:From B‘ b X lg gl B U :
From B B
x
lg gl B : From B‘ B‘ X lg gl b U :F r o m B B X l g g l b u : From B B X lg gl B’ U :
From b b x l g gl B‘ U :
From b b X lg gl B :
(1) B’/lg gl B U , ( 2 ) b/lg gl B u
( 3 ) B / k gl B
(4) B’/lg gl b u
( 5 ) B/lg gl b U
(6) B/lg gl B‘ U
( 7 ) b/lg gl B’ U
(8) b / k gl B
Additional material for cytological study was obtained from original source stocks: (9) B’ B’ and (IO) B’ B. Meiotic samples were collected from each, and the hybrids were backcrossed to lg gl b U .
Cytological examinations of samples were graciously carried out by DR. A. E. LONGLEY. No aberrancies were found in chromosome 2, particularly concentrat- ing on the short arm in the region of B, in the following numbers of plants of genotypes ( 1 ) through ( l o ) , respectively: 2,4,3, 7,2,3,3,4, 1, 1 . No other chro- mosomes appeared to have any consistent anomalies, except for the familiar in- version in chromosome 8, which was heterozygous in many plants but inde- pendent of B’ constitution. It should be commented that some suggestion of abnormalities in chromosome 2 had been seen in samples from a very limited number of B‘-bearing plants in the preceding season, but these observations were not confirmed in the present experiment.
Seedling progenies from backcrosses of genotypes ( I ) ,
(e),
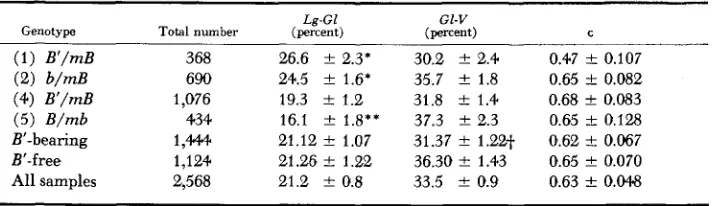
( 4 ) , and (5) were classified for Zg, gl, and U . Recombination percentages (Table 5) showed signifi-104.0 E. €1. COE, J R , TABLE 5
Recombination and coincidence in heterozygotes involving B', B and b (B is between G1 and V;
m indicates segment bearing recessive markers)
Le-GI Gl-V
Genotype Total number (percent) (percent) C
( 1 ) B'/mB 368 26.6 f 2.3' 30.2 f 2.4 0.47 t 0.107
(4) B'/mB 1,076 19.3 k 1.2 31.8 t 1.4 0.68
+
0.083 (5) B/mb 434 16.1 f 1.8** 37.3*
2.3 0.65 t 0.128B'-bearing 1,444. 21.12 f 1.07 31.37 f 1.22t 0.62 k 0.067
B'-free 1,124 21.26 t 1.22 36.30 f 1.43 0.65
+
0.070All samples 2,568 21.2 f 0.8 33.5 .+- 0.9 0.63 k 0.048
(2) b/mB 690 24.5 k 1.6' 35.7
*
1.8 0.65 t 0.082* * * Significant at 5'3, and 1 %
+'Highly significant difference from B'-free value. level, respectively, relative to total.
hancing meiotic exchange; instead they imply the kinds of effects that might be caused by an abnormal constituent in or on the chromosome.
Deletion test for time of event and chromosomal restriction of
B':
If B' repre- sents a property residing at B, deletion of the B segment should concurrently de- lete the B' properties (paramutagenicity and functional suppression) ; if B' repre- sents B plus an extrachromosomal entity, deletion of the B segment and deletion of the B' properties should not coincide. Deletions induced over a sequence of time from pre-fertilization forward in crosses of B x B' should establish whether change of B to B' has occurred up to the stage at which deletion occws.X-irradiation was applied at three stages: pollen, young zygotes, and develop- ing sporophytes. Pollen of
+
B'+
was irradiated with 1,000 to 2,000 r and used to pollinate silks of gl B +/gl B sk and gl b +/gl bsk;
the progeny were observed for loss of GI or B', and exceptional plants were classified forsk,
pollen sterility, and morphology. Young zygotes of genotypes+
B' +/gl Bsk
and+
B' + / g l b sk were irradiated on the ear with 1,000 to 2,000 r at 1 to5
days post-pollination; plants developing from these zygotes were observed for complete or sectorial loss of G1or B', and exceptional plants were observed closely for continuation of sectors and classified for
sk,
pollen sterility, and morphology. Progeny tests of exceptions from pollen and zygote treatment were carried out when possible; markers lg andU , on opposite sides of the indicated marked region, were usually present and
PA R A M U T A T I O N M E C H A N I S M 1041 the B’ segment would result in a hemizygote, gl B sk/-, which should transmit B unchanged to progeny.
No overall effect of X-irradiation on B’ expression, either in
B’
B or B‘ b plants, was observed; the plants were not color-graded since control and irradiated plants were not conspicuously different in color level. Slightly variant types, both lighter and darker than the average, were found in treatments and controls at similar frequencies. I n small, nonirradiated control populations, no losses were found. The degree of difference between B’ andB y
it should be emphasized, is great enough to permit direct classification and discrimination of B from B’ with very few errors (COE 1959.1961a, b ) .Pollen irradiation induced many losses of GI and B‘ concurrently, some con- current losses of GI, B‘, and Sky and occasional losses of GI alone, but none of B‘ alone (Table 6). Because of the design, in the cross of +/sk X
+/+
half of theGI B’ Sk losses would be identified as GZ B’ losses only, but this proportion is not crucial to the experiment. Losses of GZ alone in the B crosses (first line of Table
6) were paralleled by losses of GI in the b crosses (second line), representing breaks between GI and B’. Losses of B’ alone were not found; these were not ex- pected frequently as short intercalary deletions but might have been expected if a chromosome-independent property sensitive to X rays was essential to the B’ phe- nomenon, or if a loosely attached chromosomal appendage were involved.
