Hemagglutinin in Infected Persons during the First Wave of the 2009
Pandemic in the United States
Zhu-Nan Li,aSeh-Ching Lin,bPaul J. Carney,aJi Li,aFeng Liu,aXiuhua Lu,aMerry Liu,bJames Stevens,aMin Levine,a Jacqueline M. Katz,aKathy Hancocka
Influenza Division, National Center for Immunization and Respiratory Diseases,aand Scientific Resources Division, National Center for Emerging and Zoonotic Infectious Diseases,bCenters for Disease Control and Prevention, Atlanta, Georgia, USA
The novel influenza A(H1N1)pdm09 virus caused an influenza pandemic in 2009. IgM, IgG, and IgA antibody responses to A(H1N1)pdm09 hemagglutinin (HA) following A(H1N1)pdm09 virus infection were analyzed to understand antibody isotype responses. Age-matched control sera collected from U.S. residents in 2007 and 2008 were used to establish baseline levels of cross-reactive antibodies. IgM responses often used as indicators of primary virus infection were mainly detected in young pa-tient groups (<5 years and 6 to 15 years old), not in older age groups, despite the genetic and antigenic differences between the HA of A(H1N1)pdm09 virus and pre-2009 seasonal H1N1 viruses. IgG and IgA responses to A(H1N1)pdm09 HA were detected in all age groups of infected persons. In persons 17 to 80 years old, paired acute- and convalescent-phase serum samples demon-strated>4-fold increases in the IgG and IgA responses to A(H1N1)pdm09 HA in 80% and 67% of A(H1N1)pdm09 virus-infected persons, respectively. The IgG antibody response to A(H1N1)pdm09 HA was cross-reactive with HAs from H1, H3, H5, and H13 subtypes, suggesting that infections with subtypes other than A(H1N1)pdm09 might result in false positives by enzyme-linked immunosorbent assay (ELISA). Lower sensitivity compared to hemagglutination inhibition and microneutralization assays and the detection of cross-reactive antibodies against homologous and heterologous subtype are major drawbacks for the application of ELISA in influenza serologic studies.
I
nfluenza A(H1N1)pdm09 virus emerged in humans in North America in the spring of 2009 and quickly spread worldwide to cause the first influenza pandemic in⬎40 years (1). By 1 August, 2010,⬎214 countries and overseas territories or communities had reported laboratory-confirmed cases of A(H1N1)pdm09 virus in-fection (2). The A(H1N1)pdm09 virus was a triple-reassortant virus containing genes originating from avian, human, and swine influenza viruses, with a hemagglutinin (HA) gene from the clas-sical swine influenza virus lineage which is genetically and anti-genically distinct from genes of former seasonal H1N1 viruses (3). The enzyme-linked immunosorbent assay (ELISA) has been used to detect influenza virus-specific antibody responses follow-ing influenza infection and vaccination (4–6). To detect HA-spe-cific antibody responses, purified HA from virions and various constructs of recombinant HA, including the ectodomain of HA or the globular head domain HA1, have been used (7–9). Previous studies have demonstrated rises in HA-specific serum IgM (86 to 94%), IgG (100%), and IgA (76 to 96%) antibodies following primary influenza virus infection in children and adults (4,10). Less frequent rises in IgM (5%), IgG (68%), and IgA (74%) were seen among persons experiencing secondary infections (4).At the onset of the 2009 influenza pandemic, we investigated the potential use of detection of HA-specific IgM for identifying A(H1N1)pdm09 virus infections, in light of the antigenic differ-ences between the HA of this virus and the HA of recent seasonal H1N1 viruses that had circulated in the human population. To address this question, we measured A(H1N1)pdm09 HA (pH1 HA)-specific IgM, IgG, and IgA antibodies by ELISA in persons infected with A(H1N1)pdm09 virus during the first wave of the 2009 pandemic in the United States and in unexposed persons of similar ages.
MATERIALS AND METHODS
Expression and purification of recombinant HA.Based on H3 number-ing, cDNA corresponding to the HA ectodomains of A/Texas/05/09
[A(H1N1)pdm09] (GenBank accession numberGQ457487[HA1:11-329
and HA2:1-176]), A/Brisbane/59/07 (H1N1) (GenBank accession num-ber CY030232 [HA1:11-329 and HA2:1-176]), A/Wisconsin/67/05
(H3N2) (GenBank accession numberEU103823.1[HA1:1-329 and HA2:
1-175]), A/Vietnam/1203/04 (H5N1) (GenBank accession number
EF541404.1[HA1:11-329 and HA2:1-175]), and A/shorebird/Delaware/
68/04 (H13N9) (GenBank accession numberCY005931.1[HA1:11-329
and HA2:1-175]) were cloned into the baculovirus transfer vector
pAcGP67-A (BD Bioscience, CA). To obtain uncleaved HA0of
A/Viet-nam/1203/04 (H5N1), the original protease cleavage site QRERRRKKRG was changed to QRETRG. All recombinant HAs (HA) have amino acid residues ADP at the N terminus and a C-terminal tag with a thrombin cleavage site, a T4 fibritin foldon sequence, and 6 histidines (LVPRGSPG
SGYIPEAPRDGQAYVRKDGEWVLLSTFLGHHHHHH) (11).
Recom-binant baculoviruses were produced in Sf9 cells (Invitrogen, CA) using a BaculoGold transfection kit according to the manufacturer’s instructions (BD Bioscience, CA) and were used to infect a suspension of cultured Sf9 cells grown in SFX serum-free medium (HyClone, IL). Trimeric HAs were expressed and purified from the supernatant of recombinant
baculovirus-infected Sf9 cells as described by Stevens et al. (11). The purity,
trimeriza-Received27 February 2014 Returned for modification12 April 2014
Accepted19 May 2014
Published ahead of print28 May 2014
Editor:R. L. Hodinka
Address correspondence to Zhu-Nan Li, hix7@cdc.gov.
