271
Top Stroke Rehabil 2010;17(4):271–281 © 2010 Thomas Land Publishers, Inc. www.thomasland.com
doi: 10.1310/tsr1704-271
Intensive Care Unit Setting: Implementation
of a Quality Improvement Model
Dale M. Needham, MD, PhD,1–3 and Radha Korupolu, MBBS, MS1
1OACIS Group, Division of Pulmonary & Critical Care Medicine, Johns Hopkins University, Baltimore, Maryland; 2Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, Maryland; 3Critical
Care Physical Medicine and Rehabilitation Program, Johns Hopkins Hospital, Baltimore, Maryland
Objective: There are barriers to providing early physical medicine and rehabilitation (PM&R) in the intensive care unit (ICU). We present a specifi c model for undertaking quality improvement (QI) projects and a case study focused on QI for early PM&R in the ICU. Methods: The QI project was undertaken using a 4-step model: (1) summarizing the evidence, (2) identifying barriers, (3) establishing performance measures, and (4) ensuring patients receive the intervention. To evaluate the application and outcomes of this model, we present data collected during a 4-month QI period versus an immediately preceding 3-month control period. Results: Deep sedation was a major barrier to early PM&R that was addressed in the QI project. Compared to the control period, there was a decrease in medical ICU (MICU) days with any benzodiazepine use (73% vs 96% of days, P= .03) and narcotic use (77% vs 96%, P= .05) and improved delirium status (MICU days without delirium, 53% vs 21%, P= .003). In addition, more QI patients had physical therapy consultations (93% vs 59%, P= .004) and greater number of rehabilitation treatments with higher functional mobility (treatments involving sitting or greater mobility, 78% vs 56%, P= .03). Hospital data for the QI period demonstrated a decrease in average length of stay in the MICU (4.9 vs 7.0 days, P= .02) and hospital (14.1 vs 17.2, P= .03) compared to the prior year. Conclusion: A structured QI model can be applied to implementation of early PM&R in the ICU resulting in markedly improved delirium status, delivery of PM&R, functional mobility, and length of stay. Key words:critical care, delirium, early ambulation, health care, intensive care units, muscle weakness, quality assurance, rehabilitation
S
imilar to the sequelae faced after a stroke, patients with acute respiratory failure requir-ing mechanical ventilation in the intensive care unit (ICU) frequently experience neuromuscular weakness, impaired physical function, decreased quality of life, and delayed return to work.1–4Physical medicine and rehabilitation (PM&R) interventions delivered early have the potential to improve these complications in patients with acute stroke5 and with critical illness in the ICU
setting.6,7 However, there are many barriers
related to early PM&R in the ICU, including ICU culture, clinicians’ lack of knowledge, patients’ physiological instability, and staffi ng issues.2,8–10
In this article, we describe a quality improvement (QI) project undertaken to address early PM&R in the medical ICU (MICU) at Johns Hopkins Hospital.11 This project represents a case study for
understanding PM&R QI in the ICU setting and for illustrating how a specifi c model for QI can be applied. We will fi rst describe the context for the QI project at Johns Hopkins Hospital and then provide an overview of the QI model. Thereafter, we will discuss application of the model, step by
step, in the context of this case study, which can be generalized to improving outcomes in patients with stroke.
Context for QI Project at Johns Hopkins Hospital
Johns Hopkins Hospital is a quaternary care, academic teaching hospital with more than 1,000 beds, located in an inner city neighborhood in Baltimore, Maryland. The MICU has 16 beds and is staffed with physicians (2 attendings, 2 fellows, and 6 housestaff), registered nurses (staff to patient ratio of 1:2), and respiratory therapists (staff to patient ratio of 1:8). Physical medicine and rehabilitation and neurology physicians and physical and occupational therapy (PT and OT) consultations are available when ordered by a MICU physician. In the MICU, “bed rest” was the
model considered throughout all steps of the QI process included understanding the problem within the larger health care system and creating a multidisciplinary QI team. Moreover, there are 4 steps in the model: (1) summarizing the evidence, (2) identifying barriers to implementation, (3) establishing performance measures, and (4) ensuring that patients receive the intervention. In the last stage, a “4 Es” process was undertaken involving engaging, educating, executing, and evaluating the implementation in an iterative manner. Although described as 4 separate steps, aspects of this QI model may occur simultaneously.