Irradiated zygotes through 5 days showed concurrent loss of B’ and GZ, demon- strating that B is unchanged by B‘ during this period. One case ( 5 days, sectored loss of B’ only) was particularly informative: The main stalk was nearly but not absolutely full purple, 50 to 75% pollen-sterile, and transmitted through the fe- male the markers on the B chromosome, Zg and gl, almost exclusively, indicative of a small deficiency in the B’-bearing homolog, but the progeny were B‘, suggest- ing that B’ may have been present but prevented from suppressing B function
TABLE 6
X-ray induced deletions of B‘ and adjacent markers in crosses of
+
B’+
with recessive-marked B and b
Loss recognized’
Cross Stage irradiated GIB’ G l B ’ S k G1 B’ Numberexamined
B x B‘ pollen 23 4 4 0 3,590
b x B‘ pollen 20 1 3 0 1,100
B’ x B zygotes, 1 day 7,O 1,0 1,5 1,0 1,166
2 days 1,3 1,0 0,O 0,O 887
3 days 0,o 0,o 0,o 0,o 26
4 days 0,l 0,o 0,o 0,o 1M
5 days 0,2 0,o 0,o 0,l 43
2 days 3,l 0,O 2,2 0,O 343
B‘ x b zygotes, 1 day l,o 0,o 0,l 0,o 58
1042 E. €I. COE, JR.
fully. Two basal branches of this plant were typical B’ in phenotype, showed no sterility, and transmitted markers normally. A third basal branch was full purple ( B ) and 75% pollen-sterile, but produced no progeny as male or female from several crosses. Of all the cases from zygote irradiation, this plant was the only one in which prageny were obtained from deletion-bearing germinal tissue (main stalk). Most sectored plants have not included germinal tissue, and those which have, as well as the whole-plant cases, have failed to produce viable progeny. These failures are difficult to explain, particularly since five of the B cases from pollen irradiation have produced progeny, all consistently B and carrying mark- ers expected from a hemizygote. In the absence of progeny from B sectors, a B phenotype is assumed to represent unchanged B, uncovered by deletion of B’.
Among irradiated developing sporophytes (respectively 7, 7, 3, 1, 7, 8, and 7 plants at the 4,
5 ,
6, 7, 8, 10-leaf, and premeiotic stages), two plants had large, distinct B sectors in the cob, one from treatment at the 4-leaf and one from the 10-leaf stage. Over 3,000 pollen progeny and 3,300 ear progeny were observed, including over 500 ear progeny from premeiotic treatment, but no B individuals were found. As with the cases from zygote treatment, the anticipated B progeny appear not to have been transmitted. This failure of transmission can be attributed to somatic competition between hemizygous and normal tissues and to competi- tion among gametes derived from these tissues in this experiment; the degree of these effects is difficult to assess. Nevertheless, B sectors in cobs, a very late tissue, demonstrate persistence of unchanged B through most of the life cycle.From the above experiments it is clear that B’ is regularly deleted in concert with markers in the B region of chromosome 2, and that B is unchanged in func- tional potential throughout most of a life cycle of heterozygosity with B’. In a preceding section it was concluded, from the fact that B’ B plants are darker than B’ B’, that B is not changed to the functional equivalent of B’ until very late in the life cycle. On the basis of both analyses, B’ must suppress functional expres- sion of B, but the change of B to B‘ must be deferred until meiosis or very near meiosis.
Ultraviolet treatment of
B’
pollen: In a small test for gross effects of ultraviolet light, B’ pollen was exposed for 30 seconds to two-sided irradiation from the ger- micidal source used in this laboratory, then crossed onto b and B. Respectively, 94 and 91 plants were observed from these crosses and compared visually with controls. No gross effects on B’ expression were noted; three plants of b pheno- type, probably deletions rather than contaminations, were found among the crosses to b.P A R A M U T A T I O N M E C H A N I S M 1043
Tests for effects of chromosome aberrations: The effects of heterozygous chro- mosome aberrations are of interest inasmuch as they might affect the phenomenon through their effects on synapsis. Two aberrations were tested. B plants homo- zygous for Translocation 2-9a (breakpoints 2S.36, 9L.58; proximal to sk) or for Inversion 2a (breakpoints 2S.7, 2L.8; proximal to B) were crossed to B' plants with normal constitution. The translocation heterozygotes were selfed and back- crossed to B and b, and inversion heterozygotes were backcrossed to B; no obvious differences in color expression between normal and semisterile sibs were found in small progenies.
Since the primary trisomic for chromosome 2 was unavailable, modest attempts were made to derive tertiary trisomics homozygous for B, without success.
Independence of mutability from paramutability and paramutagenicity: The mutable allele B" simulates b, but mutates frequently to stable B and b. In hetero- zygotes with B', B" is altered, not to B' but to B"' (green plants with B' sectors, frequency similar to B sectors in B" plants). Crosses between B"' and B cause B to change to B', which is indistinguishable from the standard B' originally used to induce B"'. Whatever change occurs in paramutation is independent of the mutability, and the mechanisms of one seem not to interfere with the other.
To test the absoluteness of this independence, the following aspects were studied: ( 1 ) Paramutagenic stability of B"'; (2) tractability and paramutagenic- ity of stable b mutants from B" and B"'; ( 3 ) tractability and paramutagenicity of stable B and B' mutants from B" and B"'. In these experiments, the wide phenotypic differences among B, B', B", B"', and b were used with progeny tests as criteria of the events, rather than color grading.