Copyright © 2014, American Society for Microbiology. All Rights Reserved. doi:10.1128/CVI.00129-14
on August 17, 2020 by guest
http://cvi.asm.org/
tion, and receptor-binding activity were confirmed as previously
de-scribed (12–14).
Sources of serum samples.The serum panel from unexposed persons (3 to 79 years old) was a subset of banked serum specimens selected from different age groups of the 2007 to 2008 National Health and Nutrition
Examination Survey (NHANES) collection in the United States (15). Use
of the serum samples in the present study was approved by the National Center for Health Statistics Research Ethics Review Board. The serum panel was selected to investigate the age-specific anti-HA antibody re-sponse and was not representative of the total 2007 to 2008 NHANES serum panel or the U.S. population.
Sera from 69 A(H1N1)pdm09 virus-infected persons were collected during the first wave of the pandemic (April to July 2009) in the United States. All cases were seropositive either by the demonstration of serocon-version in the microneutralization (MN) and/or hemagglutination inhi-bition (HI) assays or by the demonstration of achieving an MN titer of
ⱖ40 and an HI titer ofⱖ20 in a convalescent-phase serum sample (16).
Many individuals were also real-time reverse transcription (RT)-PCR-positive for A(H1N1)pdm09, but not all were tested. The collection of these sera was part of the CDC public health emergency response to the pandemic and was considered a nonresearch activity that did not require CDC Institutional Review Board review.
To identify likely primary H1N1 infection among the A(H1N1)pdm09
virus-infected persons, sera from 19 individualsⱕ5 years of age were
tested by HI assay using two former seasonal H1N1 viruses (A/Brisbane/ 59/2007 [sH1 HA] and A/New Caledonia/20/1999) (data not shown).
Thirteen individuals who had sera with an HI titer ofⱕ10 to both former
seasonal H1N1 viruses were designated primary infection with A(H1N1)pdm09 virus. Thirty-nine A(H1N1)pdm09 virus-infected per-sons from 17 to 80 years old were defined as nonprimary infection perper-sons based on the age of the case and the assumption that these individuals would have had prior exposure to seasonal H1N1 viruses. Paired acute-and convalescent-phase sera from 30 out of 39 nonprimary infection per-sons were collected 1 to 7 days and 15 to 52 days after symptom onset, respectively.
ELISA.Purified, trimeric HA was captured via the C-terminal 6-his-tidine tag on HisGrab nickel-coated plates (Thermo Fisher Scientific, IL) at 200 ng/well. Standard curves were prepared using purified human IgM, IgG, and IgA standards (Thermo Fisher Scientific, IL).
Phosphate-buff-ered saline (PBS) containing 0.1% NaN3and 0.01% bovine serum
albu-min (BSA) was used to prepare immunoglobulin (Ig) standards. Each dilution of the appropriate standard series was used to coat wells of each plate at the same time the other wells were coated with HA. Plates were incubated at 4°C overnight. All sera were diluted in antibody diluent buf-fer (PBS containing 5% [wt/vol] nonfat dry milk [LabScientific, Inc., NJ] and 0.05% [vol/vol] Tween 20 [Sigma, MO] at appropriate dilutions). The WHO international A(H1N1)pdm09 antibody standard, IS/09/194 (National Institute for Biological Standards and Control [NIBSC],
Hert-fordshire, United Kingdom) (17), was included in each plate as a positive
control. The diluted sera were added to each well in duplicate followed by incubation at room temperature for 1 h. The plates were washed with wash buffer (PBS containing 0.05% [vol/vol] Tween 20), and excess horseradish peroxidase-labeled goat human IgM, IgG, or anti-IgA (Kirkegaard & Perry Laboratories [KPL], MD) was added to each well.
SureBlue TMB (3,3=,5,5=-tetramethylbenzidine) microwell peroxidase
substrate (KPL, MD) was added to each well, and after 4 min the reaction was stopped with TMB stop solution (KPL, MD). Plates were read at 450 nm with a SPECTRAmax plate spectrophotometer (Molecular Devices, CA).
The anti-HA IgM, IgG, and IgA concentrations were calculated ac-cording to the corresponding standard curve using SoftMax Pro v. 5 soft-ware (Molecular Devices, CA). To reduce intra- and interassay variability, the concentration of each test serum was divided by the concentration of the IS/09/194-positive antibody control. This test-to-positive-control ra-tio (T/P) reflects the concentrara-tion of anti-HA Ig in each serum sample.
For acute (ⱕ7 days after symptom onset) and convalescent (15 to 52 days
after symptom onset) serum samples, the fold rise of the immunoglobulin concentration was determined. In order to obtain relevant fold rise data, acute T/P values lower than 0.04 were arbitrarily set at 0.04.
Hemagglutination inhibition assay. An HI assay using the
A(H1N1)pdm09 virus was performed as described previously (18) with
updates as described by the WHO (19). Briefly, all serum samples were
treated with receptor-destroying enzyme (RDE) (Denka Seiken) over-night and heated at 56°C for 30 min. Sera were adsorbed with turkey red
blood cells (RBCs) when nonspecific agglutinins were observed (ⱖ20).
Serial 2-fold dilutions of sera were prepared in PBS starting from 1:10. The HI assay was performed in a V-shaped microtiter plate using 0.5% turkey RBCs. The titer was determined by the reciprocal of the highest serum dilution that completely inhibited hemagglutination.
Statistics.Data were graphed using Microsoft Excel and GraphPad
Prism 5 software (GraphPad Software, Inc., CA). Studenttand Fisher
exact tests were performed using GraphPad Prism 5 software and SAS 9.2
software. Differences were considered statistically significant atPvalues of
⬍0.05.