Application of QI Model for Early PM&R in the MICU
Overall considerations
It was clear that the improvement process involved a patient care system larger than the regular multidisciplinary MICU team (consisting of physicians, nurses, and respiratory therapists) and that more extensive collaboration would be needed. Moreover, based on the experience of another hospital10 and local experience in the
Johns Hopkins MICU, it was anticipated that the necessary culture change to successfully adopt early PM&R would be challenging but important for a successful QI project.20 Moreover, those involved
in the QI project wanted to rigorously evaluate it to obtain data regarding its feasibility, safety, and effectiveness. Convincing hospital leaders of these issues was considered critical to help sustain the QI process. Consequently, approximately 1 year was required for planning the QI project prior to implementing early PM&R in the MICU. We believed that appropriate planning, preparation, and collaboration were key to succeeding with a major change in culture regarding early PM&R in our MICU.
Step 1: Summarize the evidence
Relevant evidence regarding the harms of bed rest and the safety, feasibility, and potential benefi ts of early mobilization of acute and long-term ICU patients was reviewed.4,12,21–35 Moreover,
default activity level in the standard admission orders, and there were no guidelines for PT or OT consultation or treatment. Nursing care included use of standardized pain and sedation scales, with a nurse-titrated sedation protocol. Standardized delirium assessments were not part of routine nursing care.
Mobilizing mechanically ventilated patients in the ICU is feasible, safe, and benefi cial in improving physical function.7,12,13 However,
early mobilization is seldom practiced in most ICUs.14 In the MICU at Johns Hopkins
Hospital, neuromuscular complications and early mobilization became areas of interest. As the lead study site for a prospective cohort study evaluating the long-term outcomes of patients surviving acute lung injury/acute respiratory distress syndrome,15 clinicians from the Johns
Hopkins MICU became interested in these areas after witnessing the weakness and functional impairments experienced by research participants who had been discharged from the MICU. In addition, preliminary data analysis from this cohort study indicated that only 24% of ARDS patients ever received consultation for PT and/or OT in the Johns Hopkins Hospital MICU, which was almost 50% lower than for similar patients at 2 other academic hospitals in Baltimore.16 In
addition, preliminary data analysis also indicated a higher prevalence of deep sedation in the MICU patients (58% vs 27% of ICU days) and a very low proportion (<15%) of ICU days in which patients were not deeply sedated or delirious.
Based on this experience, a QI project was undertaken that focused on patients receiving mechanical ventilation for ≥4 days. Two major QI goals were (1) to improve patient sedation and delirium status and (2) to increase rehabilitation-related consults and treatments in order to improve functional mobility. The QI project was undertaken using a specifi c multistep QI model as discussed in the next section.
Description of a Model for QI
Figure 1 illustrates the established QI model used for this project. The model is briefly described here with additional details available in other publications.17–19 Key aspects of the
and delivering early rehabilitation therapy through engaging all relevant stakeholders (MICU, PM&R and neurology physicians, and RN, RT, PT, and OT) in small group meetings and presentations. In particular, our project started with gaining support of the organizational leaders (directors of PM&R, MICU, and Pulmonary and Critical Care Medicine) and supervisors for PT, OT, and RT. In addition, grassroots support was established via champions from each discipline: MICU and PM&R physicians, PT, OT, RN, and RT. A MICU physician (D.M.N.) who was well known to many of the involved clinicians and who had experience with QI methods was the overall leader for the QI project and received regular additional evidence and practice guidelines
specifically addressing early PM&R of MICU patients arose during the QI project to further solidify the evidence base.7,13,14,36 This evidence,
along with our local MICU data regarding sedation and rehabilitation practices,16 were summarized
and disseminated via newsletters, posters, and presentations.