The instability of B"' might be expected to give rise to mutants lacking para- mutagenic action. Crosses were made between B B and B"' B"'. and the progeny observed for B exceptions. None was found among 586 individuals. B"' mutates to stable alleles in about 2 to 4% of gametes, so the rate of mutation to non- paramutagenic alleles, if it occurs, is not comparably high.
To test the properties of b mutants, marked B" B" plants were crossed by b b,
and two b (green exceptions) among 96 plants were selfed and crossed onto B', B and b. In large progenies from each, no residual mutability was detected for either exception. The crosses of b-u/b X B'were testcrossed to B. Half of the proge- nies were found to be entirely B' and half 1B':lB. Segregation of gl, linked to b-u
in each case, was normal. Thus the two mutants (b-vl and b-v2) were caused to become paramutagenic (b'-vl and b'-v2) by exposure to B'.
1044
E. H . COE, JR.was found to be homozygous inviable, even though transmitted through both male and female.
B mutants from B", and B' from
E',
have been examined less thoroughly than the b mutants; two cases of B from B" have been crossed toB'
and progeny tested; both were changed to the B' level of expression. Three B' cases from B"' have been crossed toB
and progeny tested; each was paramutagenic.These experiments confirm that the change from mutable to stable does not in- fluence paramutability or paramutagenicity and that the change to the para- mutagenic condition does not influence mutability. The effects can be represented diagrammatically:
Product of B
Allele exposure to B' allele at left
t
> B"'
L
>b'-u
Product of when exposed to
Stable level B > B' B'
B'
,r
Mutable level B"
B'
J
Stable level b-v
Somatic origin of
B':
Distinct from the effects of B' on B, new mutations of B to B' occur at variable frequencies. This mutation from orthodox to unorthodox inheritance has been studied by the use of ear diagrams and a mathematical index of association designed to reveal somatic sectoring (COE 1961a). Although the evidence presented previously did not indicate sectoring, and compelled an in- terpretation that the mutation was a meiotic event, numerous plants with definite somatic sectors have since been found; several have been confirmed in progenies derived from mutant and nonmutant areas, These new observations became pos- sible as a result of the use ofP1
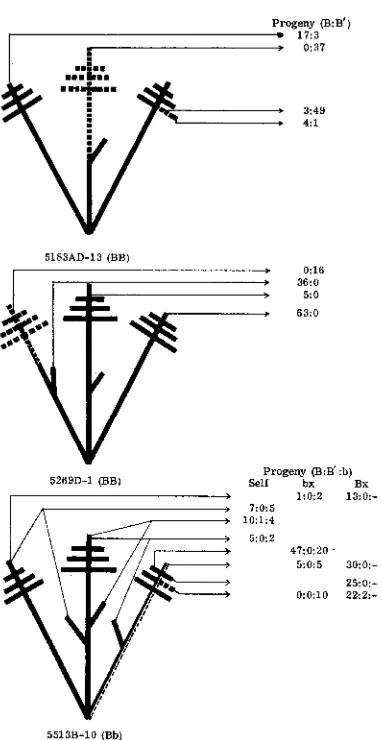
(full purple) background in place of the original p l (sun-red) ; the advantages of a background genotype permitting direct observa- tions for sectors were intimated in the earlier paper.The three sectored plants that have been most thoroughly tested are dia- grammed in figure l .
It
can be seen that B' areas transmitted B' almost without exception, while B areas transmitted mostly or entirely B . No poIlen or ear steril- ity was found in the B or B' portions. Plant No. 5513B-10 is the only case yet found which was of B b genotype, and must be considered somewhat doubtful because of abnormal transmission ratios. Areas assumed to be B' b showed high or exclusive transmission of b, while adjacent B b areas showed high transmission of B . The cause of these anomalies is not clear, and there is only circumstantial evidence (phenotype) on which to conclude that B' was tmly present. The other two exceptions were more consistent in their behavior. Exceptions in progeny (B' from B areas, B from B' areas) can be attributed to inadvertant mixing in pollen samples from B' areas, and to new B' mutations in phenotypically B areas. There is no indication of spreading of the mutation through the plant. In fact. mutant areas show patterns like those of cell lineages in morphogenesis, rather than like infectious spreading.PARAMUTATION MECHANISM 1045
Progeny (B:B‘)
I 17:3
I P 0:31
3:49
4:l
5183AD-13 (BB)
> 0:16
> 36:O
> 5:O > 63:O
-
5269D-1 (BB) Self Progeny (B:B‘:b) bx Bx
> 1 : 0 2 13:O:- , 7:0:5
, 10:1:4
> 5:0:2 47:O:ZO
5:0:5 30:O:- 25:o:-
0 : O l O 2 2 2 : -
5513B-10 (Bb)
FIGURE 1.-Three plants showing new somatic mutation to B’. B (intense) areas are repre- sented by solid lines, mutant B’ (weak color) areas by dashed lines, and linear sectoring by parallel dotted and solid lines. Ears are represented by diagonal branches, tassels by cross branches. Phenotypic ratios of progeny are indicated by numbers following arrows (1 7: 3 repre- sents 17 B and 3 B’ i n testcrosses, etc.).
tered families and a few cob-sector cases have been found. One further, unusual B B selfed progeny (5320) had nine sectored plants in 63, each of the nine with a B‘ tassel and some phenotypically B areas elsewhere. Selfed ears were obtained from 6 of these 9 plants; each cob was B’ in appearance, and progeny plants were B’. Among normal-appearing ( B ) sibs to the sectored exceptions, nine were found
1046 E. 11. COE, JR.
progeny of the remaining five were all B’; male testcross progeny of 1 were B, of 2 mixed, and of 2 all B’. Six other B plants in family 5320 showed no obvious sectoring in the cob, but female progenies were 1 B, 4 mixed, and 1 all-B’, and male progenies 3 B and 3 mixed. In the 21 tested plants a B’ tassel or ear pheno- type signaled full change of both organs to B’; sectored ear or tassel did not neces- sarily signal sectoring in the other organ, and not all tassel and ear changes were recognized before progeny tests.
