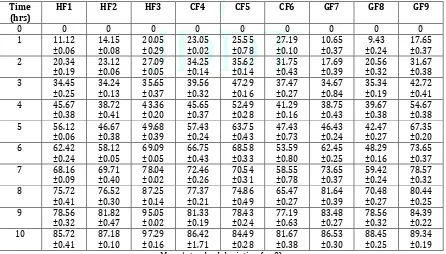
Formulation and evaluation sustained release mucoadhesive gastroretentive pantoprazole sodium sesquihydrate tablets for anti–ulcer
Full text
Figure




Related documents
The three treated samples were allowed to respire for one hour in an M/300 solution of cyanide in M/30 phosphate, when their average oxygen uptake was 8 /¿l./gm./hr., giving an
Figure 6: Activity diagram showing control flow of login system and authentication method.. Figure 7: Activity diagram showing control flow of insurance value
To the best of our knowledge, this study is the first case series in the literature, reporting successful clinical re- sults with the use of an alternative focal, anatomic,
ITACA, working together with the Italian Hereditary Angioedema Patients ’ Association, col- lected data from C1-INH-HAE patients referring to 17 centers active in Italy.. Here
In this partitioning, the transaction logs and the data access patterns are analyzed (that is, which warehouse is more probable to supply to particular warehouse). This analysis
At approximate- ly 1 year of age, health status of almost all infants was determined by physical examination, Denver Development Test, and laboratory tests and by record review
IV. has extensively regulated privacy and government without Congress finding the need to create a privacy czar to centralize and administer the process. Implementation
CDAI: Clinical disease activity index; CI: Confidence interval; CRP: C-reactive protein; csDMARDs: Conventional synthetic disease modifying anti-rheumatic drugs; DAS28: Disease