Step 2: Identify local barriers to implementation
Systematic methods for identifying and documenting barriers to QI interventions are evolving.17,37 We carefully considered the steps
involved in preparing the patient and ordering
Identify interventions associated with improved outcomes
Select interventions with the largest benefit and lowest barriers to use Convert interventions to behaviours
Observe staff performing the interventions
“Walk the process” to identify defects in each step of implementation Enlist all stakeholders to share concerns and identify potential gains and losses associated with implementation
Select measures (process or outcome) Develop and pilot test measures Measure baseline performance
Implement the “four Es” targeting key stakeholders from front line staff to executives
Envision the problem within the larger healthcare system Engage collaborative multidisciplinary teams centrally (stages 1–3) and locally (stage 4) Overall concepts
1. Summarise the evidence
2. Identify local barriers to implementation
3. Measure performance
4. Ensure all patients receive the interventions
Explain why the interventions are important Engage Educate Execute Evaluate
Regularly assess for performance measures and
unintended consequences
Share the evidence supporting the interventions Design an intervention “toolkit” targeted at barriers, standardisation, independent checks, reminders, and learning from mistakes
Figure 1. Model for quality improvement process. A model for conducting quality improvement within the health care setting. Reproduced, with permission, from Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. Copyright © 2008 by BMJ Publishing Group Ltd.
detailed in Table 1. Critical to the QI process was having the QI project leader acknowledge each global and patient-specifi c barrier raised by stakeholders and address it on a timely basis. This validation ensured that stakeholders felt that their concerns were being heard and addressed. The process of addressing the barriers often included seeking multidisciplinary input and brainstorming for solutions. During the QI process, it was emphasized that implementing this intervention required “learning” and that the approach would be continually refi ned throughout its implementation.
support and advice from the long-time MICU Director. Through a series of meetings with each clinical discipline and regular group meetings with all of the multidisciplinary champions, specifi c barriers from each discipline’s unique perspective could be obtained. Moreover, given the goal of formally evaluating the QI project, data were collected on the type and frequency of barriers to rehabilitation as well as patients’ rehabilitation-related outcomes during their hospitalization.11
Important barriers to the QI project, arising from the previously described process, are
Table 1. Barriers to early physical medicine and rehabilitation in the Johns Hopkins MICU
Barrier Strategy to overcome barrier
Lack of leadership • Designated an overall leader who was involved and committed to quality improvement (D.M.N.) • Involved a champion from every discipline, including physician, PT, OT, RN, and RT
• Obtained strong support from both MICU and PM&R leadership Lack of staffi ng & equipment • Obtained dedicated full-time OT & PT in MICU and part-time technician
• Initiated PM&R physician consultations in MICU
• Purchased 2 wheelchairs to assist with sitting patients out of bed
Lack of knowledge & training • Educated the multidisciplinary team regarding the rationale and evidence for early PM&R interventions
• Created simple guidelines for consultation of OT & PT in the MICU setting
• Consulted neurologists regarding patients with severe and/or persistent muscle weakness • Cross-trained staff, including:
• Training PTs regarding basic EKG interpretation and suctioning
• Orienting PTs and OTs to ventilator settings, use of portable ventilator, and troubleshooting alarms
• Training RNs regarding patient transfer from bed to chair
Lack of physician referrals • Project coordinator (R.K.) screened patients using pre-defi ned eligibility criteria6
• Project coordinator (R.K.) prompted physicians for timely consultations to PT and OT when screening criteria met
Oversedation • Interdisciplinary education regarding changing from continuous infusion to “as needed” boluses for sedation
• Screening patients’ sedation status using the validated RASS scale43
• For specifi c MICU patients who were deeply sedated, the project leader (D.M.N.) had: • One-on-one discussions with MICU clinical fellows and attending physicians
• Frequent bedside discussions with day and night shift nurses regarding changes in sedation practice
Delirium • Screening for delirium by PTs and OTs, using the validated CAM-ICU instrument44
• Minimized use of drugs associated with delirium (eg, benzodiazepines) • Encouraged use of antipsychotics to treat agitated delirium
Perceived pain and discomfort • Assessed pain and ordered pain medication, as needed, prior to rehabilitation therapy • Requested patient feedback on comfort during and after rehabilitation therapy Physiological instability • Created guidelines for screening patients for physiological stability for PM&R therapy6
• PT and OT screened patients on a daily basis to ensure safety before therapy initiated • Changed mechanical ventilation mode and increased FiO2 by 0.2, as needed, prior to starting
therapy
Safety • PTs and OTs ensured securement of devices prior to therapy
• Untangling of lines and tubes occurred prior to therapy to avoid accidental dislodgement
Note: CAM-ICU = Confusion Assessment Method for the ICU; EKG = electrocardiogram; MICU = medical intensive care unit; OT = occupational therapist; PM&R = physical medicine and rehabilitation; PT = physical therapist; RASS = Richmond Agitation and Sedation Scale; RN = registered nurse; RT = respiratory therapist.