Phenotypically, new B’ sectors in B B plants are quite dark; in the case of 5320, differences between B and B’ areas were relatively indistinct, but most other ex- ceptions were clearer. The level of pigmentation in sectors appears at least as dark as that of B’ B F, plants; whether it is darker than this is difficult to judge. How- ever, in plant 5513B-10 (discussed above) the presumed B’ b sector appeared to be exactly comparable to B’ b plant tissues. It seems most reasonable to conclude that newly arisen B’ is entirely like established B’, and that sectors arising in B B plants are simply as dark as B’ B, rather than unique in any way. Gametes pro- duced by B’ B sectors are all B’, indicating that paramutation in these sectors oc- curs very late in the life cycle, after the mutation rather than concurrently.
These observations demonstrate that new B’ mutations occur somatically rather than meiotically. The mutation apparently is complete in one step, and no gross infectious behavior is involved. Paramutation can occur very late, after mutation, since the gametes from a B’ B sector are all B’.
Tests of inheritance of instability (B to B’ mutability) : For several years, it was assumed that certain B B lines were unstable (represented by B”) and others stable ( B s ) , because extensive plantings were consistent within their source. Be- ginning in 1961, however, the assumed stable standard line ( B B
PI P1)
com- menced to produce B’ exceptions, independent of outcrossing, in much the same fashion as the original B B p l p l line and its PI derivatives. I n 1961, a large plant- ing from five ears, totaling 406 individuals (812 gametes) of the “stable” line, was observed closely for B’ sectors or plants, and none was found; a smaller fam- ily, however, from one of the same source ears, showed three B’ individuals in 11 plants. No consistently stable line has been derived from the “stable” line since. Although extensive tests have not been carried out on various B lines, it is possible that no B source resistant to B’ mutation exists. The following studies of inher- itance of instability, designed to analyze for segregation of B”, B8, and b, are thus of very uncertain foundation, though informative.To study instability, all possible reciprocal hybrids were made between
4-
B”4-
and gl B U testcrossed reciprocally with
4-
b+
and4-
B+,
and between B” and bcrossed reciprocally to B and testcrossed reciprocally to b and B. Table 7 shows terminal progeny data for the B”-B crosses and Table 8 for the B”-b series. Sectors, whole plants, and whole progenies of B’ phenotype were sought and tested. B’ sectors are considered to have arisen within the genotype of the sectored plant, and B’ plants and progenies within the preceding parent.
P A R A M U T A T I O N M E C H A N I S M
TABLE 7
Markers carried in H' exceptions occurring in reciprocal hybrids and progenies from unstable-B crossed with marked B, testcrossed reciprocally to b and B
1047
Cross B' sectors B' plants Observed
BU/gl B U X b
Reciprocal gl B u/BU X b
Reciprocal
BU/gl B u x B
Reciprocal
gl B u/BU x B
Reciprocal
Total exceptions
216 139 288 110 264 120 313 168
4 1618
(6/865), suggesting that B B constitution may be more important than derivation and history of the B allele. This suggestion is also strong in the Bu-b series (Table
a ) ,
where only one possible mutation to B' occurred in a B / b plant (case No.5513B-10). The most impressive indication here, however, is that instability may be transmitted maternally with greater efficiency. If it is assumed that the B line remained completely stable, six B' exceptions (Table 8 ) will be considered to have arisen in plants carrying B' derived entirely through maternal parents and three in plants carrying B" transmitted once or twice through the male. In only
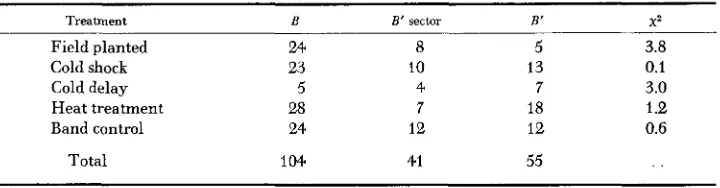
TABLE 8
Origins of B' exceptions occurring in reciprocal progenies of reciprocal hybrids between unstable-B and b, testcrossed reciprocally with b and B
Cross B' sectors B' plants B' progenies
( b / B U ) B X b
Reciprocal
Reciprocal
Reciprocal
Reciprocal
(B'/b) B
x b
B ( b / B U )
x
bB W / b )
x
b( b / B U ) B X B
Reciprocal
( B U / b ) B X B
Reciprocal
B ( b / B i l ) x B
Reciprocal
B ( B U / b ) X B
Reciprocal Total exceptions 0 0 0 0
1 B / B u ? 0 0
1 B / b
2
0 0
1 Bu/B 0 0 0
0 0
1 BU/B
0 2 Bu/B
1 Bu/B 0 0
0 1 B/Bu
6
0 0 0 1 Bu/B
0 0 0 0 0 0 0 1 Bu/B
1048 E. H. COE, JR.
the single case of 5513B-10, instability would be considered to have been trans- mitted with b through the male through two generations; this case is doubtful be- cause of anomalously low transmission of B’ to progeny, as indicated in the pre- ceding section.
These studies do not establish a clear-cut inheritance pattern; instability may be cytoplasmically inherited but could be Mendelian or nonheritable instead. In the absence of stable B lines and of direct evidence for infectious transmission of B’ or of instability, no firm conclusion can be reached. Experimental infection trials have been unsuccessful, and sectored plants have not shown any greater in- stability in progenies from nonmutant areas than have nonsectored plants. It could be conjectured that lines assumed to be stable become unstable through natural infectious transmission resulting from proximity to unstable and B’ lines, but no basis for this conjecture has been adduced from study of spatial relation- ships between lines in field plantings. Some considerations of coincidental occur- rence of B‘ mutations follow.