RESULTS
HA-specific IgM, IgG, and IgA antibody responses in influenza
A(H1N1)pdm09 cases and unexposed subjects.To understand
baseline levels of antibody to pH1 HA, the age-specific levels of pH1 HA cross-reactive antibody present in sera collected from U.S. residents prior to the pandemic (unexposed persons) were determined. In this study, the ratio of the Ig concentration of the test sample (T) to the Ig concentration of a positive serum pool (IS/09/194) (P) was less variable than the absolute Ig concentra-tion determined via a standard curve (data not shown). Therefore, the T/P ratio was used as a measurement of Ig concentration. One hundred thirty unexposed persons (3 to 79 years old) were sepa-rated into three age groups (3 to 5 years [n⫽19], 6 to 15 years [n⫽25], and 16 to 79 years [n⫽86]). The mean pH1 HA-specific IgM T/P ratios were 0.61, 0.60, and 0.87, respectively, for the three age groups (Fig. 1A). No significant difference in IgM concentra-tion was observed between any two age groups (P⬎0.1).
In contrast, the mean IgG and IgA T/P ratios increased as age increased in unexposed persons. For pH1 HA-specific IgG, the mean T/P ratios were 0.01, 0.05, and 0.15 for the age groups 3 to 5, 6 to 15, and 16 to 79 years, respectively (Fig. 1B). The ratio be-tween any two age groups was significantly different (P⬍0.01). For pH1 HA-specific IgA, the mean T/P ratios were 0.11, 0.19, and 0.31 for the same age groups, respectively (Fig. 1C). The IgA con-centration was significantly different between the youngest (3 to 5 years) and oldest (16 to 79 years) age groups (P⬍0.01), but not between the 3- to 5-year and 6- to 15-year (P⫽0.12) or between the 6- to 15-year and 16- to 79-year (P⫽0.08) age groups. For each Ig isotype, HA-specific antibody concentrations varied over a wide range, especially in the 16- to 79-year age group, as shown by the error bars indicating the standard deviations of the mean T/P ratios (Fig. 1). These results suggest that concentrations of IgG and IgA antibodies that are cross-reactive with pH1 HA increased with age in unexposed persons.
Next, we measured pH1 HA-specific IgM, IgG, and IgA antibody responses in 69 convalescent-phase sera from laboratory-confirmed A(H1N1)pdm09 virus-infected persons in 3 age groups (ⱕ5 years [n⫽19], 6 to 15 years [n⫽11], and⬎15 years [n⫽39]) and compared them with levels of age-specific baseline antibody pres-ent in unexposed persons. The mean IgM T/P ratios were 3.06, 3.30, and 1.19 for the 0- to 5-year, 6- to 15-year, and⬎15-year age groups of A(H1N1)pdm09 virus-infected persons, respectively
on August 17, 2020 by guest
http://cvi.asm.org/
(Fig. 1A). The mean IgM T/P ratios in A(H1N1)pdm09 virus-infected persons of agesⱕ5 years and 6 to 15 years were signifi-cantly higher than those in the age-matched unexposed groups (P⬍0.01) (Fig. 1A). In contrast, for A(H1N1)pdm09 virus-in-fected persons older than 15 years, there were no significant dif-ferences in mean IgM concentrations between A(H1N1)pdm09 virus-infected and age-matched unexposed individuals (P ⫽
0.14). Furthermore, using a threshold IgM T/P of 2.0, which gave a specificity of 90% for sera from unexposed persons (Table 1), 10 of 19 (53%) A(H1N1)pdm09 virus-infected personsⱕ5 years old and 4 of 11 (36%) infected persons 6 to 15 years old had detectable IgM responses. On the other hand, as few as 4 of 39 (10%) of the A(H1N1)pdm09 virus-infected persons⬎15 years old had detect-able IgM responses (Table 1). The mean IgG T/P ratios were 1.03,
1.46, and 1.14 and the mean IgA T/P ratios were 0.67, 0.98, and 1.76 in theⱕ5-, 6- to 15-, and⬎15-year-old age groups, respec-tively (Fig. 1BandC). For both IgG and IgA responses, mean T/P ratios in infected individuals were significantly higher than those in the age-matched unexposed group (P⬍0.01).
To further understand IgM responses in A(H1N1)pdm09 virus-infected persons, 13 probable primary H1N1-virus-infected persons were identified among theⱕ5-year-old age group, as described in Materials and Methods. IgM responses were significantly higher in these individuals than in those among the nonprimary infection cases (P⬍0.01) (Fig. 2). No such differences were observed be-tween primary H1N1 and nonprimary infection cases for IgG and IgA responses (P⫽0.92 andP⫽0.10, respectively) (Fig. 2). These results suggest that IgM responses were seen largely in
FIG 1A(H1N1)pdm09 HA antibody responses in an unexposed population, in the sera collected in 2007 and 2008, and in three age groups of A(H1N1)pdm09 virus-infected persons all in the United States. Immunoglobulin concentrations in convalescent-phase sera are expressed as the ratio of the concentration of the
test sample to the concentration of the positive control (IS/09/194) in ELISA. A(H1N1)pdm09- infected persons were divided into three age groups,ⱕ5 years old
(n⫽19), 6 to 15 years old (n⫽11), and⬎15 years (n⫽39). The control samples were grouped into those from 3- to 5-year-olds (n⫽19), 6- to 15-year-olds
(n⫽25), and 16- to 79-year-olds (n⫽86). For each group, the mean Ig concentration⫾the standard deviation is shown. ThePvalues between study groups
are shown. (A) IgM antibody responses. (B) IgG antibody responses. (C) IgA antibody responses.