requirement for measurement.11 Table 2 indicates
which measures were used on an ongoing basis after the intensive evaluation phase of the QI project was fi nished.
Step 4: ensure patients receive the intervention: the 4 Es model
To ensure that patients received the QI intervention, an iterative 4 Es model (engage, educate, execute, and evaluate) was used as described in this section.
Step 3: measure performance
Performance measures, or quality indicators, for the QI project can be process- or outcome-oriented as described in an accompanying article in this issue.38 As outlined in Table 2,
performance measures developed as part of the QI process were often specifi cally targeted at the previously identifi ed barriers. Not all of these performance measures were feasible to collect on an ongoing basis after the QI project, due to issues with their accuracy, feasibility, or time
Table 2. Performance measures and results for quality improvement projecta
Barrier Performance measure
Pre-QI period (n=27 patients with 312 MICU patient days)
QI period (n=30 patients with 482 MICU patient days) P Lack of staffi ng Proportion of ICU days with no therapy for patients 41% 7% .004 Lack of physician Physical therapy consultations, proportion patients 59% 93% .004 referrals Occupational therapy consultations, proportion patients 74% 90% .17 Oversedation Alert during sedation assessment (RASS score -1 to +1),
proportion of ICU days
30% 67% <.001
Benzodiazepines:
Ever receiving benzodiazepine in ICU, proportion of patients
96% 73% .03
Daily midazolam-equivalent dose, median (IQR) mg 47 (21–114) 15 (3–59) .09 Narcotics:
Ever receiving narcotics in ICU, proportion of patients 96% 77% .05 Daily morphine-equivalent dose, median (IQR) mg 71 (30–180) 24 (3–120) .01 Delirium Not delirious (CAM-ICU negative), proportion of ICU
days
21% 53% .003
Ever receiving haloperidol in ICU, proportion of patients 26% 7% .05 Daily haloperidol dose, median (IQR) mg 4 (2, 6) 2 (1, 2) .15 Perceived pain Pain scale (range 0–10), mean (SD) of daily scores 0.6 (1.9) 0.6 (1.7) .79 Physiological instability Among all of rehabilitation treatments: n=50 n=294
Change in heart rate (baseline to maximum during therapy), median (IQR) beats per minb
6 (2 to 10) 9 (2 to 16) .10 Change in systolic blood pressure (baseline to end of
therapy), median (IQR) mmHgb
3 (-4 to 13) 2 (-6 to 8) .40 Change in oxygen saturation (baseline to lowest during
therapy), median (IQR)b
-1 (-2 to 0) -2 (-3 to 0) .07 Safety Unexpected eventsc, proportion of rehabilitation
treatments
0% 1% >.99
Benefi ts Functional mobility: proportion of treatments with sitting at the edge of bed or greater
56% 78% .03
Among all MICU admissions: n=262 n=314
MICU average length of stay, days 7.0 4.9 .02
Hospital average length of stay, days 17.2 14.1 .03 In-hospital mortality, proportion of admissions 23.3% 21.0% .55
Note: CAM = confusion assessment method; ICU = medical intensive care unit; IQR = interquartile range; OT = occupational therapist; PT = physical therapist; RASS = Richmond Agitation and Sedation Scale, SD= standard deviation.
aMeasures for both the pre-QI and QI periods and the P values obtained from a published report of the Johns Hopkins medical intensive care
unit quality improvement project.19
bSample size ranges from 46 to 49 and 258 to 277 treatments for pre-QI and QI period, respectively, because of missing data.
trip to a hospital with an established ICU early rehabilitation program was undertaken by one MD, RN, and PT. Each clinician then formally and informally shared their learning experience with others in their discipline after their return. This trip provided the chance for a member from each clinician group to actually see the rehabilitation process and to speak with experienced peers.