Time clusters and coincidences in the origin of B’: New exceptions, especially in sectors, have shown many coincidental relationships. Unfortunately, new B’ exceptions have been sporadic and have not permitted quantified comparisons; in addition, progeny tests have not been possible with many sectors. The following observations assume that B‘ phenotype indicates valid B’ mutation in each case. In 1962, two adjacent plants out of 12 in family 5267.1 had small, similar-sized husk sectors; two plants out of 17 in 5267.2, planted from the same ear as 5267.1 but two weeks later, were entirely B’, and two additional entirely B’ plants were found in related families planted at the same time in another area of the field. In
the 1962-63 greenhouse planting, 9 sectored plants in 63 were found in family 5320, each with a B’ tassel and some B tissue in lower parts; a replanting from the parent ear in 1964 produced 5 sectored plants in 16, exactly like those in family 5320. In 1963, a n early planting of one ear (family 5537.1) showed 1 plant in 29 with a small tassel sector, while a same-ear planting two weeks later (5537.2) showed 3 sectored plants in 13, each with a B’ tassel and some B tissue in lower parts; two other sectored exceptions in the field could be interpreted to have oc- curred at about the same calendar time as the others. The 1964 and 1965 plant- ings did not seem to show any time clustering among the exceptions found.
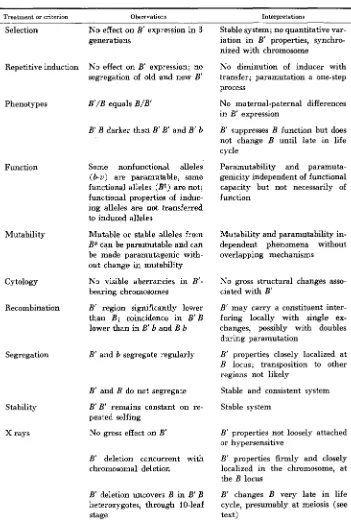
Environmental factors might influence the frequency of mutation to B’. For example, family 5320 had been exposed accidentally to below-freezing tempera- tures in the seedling stage, with severe injury, at a point morphogenetically close to that at which the mutations seemed to arise. Accordingly, a small test was conducted for environmental induction of B’ in 1964. From each of seven unstable-source ears ( B B ) and seven F, crosses ( B b ) of the same source with a uniform b line, five random 10-seed samples were taken to provide 70 kernels for each of five treatments for each genotype. Treatments included field planting, cold shock of seedlings in a deepfreeze on each of three consecutive days, cold delay of seedlings for 3 weeks under continuous low illumination at 55 F, heat treatment of seedlings for 3 weeks in a closed greenhouse, and a control planted
P A R A M U T A T I O N M E C H A N I S M
TABLE 9
New B’ exceptions arising under uariosus environmental treatments
1049
~ Treatment
~~
B B’ sector B’ X 2
Field planted 24 8 5 3.8
Cold shock 2.3 10 13 0.1
Cold delay 5 4 7 3.0
Heat treatment 28 7 18 1 .e
Band control 24 12 12 0.6
Total 104 41 55
at the time of flowering. No B’ exceptions occurred in the B b plants. Table 9 presents the results for B B plants; none of the treatments had significant effect
( x z
at5%
level, 2 df, is 5.99). Survival was lowest, as might be expected, in the cold-delay treatment; these plants were severely shocked by transplanting directly to the field without adaptation. Better survival of plants with less pig- ment might be expected under high light intensities, explaining the small devia- tion in the direction of a treatment effect.No experimental evidence has been obtained for the induction of B’ by specific environmental conditions. The tendency for coincidental time clusters in the origin of B’ suggests induction or enhancement by particular conditions but could not be said to prove it.
DISCUSSION
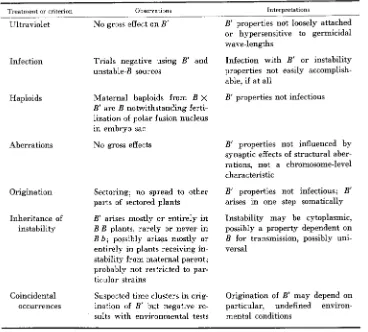
The lengthy considerations presented above, combined with a few observations from previous reports (COE 1959a, 1961b), are summarized in Table IO. Es-
pecially pertinent features for interpretation of the mechanism of the
B’
phenom- enon are that the B’ properties are closely and firmly localized at the B locus, that “conversion” (paramutation) is a late event possibly at meiosis, that the phe- nomenon affects functional capacity but is essentially independent of function and mutability, that the presence of B’ may influence meiotic exchange, and that instability (the tendency to mutate from orthodox to unorthodox inheritance) may be cytoplasmically inherited yet possibly gene-dependent, giving rise to B’ somatically.1050 E. H . COE, JR.