TABLE 1Sensitivities and specificities of ELISA to detect anti-A(H1N1)pdm09 HA IgM, IgG, and IgA antibodies in infected and unexposed
personsa
Isotype
Sensitivityb(%) for age group: Sensitivityc(%) for: Specificity (%) for age group:
ⱕ5 yr
(n⫽19)
6–15 yr
(n⫽11)
16–80 yr
(n⫽39)
Primary infection
(n⫽13)
Nonprimary
infection (n⫽39)
3–5 yr
(n⫽19)
6–15 yr
(n⫽20)
16–79 yr
(n⫽86)
IgM 53d 36 10d 62e 10e 95 100 90
IgG 79 91 72 85 72 100 100 93
IgA 53f 55 82f 46g 82g 100 96 91
a
Assay sensitivities were based on antibody responses obtained in convalescent-phase sera from A(H1N1)pdm09-infected persons. All had MN antibody titers ofⱖ40 and HI antibody titers ofⱖ20 (Veguilla et al. [24]). Sera were grouped by age or by primary or nonprimary infections status determined as described in Materials and Methods. Individuals experiencing a primary infection wereⱕ5 years old, and those with nonprimary infections were⬎16 years old. Assay specificities were determined using sera from healthy age-matched individuals collected in 2007 and 2008 prior to the emergence of the A(H1N1)pdm09 virus. Seropositivity was based on an immunoglobulin concentration ratio of test serum sample to the concentration of the positive control, IS/09/194 (T/P ratio), that achieved at least 90% specificity for all age groups. The T/P ratio thresholds were 2.0, 0.4, and 0.6 for the IgM, IgG, and IgA responses, respectively.
b
The sensitivity was determined in 3 age groups (ⱕ5 years, 6 –15 years, and 16 – 80 years).
cThe sensitivity was determined in persons with primary infection and those with nonprimary infection. d
Statistically significant at aPvalue of⬍0.05.
eStatistically significant at aPvalue of⬍0.05. f
Statistically significant at aPvalue of⬍0.05.
gStatistically significant at aPvalue of⬍0.05.
on August 17, 2020 by guest
http://cvi.asm.org/
A(H1N1)pdm09 virus-infected persons experiencing the H1N1 subtype for the first time.
Evaluation of acute- and convalescent-phase serum samples via ELISA with pH1 HA and seasonal H1N1 A/Brisbane/59/2007 HA.As most of the human population has had prior exposure to influenza viruses, paired human sera are generally required to differentiate recent infection from residual antibodies resulting from prior infection or vaccination, by detecting a rise in antibody titer in a convalescent-phase serum sample compared to an acute-phase sample. We analyzed 30 paired sera (acute,ⱕ7 days; con-valescent, 15 to 52 days) from A(H1N1)pdm09 virus-infected per-sons with ages ranging from 17 to 80 years who were presumed to have prior exposure to former seasonal H1N1 viruses and there-fore were considered to have mounted a nonprimary infection in response to the A/(H1N1)pdm09 virus. To understand better the cross-reactive antibody responses to the HAs used in ELISA, we used pH1 and sH1 HAs. Using the criterion of a demonstration of aⱖ4-fold rise in antibody concentration as an indication of a recent infection, an IgM response against pH1 or sH1 HAs was detected in only 3% of nonprimary infections (Table 2). On the
other hand, aⱖ4-fold rise in IgG antibody concentrations was seen in 80% and 67% of A(H1N1)pdm09 virus-infected persons, respectively, when IgG and IgA were measured using pH1 HA. The proportion with IgG and IgA responses increased to 90% and 77%, respectively, if a less stringent 3-fold rise in antibody con-centration was used (Table 2). However, IgG responses were par-ticularly cross-reactive in that 33% of A(H1N1)pdm09 virus-in-fected persons also exhibited a ⱖ4-fold rise in antibody concentration detected by sH1 HA and 43% exhibited aⱖ3-fold rise to sH1 HA (Table 2). A similar trend was observed in IgA responses (Table 2). These results demonstrated that IgG and IgA, but not IgM, antibody responses were detected in the majority of persons experiencing A(H1N1)pdm09 virus infection as a nonpri-mary H1N1 infection.
Heterosubtypic antibody response detected by IgG ELISA.
To characterize cross-subtype antibody reactivity of the IgG re-sponse in ELISA, we selected paired sera from 6 individuals (ages ranging from 4 to 80 years) with aⱖ4-fold rise in HI and MN antibody titer to A(H1N1)pdm09 virus and performed ELISA us-ing HAs from A(H1N1)pdm09, A/Brisbane/59/2007 (H1N1), A/Wisconsin/67/2005 (H3N2), A/Vietnam/1203/2004 (H5N1), and A/shorebird/Delaware/68/2004 (H3N9) viruses. All six sera showed the highest fold rise for pH1 HA-specific IgG compared to the other HAs. Nevertheless,ⱖ4-fold rises in IgG concentrations were observed with some other HAs, in particular with H5 HA and to a lesser extent with H3 HA (Table 3). These results indicate that although the most robust ELISA IgG responses were detected us-ing the homologous pH1 HA subtype, cross-reactivity is readily detected, at least when the ectodomain HA is used as the antigen.
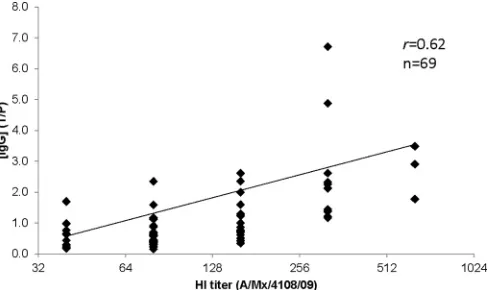
HI titer to A(H1N1)pdm09 virus and pH1 HA-specific IgG concentration.To better understand the relationship between the antibody detected by ELISA and that detected by the HI assay, the correlation coefficient (Pearson’sr) between the A(H1N1)pdm09 virus HI titer and the pH1 HA-specific IgG concentration ratio in the convalescent-phase sera from 69 A(H1N1)pdm09 virus-in-fected persons was calculated. There was a positive association between the pH1 HA-specific IgG antibody response and the HI titer to A(H1N1)pdm09 virus (r⫽0.62) (Fig. 3). Low correlation coefficient values were obtained for HI and IgM/IgA (r⫽0.11/ 0.15) (data not shown). Furthermore, HI titers against A(H1N1)pdm09 virus were poorly correlated with sH1-specific IgM, IgG, and IgA responses (allr⬍0.29) (data not shown).
DISCUSSION
The A(H1N1)pdm09 pandemic highlighted the public health need for simpler and more rapid serologic assays to detect
influ-FIG 2Primary A(H1N1)pdm09 virus-infected persons were allⱕ5 years old
(n⫽13, HI titerⱕ10 to seasonal H1N1). Nonprimary infected persons were
⬎15 years old (n⫽39). For each group, the mean Ig concentration ratio to
IS09/194⫾the standard deviation is shown. ThePvalues between study
groups are shown.