Execute
Execution of the QI project required specifi c interventions to overcome the identifi ed barriers. General approaches to successfully addressing barriers included (a) standardizing care, (b) using independent checks and reminders, (c) maximizing convenience and simplicity, and (d) learning from situations in which there were problems with implementation.17 Table 1 describes some specifi c
interventions used to address barriers identifi ed in our QI project.
This approach resulted in the delivery of 294 PT and OT treatments for 30 patients during their MICU stay. These treatments involved the following specifi c mobilization activities: moving from supine to sit (72% of treatments), sitting at the edge of bed (77%), transfer from bed to chair (38%), transfer from sitting to standing (49%), and ambulation (13%). These mobilization activities were performed by a PT or OT with the assistance of an RN or technician, as needed. In general, RNs did not routinely initiate any of these activities in mechanically ventilated patients. To ensure safety, all patients were screened, on a daily basis, by PT and OT before initiating any mobilization activities as described in Figure 2.
Evaluate
Via weekly multidisciplinary meetings, problems arising during the QI intervention were discussed and resolved on a timely basis. A summary of key results from the QI project are presented in Table 2. These results indicate what was achieved during a 4-month QI period compared to either a 3-month period immediately prior to the QI period or to the same 4-month
Engage
For education to be benefi cial and clinical practice to change, it was key that all stakeholders (from leaders and supervisors to frontline clinical staff) were engaged in the QI project and understood why the interventions were important. Engagement for our QI project occurred via many routes, including (a) sharing patient anecdotes, (b) having patients return to the MICU to share their stories of diffi culties encountered during the recovery process (eg, muscle weakness, physical impairment), (c) providing local data regarding MICU performance compared to peer hospitals, and (d) having guest speakers from another hospital experienced in ICU early mobility10,12 discuss their approach
with our staff.
Educate
For busy clinicians, education must occur in an easy-to-access manner. Full research papers are often not readily accessible to clinicians. Hence, brief summaries of important research for educating clinicians were disseminated via a MICU newsletter, posters, bulletin boards, and invited speakers giving brief presentations. In addition, assessment of the local barriers for our QI project (step 2) revealed some specific educational barriers that required intervention during the QI implementation. For example, PTs received training on basic EKG interpretation and general orientation to ventilator modes, settings, and alarms in preparation for ambulating mechanically ventilated patients. RTs provided specific orientation to PTs regarding the portable ventilator (iVent201; VersaMed GE Healthcare, Piscataway, NJ) used for ambulation of mechanically ventilated patients. PTs trained RNs regarding transfer techniques and use of wheelchairs for seating patients out of bed. To educate RNs regarding the necessary changes in sedation and the rehabilitation interventions by PT and OT, 16 small group meetings were held by the MICU physician leaders. Two large group training sessions were held with RTs, and 5 presentations were given to the MICU physicians. In addition, a 2-day educational
Attempt to decrease sedation via: - interrupting continuous infusions - changing from continuous infusion to “as needed” bolus doses - using anti-psychotic medication for treatment of hyperactive delirium
Neurologic criteria: Opens eyes to verbal stimulation
Yes No Primary CNS etiology Sedation-related medication Reassess tomorrow Other criteria:
Hypoxemia: Pulse oximetry <88%, Tachypnea: Respiratory rate >45 bpm, Acidosis: Arterial pH <7.25,
Hypotension: Mean arterial pressure <55 mmHg, Hypertension: Mean arterial pressure >140 mmHg, or New deep venous thrombosis
Carefully initiate activity
Discuss with ICU physician regarding appropriateness for therapy Carefully evaluate appropriateness of therapy Respiratory criteria: FiO2 > 0.6 PEEP > 10 cm H2O Circulatory criteria:
New or increased vasopressor dose within past 2 hrs Continuous infusion of a vasodilator medication Addition of a new anti-arrhythmic agent New cardiac ischemia
Consult therapist for rehabilitation
No Yes to 1 or
more
No Yes
Figure 2. A screening algorithm for patient mobilization in the intensive care unit. CNS = central nervous system; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; PEEP = positive end expiratory pressure. Reproduced, with permission, from Korupolu R, Gifford JM, Needham DM. Early mobilization of critically ill patients: reducing neuromuscular complications after intensive care. Contemp Crit Care. 2009; 6(9):1–12. Copyright 2009 Lippincott Williams & Wilkins.