TABLE 10
A summary of observations and interpretations relating to the properties, mechanism and origin of B'
Treatment or criterion Observations Interpretations
Selection No effect on B' expression in 3 generations
Repetitive induction No effect on B' expression; no segregation of old and new B'
Phenotypes B'/B equals B/B'
B' B darker than B' B' and B' b
Function
Mutability
Some nonfunctional alleles ( b - v ) are paramutable, some functional alleles ( B b ) are not; functional properties of induc- ing alleles are not transferred to induced alleles
Mutable or stable alleles from B v can be paramutable and can be made paramutagenic with- out change in mutability
No visible aberrancies in B'-
bearing chromosomes Cytology
Recombination B' region significantly lower than B; coincidence in B'B lower than in B' b and B b
Segregation B' and b segregate regularly
Stability
X rays
B' and B do not segregate B'B' remains constant on re- peated selfing
No gross effect on B'
B' deletion concurrent with chromosomal deletion
B' deletion uncovers B in B' B heterozygotes, through IO-leaf stage
Stable system; no quantitative var- iation in B' properties, synchro- nized with chromosome
No diminution of inducer with transfer; paramutation a one-step process
No maternal-paternal differences in B' expression
B' suppresses B function but does not change B until late in life cycle
Paramutability and paramuta- genicity independent of functional capacity but not necessarily of function
Mutability and paramutability in- dependent phenomena without overlapping mechanisms
No gross structural changes asso- ciated with B'
B' may carry a constituent inter- fering locally with single ex- changes, possibly with doubles during paramutation
B' properties closely localized at B locus; transposition to other regions not likely
Stable and consistent system
Stable system
B' properties not loosely attached or hypersensitive
B' properties firmly and closely localized in the chromosome, at the B locus
P A R A M U T A T I O N M E C H A N I S M
TABLE IO-Continued
A summary of observations and interpretations relating to the properties, mechanism and origin of B‘
- ‘Treatment or criterion Observations
Ultraviolet No gross effect on B’
Infection Trials negative using B’ and unstable-B sources
Haploids
Aberrations
Maternal haploids from B X B’ are B notwithstanding ferti- lization of polar fusion nucleus in embryo sac
No gross effects
Origination Sectoring; no spread to other parts of sectored plants
B’ arises mostly or entirely i n B B plants, rarely or never in B b; possibly arises mostly or entirely in plants receiving in- stability from maternal parent; probably not restricted to par- ticular strains
Suspected time clusters in orig- ination of B‘ but negative re- sults with environmental tests Inheritance of
instability
Coincidental occurrences
Interpretations
1051
B’ properties not loosely attached or hypersensitive to germicidal wave-lengths
Infection with B’ o r instability properties not easily accomplish- able, if at all
B’ properties not infectious
B’ properties not influenced by synaptic effects of structural aber- rations, not a chromosome-level characteristic
B’ properties not infectious; B’ arises in one step somatically
Instability may be cytoplasmic, possibly a property dependent on B for transmission, possibly uni- versal
Origination of B’ may depend on particular, undefined environ- mental conditions
occurring in well defined somatic sectors; ear diagrams and a numerical index of association (COE 1960, 1961a) had earlier indicated that sectors were not occurring. Chance must have led to an erroneous conclusion previously.
BRINK (1964b) has reviewed recently the extensive studies on paramutation at
1052 E. H. COE, J R .
(mostly or entirely in B B homozygotes)
,
but the change is discrete and definitive, giving rise to conspicuous, well defined sectors. This single-step change agrees with the selection and repetitive-induction experiment (on B' itself) in indicating a unitary system, rather than a quantitative one. Comparable observations for theR
system indicate quantitative variation (BRINK 1964b).BRINK (1964b) has concluded that
Rat
changesR'
in somatic cells, rather thanTABLE 1 1
A summary of Observations and interpretations relating to the properties and mechanism of paramutation at the R locus
Observations
Heritable differences in level of expression of R+ exist within plants of Rat R';1--4,29 rever- sion of R' occurs irregularly and to various levels;3-5 cumulative changes on repeated in- duction; 3,697 continuous variation in level of
paramutation possible8
R s t unaffected by paramutagenesis4J
r r/R' R' much less strongly pigmented than
R' R/r,499 heritability to be determined
Rat RSt R+ endosperms dark, Rst R8t Rr:st
1ight;'o but R' preconditioned by weakly paramutagenic alleles is detectably altered by stronger alleles in immediate endosperms6 Mutation of Rat to stable functional and non- functional alleles sometimes is accompanied by loss of paramutagenicity;4,8911-14 paramu-
tability and paramutagenicity are mutually exclusive for many but not all alleles$ muta- tion rate of paramutant alleles parallels their functional level;l3 variegation in diverse races is associated with paramutagenicityl.28
R' and r segregate regularly, as do Rst and
r, R' and R29395.159'6 (except that barely de- tectable secondary paramutation of R is foundl,li,l8) ; not all Rr gametes from Rat Rr are paramutant4
X rays have gross effects on paramutagenic properties of Rat and Rmb, and on paramuta- bility of R719
Aberrations (Translocations, Abnormal-10) have profound and persistent but not perma- nent effects on Rr, enhancing function and decreasing paramutability ; structural hetero- zygosity not required 2 0 - 2 5
Interpretations
Metastable system;4 paramutation probably not a one-step process;4 R' properties quanti- tative, replication not necessarily synchro- nized with chromosome4
No diminution of inducer with transfer
Maternal-paternal effect on R or R' expres- sion,4 though not on paramutagenesis in usual genotypes5
Paramutation is delayed or restricted during
first generation;4 possibly weak suppression of
R+ function by Rat rather than heritable change (see text)
Function, mutability, paramutability, and paramutagenicity are complexly interrelated
Properties localized i n R region$ not cytu- plasmic;495 quantitative system with irregular events, possibly a reflection of metastability4
Loosely attached or hypersensitive system
P AR AM UT AT I ON M E C H A N I S M
TABLE 1 l-Continued
A summary of observations and interpretations relating to the properties and mechanism of paramutation at the R locus
1053
Observations Interpretations
Trisomics ( R s t Rr r g ) yield all or mostly Zygotene pairing, if strictly two-by-two, is
Rr:stlO not a requirement for paramutation;lO para-
mutation a somatic process4 (see text)
System not strictly gene-limited, possibly a
chromosome-level propertyz6 Plant-color element (P) as well as seed-color
element (S) of R r is affected by RStzG
'BRINK 1960
* BRINK, KERMICLE and BROWN COOPER 1964
BRINK 1964h BRINK 1956a, b, c; 1957 6 MCWHIRTER and BRINK ? M I K U L A 1961
8 MCWHIRTER and BRINK KERMICLE 1963
BRINK 1959
"BRINK 1958a c
li ASHMAN 196b
BRAY 1964
'* ASHMAN 1965 BRINK and WEYERS 1957
1963 1962
1964
BRIKK 1958b
17 BRINK, BROWN, KERMICLE and WEYERS 1960
'8 BROWN and BRINK 1960
'9 LINDEN 1963 1964
20 BRINK, B ~ ~ c ~ w o o o and NOTANI 1960
21 BRINK and WEYEHS 1960
22 BRINK and BUCKWWD 1961 BRINK 1961
*4 BRINK and NOTANI 1961 26 BRINK and MIKULA 1958 2' BRINK 1964a
28 LINDEN and RODRIQUEZ 1965
25 BRINK and VENKATESWARLU 1965
SASTRY, CWPER and BHINK 1965
during meiosis. The present data have led to the conclusion that B' changes B very late in the life cycle, presumably during meiosis when intimate chromosome pairing occurs. The R s t conclusions rest on three grounds. First, BRINK (1959) has shown that all or most R' gametes from trisomic RSt R' rg plants have been
altered to R r ' s t . Thus meiotic pairing cannot be a requirement for paramutation if such pairing is strictly two-by-two and not repetitive (which may not be the case, as BRINK points out). This purposive and informative experiment in itself does not exclude meiosis as the stage of paramutation, since some meiotic process other than pairing, or extra pairing events such as those proposed for Drosophila