TABLE 2Demonstration ofⱖ3-fold orⱖ4-fold rise in specific
antibody concentration between acute-aand convalescent-phasebserum
samples in nonprimary A(H1N1)pdm09 cases
Isotype Fold rise
Ig response (% [no. of responses/ no. of paired sera]) to:
pH1 HA sH1 HA
IgM 3 7 (2/30) 3 (1/30)
IgM 4 3 (1/30) 3 (1/30)
IgG 3 90 (27/30) 43 (13/30)
IgG 4 80 (24/30) 33 (10/30)
IgA 3 77 (23/30) 17 (5/30)
IgA 4 67 (20/30) 17 (5/30)
a
Acute-phase sera collected 1 to 7 days after symptom onset.
bConvalescent-phase sera collected between 15 and 52 days after symptom onset.
TABLE 3Evaluation of increase in HA IgG antibody detected by ELISA in response to a panel of HAs in A(H1N1)pdm09 virus-infected individuals
Infected person
Age (yr)
Fold rise ([␣-HA IgG] in convalescent-phase
serum/[␣-HA IgG] in acute-phase serum)
pH1a sH1 H3 H5 H13
A 4 9.7 5.9 2.3 8.7 5.2
B 6 16.5 2.4 1.2 10.4 3.6
C 22 3.6 1.8 1.4 3.4 3.5
D 22 13.8 1.6 1.0 4.6 3.4
E 49 10.1 6.3 2.4 6.5 6.0
F 80 10.2 4.7 2.1 4.2 3.3
a
The highest fold rise for each case is indicated in bold type.
on August 17, 2020 by guest
http://cvi.asm.org/
enza subtype-specific antibody responses as markers for infection and immunity against novel influenza viruses. Timely serologic studies detect both symptomatic and asymptomatic infections and provide more robust estimates of total infections within a population (20). Most seroepidemiological studies conducted during the 2009 pandemic relied on virus neutralization and/or HI gold standard assays, which typically require virus propagation expertise and appropriate biocontainment. Other alternative as-says such as pseudotype virus neutralization asas-says were also used in some laboratories; this approach requires technical expertise to generate pseudotype viruses. In contrast, ELISA is a relatively sim-ple assay and is available in many laboratories. The early and tran-sient nature of serum IgM responses makes them ideal biomarkers for many viral infections (21). However, detection of IgM re-sponses against influenza viruses is complicated by repeated ex-posure to influenza antigens (22). To better understand the fre-quency of IgM, IgG, and IgA responses to the novel pH1 HA, we compared age-related anti-pH1 HA IgM, IgG, and IgA responses by ELISA in sera collected from A(H1N1)pdm09 virus-infected or nonexposed persons.
Our findings suggested that HA-specific IgM antibodies were mainly detected in younger A(H1N1)pdm09 virus-infected per-sons; therefore, detection of IgM is not a viable approach for iden-tifying a nonprimary influenza virus infection (4,22).
We detected anti-HA IgM responses in 53% of A(H1N1)pdm09 virus-infected childrenⱕ5 years old, 36% of infected persons 6 to 15 years old, and only 10% of infected persons⬎15 years old (Table 1). In a smaller number of childrenⱕ5 years old who had no detectable HI antibody to seasonal H1N1 viruses and were presumed to be undergoing a primary H1N1 infection, we found a 62% anti-pH1 HA IgM response rate (Table 1). These results are consistent with previous studies which demonstrated an IgM re-sponse in the majority of children or adults experiencing a pri-mary H1N1 infection following experimental inoculation or nat-ural infection with H1N1 or H3N2 viruses and a very low frequency of IgM responses in adults experiencing a secondary infection (4, 10). However, there are several limitations in our study. We may have overestimated primary infections and
under-estimated the frequency of IgM responses in a primary A(H1N1)pdm09 virus infection. This may have occurred if (i) prior exposure to seasonal H1N1 virus had occurred but no anti-bodies to contemporary seasonal viruses were detected by the HI assay, (ii) collection of the serum samples did not coincide with the peak IgM response, and (iii) there was a sampling bias due to the small number of primary infection cases evaluated in this study.
Recently, Qiu et al. investigated antibody responses in 131 A(H1N1)pdm09-infected persons ranging in age from 0 to 55 years and reported an anti-influenza A IgM response in 84.7% of cases (23). The IgM antibody response was not age related and in the majority of cases occurred in individuals who by virtue of their age had prior H1N1 infection. In addition, IgM antibody was de-tected as early as 2 days after symptom onset, with only a modest rise in mean geometric mean titers through day 10 after symptom onset (23). One reason for the discrepancies between the studies is that Qiu et al. used a commercial ELISA kit containing seasonal (late 1990s) influenza A(H1N1) and A(H3N2) vaccine antigens and as such likely detected cross-reactive IgM antibody recogniz-ing HA as well as other viral proteins.
IgM detection may be affected by the presence of rheumatoid factor (RF) and/or excess IgG antibody; removal of either IgG or RF has been used to overcome these issues (24). In our studies with paired acute- and convalescent-phase serum samples, IgM concen-tration did not increase following removal of⬎99% of IgG in the serum by protein G adsorption (data not shown). Therefore, we be-lieve that detection of pH1 HA-specific IgM antibody in this study was not hindered by the presence of RF and IgG antibody.
Influenza A(H1N1)pdm09 virus infection was not primary for most of the U.S. population who had previous exposure to former seasonal H1N1 infection, even though A(H1N1)pdm09 HA was significantly different from seasonal H1N1 HA, with only 50% amino acid identity in HA1 antigenic sites (3,25). Further inves-tigations are necessary to evaluate HA-specific IgM detection for other novel HA subtype virus infections, such as H7N9 and H5N1. Similar to patterns observed with neutralizing antibodies cross-reactive with A(H1N1)pdm09 (26, 27), we found an in-crease in IgG and IgA cross-reactive antibodies with increasing age of the serum donor for samples collected prior to the emergence of the A(H1N1)pdm09 virus (Fig. 1BandC). These results highlight the need to evaluate age-specific baseline levels of antibody in unexposed human populations, when evaluating the antibody re-sponse to a novel influenza virus with any assay, in order to un-derstand age-specific cross-reactive antibodies that might compli-cate interpretation of seropositivity and inferred infection status. In this study, antibody responses to influenza HA protein were detected by ELISA using correctly folded and functional HA trimers. Recombinant HAs bound to nickel-coated plates are expected to re-semble the presentation of HA on the virion surface, exposing sub-type-specific epitopes on the HA globular domain and potentially restricting antibody access to conserved epitopes on the HA stalk region. This approach reduced but did not eliminate cross-reactions between HA subtypes in ELISA (Table 3and data not shown).
Antibody binding sites have been identified throughout the HA sequence (28,29), and it has been observed that the amino acid se-quence of the HA2 region of the HA molecule is more conserved between subtypes than the amino acid sequence of the HA1 region (30,31). The antigenic sites that are subject to antigenic drift are in the HA1 region (32–35). Some cross-subtype neutralizing epitopes in
FIG 3Correlation coefficient (Pearson’sr) between HI titer and IgG concen-tration. Convalescent-phase sera from 69 A(H1N1)pdm09 virus-infected per-sons were tested by HI using A/Mexico/4108/2009 [A(H1N1)pdm09] virus
and ELISA using pH1 HA. The log2scale HI titers are shown at thexaxis. IgG
concentrations in ELISA are expressed as the ratio of the concentration of the test sample to the concentration of the positive control (IS/09/194). The linear
regression line and the correlation coefficient Pearson’srare shown.
on August 17, 2020 by guest
http://cvi.asm.org/
HA2 have been characterized (36–40). One approach to reducing the cross-reactivity observed in the HA ELISA is to eliminate the more conserved HA stalk, primarily the HA2 region of the HA, and use the globular head domain HA1 as the antigen. Others have shown the feasibility of this approach for the detection of antibody responses to the A(H1N1)pdm09 virus (7,41).
The detection of the binding antibodies to HA via ELISA or a similar assay is much simpler than detection via functional HI and MN assays. When appropriately paired serum samples are used, the demonstration of a significant rise in antibody titers indicates recent infection or successful vaccination (4–6,10). Although we demonstrated ELISA antibody responses against pH1 HA in per-sons infected with A(H1N1)pdm09 virus during the first wave of the pandemic, stringent threshold criteria were needed for opti-mal assay specificity based on sera from non-A(H1N1)pdm09-exposed persons. Now that A(H1N1)pdm09 viruses have circu-lated among humans for⬎4 years, interpretation of ELISA and other serological assays will need to allow for increased baseline levels of antibodies in the population due to extensive exposure to A(H1N1)pdm09 virus through infection or vaccination.
Overall detection of anti-HA IgM is an insensitive means of de-tecting infection even in primary infection cases (Fig. 2andTable 1). When the specificity for the age-matched unexposed population was
⬎90%, IgG and IgA ELISAs were both less sensitive (Table 1) than the MN and HI combination criteria reported previously (16). The major limitation for HA ELISA is the detection of antibodies that are cross-reactive with other HA strains and even other HA subtypes (Table 2,
Table 3, and data not shown). This phenomenon was also observed in previous studies (42,43). This limitation suggests that in the case of cocirculating influenza virus subtypes, an infection with one virus might result in an antibody response to the HAs of other virus sub-types in ELISA, making it difficult to determine which influenza virus caused the infection (Table 3). Indeed, we observed in this study that although rises in IgG were always highest against pH1 HA among paired sera from selected infected persons, 4-fold rises were also de-tected to other HAs, most notably sH1 and H5, suggesting that A(H1N1)pdm09 virus infection might yield false positives for antibody responses to novel subtypes like H5N1 in ELISA or other direct antibody binding assays. The presence of cross-reactive antibodies might also be associated with the phenom-enon of “original antigenic sin” (OAS), in which reexposure to the same subtype influenza induces an HI antibody response to the original virus (44). It is possible that the OAS contributes to those cross-reactive IgM, IgG, and IgA antibodies to seasonal H1 HA (Table 2).
In summary, we detected IgM responses in just over half of the young children tested, including those who lacked evidence of HI antibodies to seasonal H1N1 viruses and were presumably experienc-ing their first H1N1 subtype infection. HA-specific IgG and IgA an-tibodies were detected in the serum samples from all age groups of A(H1N1)pdm09 virus-infected persons. However, unexposed individuals had a background level of reactivity to the pH1 HA that increased with age. The IgG antibody response to pH1 HA was cross-reactive with HAs from sH1, H5, and H13 subtypes, suggesting that infections with subtypes other than A(H1N1)pdm09 virus might result in false positives in ELISA. The development of simpler and more rapid serological assays for de-tection of recent influenza virus infection remains an urgent pub-lic health need. Well-defined age-matched control sera from non-exposed individuals are necessary to evaluate the specificity of any
improved serological assay. The evaluation of the globular head domain HA1 as an antigen in ELISA and other antibody detection platforms is under way in our laboratory.
ACKNOWLEDGMENTS
We thank state and CDC epidemiologists and the Naval Health Research Center, San Diego, CA, for contributions of sera used in this study. We thank Carrie Reed (Influenza Division) for facilitating the use of NHANES sera. We thank Heather Tatum, Leilani Thomas, and Peter Browning for so ably managing the serum samples.
This study was fully supported by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
We declare that we have no conflicts of interest.
REFERENCES
1.World Health Organization.2009. World now at the start of 2009 influenza pandemic: statement to the press by WHO Director-General Dr Margaret
Chan. World Health Organization, Geneva, Switzerland.http://www.who.int
/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en /index.html.