demonstrate that the extensive planning was successful in changing culture to make early PM&R in the MICU feasible, safe, and benefi cial to patients.
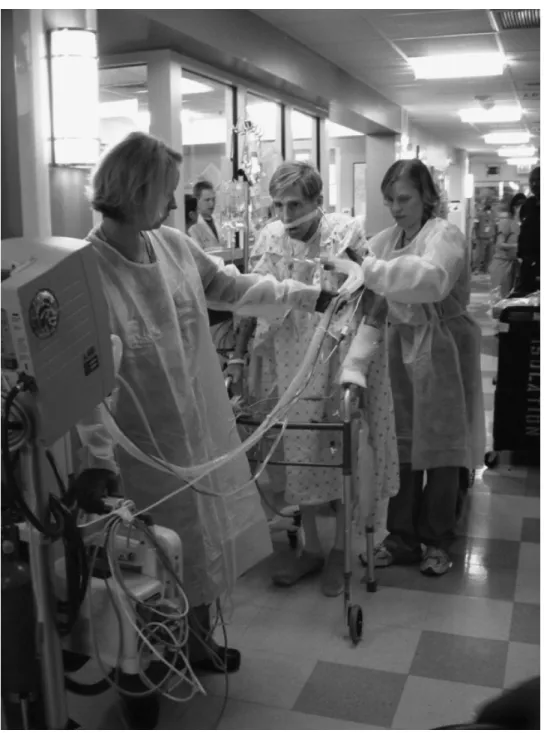
period in the prior year. A photograph illustrating successful ambulation of a mechanically ventilated patient in the MICU is provided in Figure 3. The magnitude of these improvements helped
Figure 3. Early mobilization of a mechanically ventilated patient in the Johns Hopkins Hospital medical intensive care unit (MICU). Photograph of a 56-year-old man with severe chronic obstructive pulmonary disease and acute renal failure who is ambulating 4 days after admission to the Johns Hopkins Hospital MICU. He is ambulating while receiving mechanical ventilation via an oral endotracheal tube with a Physical Therapist supporting the patient from behind and a Respiratory Therapist in front with a portable mechanical ventilator.
Reproduced, with permission, from Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. J Am Med Assoc. 2008;300:1685–1690 Copyright © 2008 American Medical Association. All rights reserved.
available online39), new biomedical engineering
devices to assist with ambulation of mechanically ventilated patients (video available online40), and
a randomized controlled trial of neuromuscular electrical stimulation therapy.41
Conclusion
Stroke rehabilitation involves a diverse set of professional skills delivered in a team approach.42
Delivery of coordinated, patient outcome-oriented, intensive physical medicine and rehabilitation therapies to achieve maximum functional improvement is key for successful stroke rehabilitation.
Efforts to introduce similar early PM&R interventions in the MICU at Johns Hopkins Hospital involved many challenges that may help inform QI efforts in the stroke rehabilitation setting. In our experience, these challenges were overcome using a structured QI process. Our QI project resulted in a reduction in deep sedation and increased early PM&R activities for mechanically ventilated patients. Through these changes, substantial improvement was observed in the prevalence of ICU delirium and in patients’ functional mobility in the ICU, with a substantial decrease in MICU and hospital length of stay. The positive results of our QI project were sustained after its conclusion and resulted in a hospital-funded program for early PM&R and stimulated changes in other ICUs within our institution and at our peer hospitals.