by GRELL (1964) and NOVITSKI ( 1964), or unorthodox pairing in this unusual
1054 E . H. COE, J R .
ments, in which the full functional capacity of B is present at the time of maturity following earlier deletion of the B’ chromosome. In view of the considerable differences in other properties between B’ and R S t , there is no compelling reason to assume that the changes they induce occur at the same stage; the foregoing considerations rather emphasize the interpretive difficulties encountered using indirect evidence derived in systems with unorthodox properties.
HAGEMANN (1958b) found changes in sulf+ induced by sulfPura and sulpg to
occur in somatic sectors in tomato, suggesting the mutational events to be pre- meiotic. It is possible, however, that the inducing allele changes from a non- inducing to an inducing state somatically, suppressing sulf+ function in a sector, and that the heritable change in sulf + occurs at meiosis, in parallel with the B‘ situation. HAGEMANN ( 1958b) considers thoroughly the application of the term conversion ( WINKLER 1930) to the phenomenon; later studies (HAGEMANN
1961b) show that su1fPUra induces changes of sulff both to sulfPura and to s u l p g ,
demonstrating that the changes are not directed and are not purely conversion. Somatic exchange caiinot be the cause of the events (HAGEMANN 1961a) because the reciprocal products of such an exchange (sulf+/sulf+) are not found. No in- tractable sulf+ alleles were found in a survey of related species (HAGEMANN
1 9 6 1 ~ ) .
SANDLER and
HIRAIZUMI
(1961 ) have described an interesting effect connectedwith segregation distortion in Drosophila, in which the second-chromosome factor SD, in certain strains, directs a regular change i n X-chromosome condition which in turn modifies the effects of
SD
on transmission of the second chromosome. The stage at which this “nonallelic conversion” event occurs could not be clearly fixed. The term “conversion-type,” used in this paper to designate an extreme form of paramutation, implies only supplanting of one property by another, in the abstract sense. The inducing properties of B’ are regularly and fully conferred on B : the induced B’ is not recognizably different from its inducer, and the inducing property continues to be transferred in subsequent inductions. That the phenom- enon does not involve concrete replacement of one allele by another is clearly shown by the fact that Bu andb-v
can receive the inducing property without being altered in functional capacity or mutability, and that they then transmit the inducing property without transmitting functional capacity or mutability. The RSt system is even more clearly not a replacement phenomenon, since continuing transfer is absent: neither the functional properties nor the inducing properties of Rst are fully conferred on R‘. It is interesting to note that initial observation of unorthodox inheritance in a B” strain might not have suggested “conversion-type” as a term. In the B system, no connection or interaction with mutability ( B ” ) is apparent; in the R system, on the other hand, mutability appears to be complexly related to paramutability (BRINK 1964b; LINDEN and RODRIGUEZ 1965), yet mutability and functional capacity are not transferred in paramutation.P A R A M U T A T I O N MECHANISM 1055 A gene-extrinsic system, pictured to be of the type represented by gene-de- pendent particles such as kappa in Paramecium (SONNEBORN 1959), can be con- sidered likely to show certain specific properties in the experiments performed. To simplify the presentation, agreement with the observations and interpreta- tions given in Table 10 is represented in the following by (+)
,
disagreement by (--). Selection should be effective for or against the particle either through quantitative variation (asynchrony),
instability, or elimination (-).
Repetitious transmission of the particle could dilute it (-).
Maternal-paternal differences would be expected as a result of the great difference in cytoplasmic transmission between the sexes in maize (-).
The consequences of the presence of the particle would be fixed at fertilization, causing the phenotype of B’ B to be equal to that of B’ b and B’ B‘ i-). Functional capacity of alleles at the B locus, on which the particle should depend in the way mu in Paramecium is dependent on its metagon (GIBSON and SONNEBORN 1964), ought to be related to their capacity for particle maintenance (-).
Mutability might or might not be related to maintenance and transmission of the particle ( ?).
No chromosome abnormalities would be expected(-t)
; microscopically visible elements in the cytoplasm might or might not be found ( ? ).
Recombination and coincidence would be unaffected (-).
Segrega- tion of B’ and b should be regular if the particle is stable (+).X
rays should delete the particle independently of chromosome segments (-) ; deletion of “the B’ segment of chromosome” in B’ B heterozygotes would not eliminate the parti- cle concurrently, since B would remain for its maintenance (--). B’ should “change” B a t fertilization (-).