2.World Health Organization.2010. Global alert and response (GAR). Pandemic (H1N1) 2009 — update 2012. World Health Organization,
Ge-neva, Switzerland.http://www.who.int/csr/don/2010_08_06/en/.
3.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ.2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science
325:197–201.http://dx.doi.org/10.1126/science.1176225.
4.Burlington DB, Clements ML, Meiklejohn G, Phelan M, Murphy BR.
1983. Hemagglutinin-specific antibody responses in immunoglobulin G, A, and M isotypes as measured by enzyme-linked immunosorbent assay after primary or secondary infection of humans with influenza A virus.
Infect. Immun.41:540 –545.
5.Karron RA, Talaat K, Luke C, Callahan K, Thumar B, Dilorenzo S, McAuliffe J, Schappell E, Suguitan A, Mills K, Chen G, Lamirande E, Coelingh K, Jin H, Murphy BR, Kemble G, Subbarao K.2009. Evalu-ation of two live attenuated cold-adapted H5N1 influenza virus vaccines
in healthy adults. Vaccine 27:4953– 4960. http://dx.doi.org/10.1016/j
.vaccine.2009.05.099.
6.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, Lamirande EW, Jin H, Coelingh KL, Murphy BR, Kemble G, Subbarao
K.2009. A live attenuated H7N3 influenza virus vaccine is well tolerated
and immunogenic in a phase I trial in healthy adults. Vaccine27:3744 –
3753.http://dx.doi.org/10.1016/j.vaccine.2009.03.082.
7.Alvarez MM, Lopez-Pacheco F, Aguilar-Yanez JM, Portillo-Lara R, Mendoza-Ochoa GI, Garcia-Echauri S, Freiden P, Schultz-Cherry S, Zertuche-Guerra MI, Bulnes-Abundis D, Salgado-Gallegos J, Elizondo-Montemayor L, Hernandez-Torre M.2010. Specific recognition of in-fluenza A/H1N1/2009 antibodies in human serum: a simple virus-free
ELISA method. PLoS One 5:e10176.http://dx.doi.org/10.1371/journal
.pone.0010176.
8.Arankalle VA, Virkar RG, Tandale BV, Ingle NB.2010. Utility of pandemic H1N1 2009 influenza virus recombinant hemagglutinin protein-based en-zyme-linked immunosorbent assay for serosurveillance. Clin. Vaccine
Im-munol.17:1481–1483.http://dx.doi.org/10.1128/CVI.00223-10.
9.Murphy BR, Phelan MA, Nelson DL, Yarchoan R, Tierney EL, Alling DW, Chanock RM.1981. Hemagglutinin-specific enzyme-linked immu-nosorbent assay for antibodies to influenza A and B viruses. J. Clin.
Mi-crobiol.13:554 –560.
10. Murphy BR, Nelson DL, Wright PF, Tierney EL, Phelan MA, Chanock
on August 17, 2020 by guest
http://cvi.asm.org/
RM.1982. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect. Immun.
36:1102–1108.
11. Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA.
2004. Structure of the uncleaved human H1 hemagglutinin from the
ex-tinct 1918 influenza virus. Science303:1866 –1870.http://dx.doi.org/10
.1126/science.1093373.
12. Yang H, Carney P, Stevens J. 2010. Structure and receptor binding properties of a pandemic H1N1 virus hemagglutinin. PLoS Curr. 2:RRN1152.
13. Yang H, Carney PJ, Chang JC, Villanueva JM, Stevens J.2013. Struc-tural analysis of the hemagglutinin from the recent 2013 H7N9 influenza
virus. J. Virol.87:12433–12446.http://dx.doi.org/10.1128/JVI.01854-13.
14. Yang H, Chang JC, Guo Z, Carney PJ, Shore DA, Donis RO, Cox NJ, Villanueva JM, Klimov AI, Stevens J.2014. Structural stability of
influ-enza A(H1N1)pdm09 virus hemagglutinins. J. Virol.88:4828 – 4838.http:
//dx.doi.org/10.1128/JVI.02278-13.
15. Centers for Disease Control and Prevention.2014. National health and nutrition examination survey. Centers for Disease Control and Prevention,
Atlanta, GA.http://wwwn.cdc.gov/nchs/nhanes/search/nhanes07_08.aspx.
16. Veguilla V, Hancock K, Schiffer J, Gargiullo P, Lu X, Aranio D, Branch A, Dong L, Holiday C, Liu F, Steward-Clark E, Sun H, Tsang B, Wang D, Whaley M, Bai Y, Cronin L, Browning P, Dababneh H, Noland H, Thomas L, Foster L, Quinn CP, Soroka SD, Katz JM.2011. Sensitivity and specificity of serologic assays for detection of human infection with
2009 pandemic H1N1 virus in U.S. populations. J. Clin. Microbiol.49:
2210 –2215.http://dx.doi.org/10.1128/JCM.00229-11.
17. Wood JM, Major D, Heath A, Newman RW, Hoschler K, Stephenson I, Clark T, Katz JM, Zambon MC.2012. Reproducibility of serology assays for pandemic influenza H1N1: collaborative study to evaluate a candidate
WHO International Standard. Vaccine30:210 –217.http://dx.doi.org/10
.1016/j.vaccine.2011.11.019.
18. Kendal AP, Pereira MS, Skehel JJ.1982. Concepts and procedures for laboratory-based influenza surveillance. Centers for Disease Control and Prevention, Atlanta, GA.
19. WHO Global Influenza Surveillance Network.2011. Manual for the laboratory diagnosis and virological surveillance of influenza. WHO Press, Geneva, Switzerland.
20. Laurie KL, Huston P, Riley S, Katz JM, Willison DJ, Tam JS, Mounts AW, Hoschler K, Miller E, Vandemaele K, Broberg E, Van Kerkhove MD, Nicoll
A.2013. Influenza serological studies to inform public health action: best
practices to optimise timing, quality and reporting. Influenza Other Respir.
Viruses7:211–224.http://dx.doi.org/10.1111/j.1750-2659.2012.0370a.x.