Acknowledgments
All members of the multidisciplinary team involved in the Johns Hopkins Hospital Medical Intensive Care Unit quality improvement project provided important contributions to the early mobility program. The team members were Roy Brower, MD; Nancy Ciesla, PT; Victor Dinglas, BS; Eddy Fan, MD; Kenroy Greenidge, OT; Aline Hauber, OT; Kashif Janjua, MD; Landon King, MD; Chris Moghimi, OT; David Pitts, MD; Jeffrey B. Palmer, MD; Pranoti Pradhan, MBBS, MPH; Maggie Price, OT; Didi Rosell-Missler, RN; Jessica Rossi, PT, DPT; Janette Scardillo, PT; Edwin Szetela, OT; Lauren Waleryszak, RN; Mohommad Yavari Rad, MD; and Jennifer Zanni, PT, MSPT. Follow-up From the QI Project
Based on our rigorous evaluation of the QI project and the substantial decrease in MICU and hospital length of stay (Table 2), the hospital funded a comprehensive Critical Care Physical Medicine and Rehabilitation program after completion of the QI project. This program started July 1, 2008, and provided funding for the following staff dedicated to early PM&R in the MICU: 2 full-time PTs who provide services 6 days per week, 1 full-time technician to assist the PTs 5 days per week, 1 full-time clinical coordinator (R.K.), and 1 full-time assistant to the coordinator. Given a strong focus on early mobility and the availability and skills of existing rehabilitation staff within the hospital, the staffi ng pattern was changed from 1 OT and 1 PT during the QI project to 2 PTs for this new hospital-funded program. The technician assisting with early PM&R also changed from a part-time to a full-time member of the team. The program includes support for MICU physicians to continue efforts to sustain and improve the culture change, including a MICU physician to serve as Medical Director for the multidisciplinary Critical Care Physical Medicine and Rehabilitation program (D.M.N.). The program continues to collect data on performance measures regarding both PM&R activities and length of stay for both clinicians and hospital administrators (Table 2).
To further improve the early PM&R program, a new sedation protocol was created that formalized the approach undertaken during the QI project. In addition, delirium assessment became part of routine practice for MICU RNs and a delirium quality improvement project was undertaken. These quality improvements have also spread to 4 other ICUs at Johns Hopkins Hospitals using the foundation created from the MICU QI project. Building on this success, a new QI project aimed at improving the quality of patients’ sleep in the MICU, in order to improve patients’ cognition and participation with early PM&R activities, has been started. There is growing enthusiasm among many of the multidisciplinary clinicians working in the MICU to suggest quality improvements for other aspects of clinical care. Early PM&R is also being continuously refi ned, such as with the introduction of cycle ergometry (video
REFERENCES
1. Dowdy DW, Eid MP, Dennison CR, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. 2. Needham DM. Mobilizing patients in the intensive
care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690. 3. Herridge MS, Cheung AM, Tansey CM et al.
One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348: 683–693.
4. Stevens RD, Dowdy DW, Michaels RK, Mendez-Te l l e z PA , P r o n o v o s t P J , N e e d h a m D M . Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med.
2007;33:1876–1891.
5. Hu MH, Hsu SS, Yip PK, Jeng JS, Wang YH. Early and intensive rehabilitation predicts good functional outcomes in patients admitted to the stroke intensive care unit. Disabil Rehabil. 2010. 6. Korupolu R, Gifford J, Needham DM. Early
mobilization of critically ill patients: reducing neuromuscular complications after intensive care.
Contemp Crit Care 2009;6:1–12.
7. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373: 1874–1882.
8. Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med. 2007;35:393–401.
9. Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable.
Intensive Care Med. 2003;29:368–377.
10. Hopkins RO, Spuhler VJ, Thomsen GE. Transforming ICU culture to facilitate early mobility. Crit Care Clin.
2007;23:81–96.