Ultraviolet light might eliminate the particle(--)
.
Infection should be relatively easy to accomplish, either artificially or in maternal haploid formation or between areas in sectored plants (-).
Chromo- some aberrations should not affect the system (+). Origin of the system might be by infection ( ? ).
Origin by mutation of an indigenous particle or by infection might or might not be dependent on genotype or environment ( ?) ; an indigenous particle might show maternal inheritance (+).
The weight of evidence is against a system of this general type. The most important contradictions are in the lack of maternal-paternal differences, the concurrence of deletion of chromosomal seg- ments and of B‘ properties, and absence of infectiousness according to three in- dependent diagnoses. The criteria for cytoplasmic inheritance emphasized by MICHAELIS (1958), JINKS (1963) and NANNEY (1963), as well as certain of the approaches described by POULSON ( 1963), adapted to maize in the foregoing, are unfulfilled. ALTENBURG (1959) has urged that this type of system be given care- ful consideration for the R case and, by extension, for the B case. BRINK ( 1 9 5 6 ~ ~1964b) ; BRINK, BROWN, KERMICLE and WEYERS (1960) ; and BRINK, KERMICLE and BROWN (1 964) have presented strong circumstantial evidences against a gene-maintained particle, primarily hinging on the lack of maternal-paternal differences, as far as these can be tested in the R system, and on the fact that paramutant (R’) and paramutable ( R ) alleles segregate from each other, as opposed to their sharing a particle that both should be able to maintain.
A gene-intrinsic system can be represented by enforced copying ( COE 1959a),
1056 E. H. COE, JR.
nonreciprocally, whether by induced mutation, copy choice, or replacement, has been considered a reasonable basis for rare conversion-type events in several systems (ROMAN 1956 and discussants), but the present system would require regular and complete substitution instead. There is no adequate precedent physi- cally, however, for the mechanism of rare events of this type, as LAUGHNAN
(1961) has emphasized, or for regular events. Selection should be ineffective inasmuch as no quantitative variation or asynchrony would be anticipated (+)
.
Repetitive induction should have no influence (+)
.
No maternal-paternal differ- ences would be expected (+) ; functional suppression of B by B’ is unlikely (-).Functional properties of inducing alleles would be transferred to induced alleles, and restrictive relationships between functional capacity and paramutation would be expected (-)
.
Mutability, depending on its mechanism, might or might not be transferred, and might or might not be altered by paramutation ( ? ).
Chromo- somal abnormalities ‘would be unlikely (+). Recombination should not be in- fluericed by the presence of an inducer (-), but meiotic copying might greatly increase crossing over in the region (-) ; a replacement or substitution mecha- nism might decrease crossing over and coincidence (+).
Segregation of B’ and b should be regular if the system is stable (+).
X-rays should cause concurrent deletion of B’ and its chromosome segment (+). B’ should change E at meiosis, during synapsis (+).
Ultraviolet light should not have gross effects on B’ (+).
Infectious transmission would not be expected (+)
.
Chromosome aberrations might or might not influence copying (?). Origin of the system could be by somatic or meiotic mutation (+).
Instability should be either universal or simply inherited (?), not cytoplasmic (-) and probably not dependent on genotype or environment (-).
The two properties most specific to this sort of system, transfer of functional properties of the inducer and a particular pattern of effects on recombination, are not fulfilled; most other properties are satisfied to an accept- able degree but do not balance the unfulfilled two.A combination system can be considered typified by a gene-associated but transferable element (or material), represented by the controlling-element con- cept of MCCLINTOCK ( 195 1 ), for which physicochemical precedent has been established in bacteria with the development of the episome concept (review, CAMPBELL 1962). Two possibilities will be incorporated: The element may be innate (perhaps even chromosomal i n all conditions) or adventive. Selection should be ineffective against an appended or inserted single element, which should be synchronized with the chromosome except at the time of transfer (+)
.
Repetitive transfer of the element need not dilute it, and transfer should be a one-step process (+) . No maternal-paternal differences would be expected (+) ;
P A R A M U T A T I O N M E C H A N I S M 1057
(I-)
; coincidence might be decreased by physical interference during transfer (+).
Segregation of B’ and b should be regular if the system is stable (+). High stability would be unlikely, since occasional errors in replication and transfer, or transposition of the element to other sites, might be anticipated (-).
X rays might be expected to dislodge the element, causing either gross effects on B’ expression or frequent reversion to B (-).
Deletion of the chromosome segment bearing the B locus would delete the element concurrently (+) . The element might possibly be transferred at any stage, but either fertilization (zygotic induction) or meiosis would be most likely to induce transfer, with meiotic synapsis being a more prob- able stage (+). Ultraviolet light might be expected to dislodge an appended element if it were a nucleic acid (-).
Infectious transmission would be accom- plished only with difficulty, since the element would be limited in quantity ( ?) ;maternal haploids and sectors should not show infection if release and transfer occur only at meiosis (+)
.
Chromosome aberrations might or might not influence transfer ( ? ).
Origination of the system would be by integration or mutation of the element, forming sectors following a somatic event(I-);
the element might be introduced into the cell by infection (?), or might be carried in certain lines, where it could show maternal inheritance if free, or various patterns of inheri- tance if chromosomal ( ? ),
and might show gene dependence (+).
Environmental circumstances should influence the time and frequency of origination of un- orthodoxy (+).
A number of the properties anticipated for this type of system are not fulfilled. The reader will recognize, however, that there are few compel- ling predictions of a model based on gene-associated but transferable elements.Variations can be postulated in the time, control, and efficiency of element integration, removal, and transposition, in the number of elements, their precise sites and modes of attachment, and in infectiousness and synchrony, to mention only a few. As emphasized by NEUFFER (1966)