21. Erdman DD, Anderson LJ, Adams DR, Stewart JA, Markowitz LE, Bellini WJ.1991. Evaluation of monoclonal antibody-based capture en-zyme immunoassays for detection of specific antibodies to measles virus. J.
Clin. Microbiol.29:1466 –1471.
22. Zambon M.1998. Laboratory diagnosis of influenza. Blackwell Science, Oxford, United Kingdom.
23. Qiu C, Tian D, Wan Y, Zhang W, Zhu Z, Ye R, Song Z, Zhou M, Yuan S, Shi B, Wu M, Liu Y, Gu S, Wei J, Zhou Z, Zhang X, Zhang Z, Hu Y, Yuan Z, Xu J.2011. Early adaptive humoral immune responses and virus clearance in humans recently infected with pandemic 2009 H1N1
influ-enza virus. PLoS One6:e22603.http://dx.doi.org/10.1371/journal.pone
.0022603.
24. Salonen EM, Vaheri A, Suni J, Wager O.1980. Rheumatoid factor in acute viral infections: interference with determination of IgM, IgG, and
IgA antibodies in an enzyme immunoassay. J. Infect. Dis.142:250 –255.
http://dx.doi.org/10.1093/infdis/142.2.250.
25. Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr, Wilson IA.2010. Structural basis of preexisting immunity to the 2009 H1N1 pandemic
influenza virus. Science328:357–360.http://dx.doi.org/10.1126/science
.1186430.
26. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz
JM.2009. Cross-reactive antibody responses to the 2009 pandemic H1N1
influenza virus. N. Engl. J. Med.361:1945–1952.http://dx.doi.org/10
.1056/NEJMoa0906453.
27. Reed C, Katz JM, Hancock K, Balish A, Fry AM, H1N1 Serosurvey Working Group.2012. Prevalence of seropositivity to pandemic influenza A/H1N1 virus in the United States following the 2009 pandemic. PLoS
One7:e48187.http://dx.doi.org/10.1371/journal.pone.0048187.
28. Zhao R, Cui S, Guo L, Wu C, Gonzalez R, Paranhos-Baccala G, Vernet
G, Wang J, Hung T.2011. Identification of a highly conserved H1 sub-type-specific epitope with diagnostic potential in the hemagglutinin
pro-tein of influenza a virus. PLoS One6:e23374.http://dx.doi.org/10.1371
/journal.pone.0023374.
29. Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honor-kiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H.
2010. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med.
2:15ra15.http://dx.doi.org/10.1126/scitranslmed.3000624.
30. Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K.
1991. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza
A viruses. Virology 182:475– 485. http://dx.doi.org/10.1016/0042
-6822(91)90588-3.
31. Krystal M, Elliott RM, Benz EW, Jr, Young JF, Palese P.1982. Evolution of influenza A and B viruses: conservation of structural features in the
hemagglutinin genes. Proc. Natl. Acad. Sci. U. S. A.79:4800 – 4804.http:
//dx.doi.org/10.1073/pnas.79.15.4800.
32. Caton AJ, Brownlee GG, Yewdell JW, Gerhard W.1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype).
Cell31:417– 427.http://dx.doi.org/10.1016/0092-8674(82)90135-0.
33. Wilson IA, Skehel JJ, Wiley DC.1981. Structure of the haemagglutinin
membrane glycoprotein of influenza virus at 3 A resolution. Nature289:
366 –373.http://dx.doi.org/10.1038/289366a0.
34. Tsuchiya E, Sugawara K, Hongo S, Matsuzaki Y, Muraki Y, Li ZN, Nakamura K.2001. Antigenic structure of the haemagglutinin of human
influenza A/H2N2 virus. J. Gen. Virol.82:2475–2484.
35. Wiley DC, Skehel JJ.1987. The structure and function of the hemagglu-tinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem.
56:365–394.http://dx.doi.org/10.1146/annurev.bi.56.070187.002053.
36. Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. 2009. Structural and functional bases for broad-spectrum neutralization of
avian and human influenza A viruses. Nat. Struct. Mol. Biol.16:265–273.
http://dx.doi.org/10.1038/nsmb.1566.
37. Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Thro-sby M, Goudsmit J, Wilson IA.2009. Antibody recognition of a highly
conserved influenza virus epitope. Science324:246 –251.http://dx.doi.org
/10.1126/science.1171491.
38. Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J.2011. A highly conserved neutralizing epitope
on group 2 influenza A viruses. Science333:843– 850.http://dx.doi.org/10
.1126/science.1204839.
39. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A.2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science
333:850 – 856.http://dx.doi.org/10.1126/science.1205669.
40. Okuno Y, Isegawa Y, Sasao F, Ueda S.1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1
and H2 strains. J. Virol.67:2552–2558.
41. Koopmans M, de Bruin E, Godeke GJ, Friesema I, van Gageldonk R, Schipper M, Meijer A, van Binnendijk R, Rimmelzwaan GF, de Jong MD, Buisman A, van Beek J, van de Vijver D, Reimerink J. 2012. Profiling of humoral immune responses to influenza viruses by using
pro-tein microarray. Clin. Microbiol. Infect.18:797– 807.http://dx.doi.org/10
.1111/j.1469-0691.2011.03701.x.
42. Burlington DB, Wright PF, van Wyke KL, Phelan MA, Mayner RE, Murphy BR.1985. Development of subtype-specific and heterosubtypic antibodies to the influenza A virus hemagglutinin after primary infection
in children. J. Clin. Microbiol.21:847– 849.
43. Stelzer-Braid S, Wong B, Robertson P, Lynch GW, Laurie K, Shaw R, Barr I, Selleck PW, Baleriola C, Escott R, Katsoulotos G, Rawlinson
WD.2008. A commercial ELISA detects high levels of human H5 antibody
but cross-reacts with influenza A antibodies. J. Clin. Virol.43:241–243.
http://dx.doi.org/10.1016/j.jcv.2008.06.012.
44. Francis T.1960. On the doctrine of original antigenic sin. Proc. Am.
Philos. Soc.104:572–578.