11. Zanni JM, Korupolu R, Fan E et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care.
2010;25(2):254–262.
12. Bailey P, Thomsen GE, Spuhler VJ et al. Early activity is feasible and safe in respiratory failure patients.
Crit Care Med. 2007;35:139–145.
13. Morris PE, Goad A, Thompson C. et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238– 2243.
14. Gosselink R, Bott J, Johnson M, et al. Physiotherapy for adult patients with critical illness: recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients. Intensive Care Med. 2008;34:1188–1199.
15. Needham DM, Dennison CR, Dowdy DW, et al. Study protocol: The Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2005;10:R9 http://ccforum.com/content/10/1/R9.
16. Needham DM, Wang W, Desai SV, et al. Intensive care unit exposures for long-term outcomes
research: development and description of exposures for 150 patients with acute lung injury.
J Crit Care. 2007;22:275–284.
17. Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. 18. Needham DM. Patient safety, quality of care, and
knowledge translation in the ICU. Respir Care.
2010;50:922–928.
19. Needham DM, Korupolu R, Zanni JM et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–543. 20. Thomsen GE, Snow GL, Rodriguez L, Hopkins
RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med.
2008;36:1119–1124.
21. Stiller K. Safety issues that should be considered when mobilizing critically ill patients. Crit Care Clin.
2007;23:35–53.
22. Stiller K, Phillips AC, Lambert P. The safety of mobilisation and its effects on haemodynamic and respirator y status of intensive care patients. Physiotherapy Theory Pract. 2004;20: 175–185.
23. Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther.
2006;86:1271–1281.
24. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627-E633.
25. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297: 1772–1774.
26. Winkelman C. Inactivity and inflammation in the critically ill patient. Crit Care Clin.
2007;23:21–34.
27. Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol.
2007;27:2650–2656.
28. Fortney SM, Schneider VS, Greenleaf JE. The physiology of bed rest. In: Fregly MJ, Blatteis CM, eds. Handbook of Physiology. New York: Oxford University Press; 1996;889–939.
29. Convertino VA, Bloomfi eld SA, Greenleaf JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
30. Hung J, Goldwater D, Convertino VA, McKillop JH, Goris ML, DeBusk RF. Mechanisms for decreased exercise capacity after bed rest in normal middle-aged men. Am J Cardiol. 1983;51:344–348. 31. Nava S. Rehabilitation of patients admitted to
a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
32. Make B, Gilmartin M, Brody JS, Snider GL. Rehabilitation of ventilator-dependent subjects with lung diseases. The concept and initial experience. Chest. 1984;86:358–365.
33. Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation.
Crit Care Med. 2005;33:2259–2265.
34. Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997;82:182–188.
35. Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135: 1824S-1828S.
36. Morris PE. Moving our critically ill patients: mobility barriers and benefi ts. Crit Care Clin. 2007; 23:1–20. 37. Gurses AP, Murphy DJ, Martinez EA, Berenholtz
SM, Pronovost PJ. A practical tool to identify and eliminate barriers to compliance with evidence-based guidelines. Jt Comm J Qual Patient Saf.
2009;35:526–32, 485.
38. Zorowitz R. Stroke rehabilitation quality indicators: raising the bar in the inpatient rehabilitation facility. Top Stroke Rehabil. 2010;17(4):294–304.
39. Johns Hopkins Medicine. Research update: ICU early exercise. http://www.youtube.com/watch? v=n9QMqcYpbbw&feature=channel_page. Accessed August 20, 2010.
40. American Institute of Physics. Discoveries and breakthroughs: inside science. Moving in the ICU. http://www.aip.org/dbis/APS/stories/18110.html. Accessed August 20, 2010.
41. Needham DM, Truong AD, Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37:S436–S441. 42. Stevens AB, Strasser DC, Uomoto J, Bowen SE,
Falconer JA. Utility of treatment implementation methods in clinical trial with rehabilitation teams. J Rehabil Res Dev. 2007;44:537–546. 43. Ely EW, Truman B, Shintani A, et al. Monitoring
sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983–2991.
44. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA.