Neuroimaging of Intraparenchymal Lesions Predicts
Outcome in Shaken Baby Syndrome
Christine Bonnier, MD*; Marie-Ce´cile Nassogne, MD, PhD*; Christine Saint-Martin, MD‡; Bettina Mesples, MD§; Hazim Kadhim, MD, PhD*储; and Guillaume Se´bire, MD, PhD*
ABSTRACT. Objective. Studies of long-term outcome on nonaccidental head injury (NAHI) in young children have shown severe neurodevelopmental sequelae in most cases. For improving the knowledge of outcome and for identifying prognostic factors, additional clinical and cerebral imaging data are needed. The aim of this study was to describe clinical and imaging features over time and to consider their value for predicting neurodevelop-mental outcome.
Methods. A retrospective medical record review was conducted of 23 children with confirmed NAHI, for whom an extended follow-up of 2.5 to 13 years (mean: 6 years) was contemplated. Glasgow Coma Scale scores, severity of retinal hemorrhages, presence of skull frac-tures, cranial growth deceleration, and sequential neuro-imaging data (computed tomography and/or magnetic resonance imaging) were compared with patterns of clin-ical evolution assessed by the Glasgow Outcome Scale.
Results. Clinical outcome showed that 14 (61%) chil-dren had severe disabilities, 8 (35%) had moderate dis-abilities, and 1 (4%) was normal. A low initial Glasgow Coma Scale score, severe retinal hemorrhages, presence of skull fracture, and cranial growth deceleration were significantly associated with poor developmental out-come. Eighteen of the 23 patients had abnormal magnetic resonance imaging scans. This examination disclosed at-rophy when performed beyond 15 days of injury. Atro-phy seemingly resulted from various brain lesions, namely, contusions, infarcts, and other lesions within the white matter. Presence of intraparenchymal brain lesions within the first 3 months was significantly associated with neurodevelopmental impairment. Severity of motor and cognitive dysfunctions was related to the extent of intraparenchymal lesions.
Conclusions. Early clinical and radiologic findings in NAHI are of prognostic value for neurodevelopmental outcome.Pediatrics2003;112:808 – 814;nonaccidental head injury, traumatic brain injury, child abuse, shaken-infant syndrome, neuroimaging, outcome.
ABBREVIATIONS. NAHI, nonaccidental head injury; SBS, shak-en-baby syndrome; CT, computed tomography; MRI, magnetic resonance imaging; GCS, Glasgow Coma Scale; GOS, Glasgow
Outcome Scale; GR, good recovery; MD, moderate disability; SD, severe disability; PVS, persistent vegetative state.
N
onaccidental head injury (NAHI) includes 1 or more of the following: shaking injury, le-sions as a result of direct impact, compres-sion, and penetrating injuries.1 The most frequentform of NAHI, the so-called shaken baby syndrome (SBS), occurs during the first year of life.2 Death
occurs in 10% to 40% of patients, and most survivors have poor neurologic outcome.3–10 Neurologic
se-quelae include cognitive and behavioral distur-bances, cerebral palsy, blindness, and epilepsy.11–14
Factors of adverse prognostic significance identified to date include ophthalmologic symptoms,7,15–17
sei-zures early after injury,18 high intracranial
pres-sure,19 cranial growth impairment,11,12 and major
neuroradiologic abnormalities such as the “big black brain,” indicating severe edema on computed to-mography (CT) scans.20 Recent prospective studies
established a correlation between neuroimaging and short-term outcome.21,22To our knowledge, there are
no studies correlating the long-term neurodevelop-mental outcome with intraparenchymal lesions in-vestigated by combined CT and magnetic resonance imaging (MRI) of the brain. Thus, the aim of this study was to correlate both early (see “Patients and Methods”) clinical and radiologic findings during the first 3 months after injury, with long-term neu-rodevelopmental outcome in children with a history of NAHI.
PATIENTS AND METHODS Study Population
The medical charts of all patients who were admitted between 1985 and 1998 (n ⫽ 25) to Cliniques Universitaires Saint-Luc in Brussels, Belgium, with a diagnosis of NAHI were reviewed retrospectively. The “Child Protection Team” in our hospital es-tablished the diagnosis in each patient according to Duhaime criteria.4These include acute encephalopathy with subdural
hem-orrhages, cerebral edema, retinal hemhem-orrhages, and fractures, in the context of an inappropriate or inconsistent history, commonly with additional evidence of other malicious injuries. Two infants died during the acute period. Brain imaging was not available for 1 of them; these 2 patients were not included in our study because the goal was to correlate imaging with long-term developmental evolution. The remaining 23 patients underwent the full follow-up procedure described in “Outcome Assessment.” Table 1 summa-rizes the clinical and radiologic findings at the acute phase and the long-term outcome in the 23 patients. Mean age at injury was 4.2 months (range: 3 weeks to 13 months). The male-to-female ratio was 3:1. To improve the sensitivity of our study for detecting long-term sequelae, we included only patients who were 3 years
From the *Service de Neurologie Pe´diatrique, ‡De´partement de Radiologie, Cliniques Universitaires Saint-Luc, Universite´ Catholique de Louvain, Brus-sels, Belgium; §Service de Pe´diatrie Ge´ne´rale, Hopital Robert-Debre´, Faculte´ Xavier-Bichat, Universite´ Paris VII, Paris, France; and储Service de Neuropa-thologie, CHU Brugmann, ULB, Brussels, Belgium.
Received for publication Mar 29, 2002; accepted Feb 12, 2003.
of age or older at the last evaluation. Mean follow-up duration was 6 years (range: 2.5–13 years). The severity of injury was determined using the Glasgow Coma Scale (GCS)23and the
du-ration of impaired consciousness, adapted for infants by Ewing-Cobbs.21,22,24When 2 different GCS scores were available during
the first 24 hours, the lowest one was considered. The initial GCS was always performed in nonsedated patients. In alignment with other studies of brain injury in young children,24we separated our
patients into 2 groups: 1) patients with GCS 9 to 15 with conscious-ness impairment for⬍24 hours and 2) patients with GCS 3 to 8 for at least 6 hours or consciousness impairment for⬎24 hours. All children underwent skeletal radiographs and optic funduscopy within the first 3 days after admission.
Neuroradiologic Assessment
All children had “initial” (ie, within 2 days after admission) clinical and paraclinical investigations. These included at least 1 CT scan. “Early” and “late” imaging (CT and MRI) studies were defined as studies done before and after 3 months, respectively. Early MRI of the brain (T1- and weighted axial, T1- or T2-weighted coronal and sagittal sections) was done in 12 patients, and late MRI in was done 20 patients. Six of 7 infants (patients 3, 5, 7, 13, 15, 19, and 23) each had 1 MRI during the first 15 days; in patient 15, MRI was repeated (days 2 and 4). One MRI was performed in each of the 9 children (patients 3, 4, 7, 9, 16, 17, 19, 21, and 23) between days 15 and 90. All images were reviewed by an independent investigator who was unaware of clinical out-come. “Diffuse” lesions were defined as extensive lesions on both sides of the brain affecting at least 3 lobes. We described as “infarcts” lesions presenting the following criteria: 1) MRI show-ing brain parenchymal signal abnormalities correspondshow-ing to isch-emic lesions and 2) a topography strictly localized to an arterial territory.25
Outcome Assessment
The Glasgow Outcome Scale (GOS),26adapted for infants by
Ewing-Cobbs, was used to assess the overall developmental out-come at last evaluation.21,22 In this score, good recovery (GR)
refers to a return to age-appropriate or preinjury levels of func-tioning; moderate disability (MD) is assigned if the child 1) has a significant reduction in cognitive functioning from estimated pre-morbid levels, 2) has motor deficits including hemiparesis
inter-fering with daily living activities, or 3) is referred for outpatient rehabilitation therapies. Severe disability (SD) is assigned when 1) cognitive scores are in the deficient range; 2) severe motor deficits are present, such as lack of appropriate postural control or ambu-lation; or 3) the child is referred for inpatient rehabilitation. Per-sistent vegetative state (PVS) is defined as total dependence.21,22
TheWechsler Preschool and Primary Scale of Intelligence27and the
Kaufman Assessment Battery for Children28 developmental scales
were used in children aged 3 to 6 years at the time of evaluation, and theWechsler Intelligence Scale for Children-Revised29 and the
Kaufman Assessment Battery for Childrenscales were used in older children.
Statistical Analysis
Student’sttests for unequal variances were used to compare the means of GOS scores (the 4 GOS categories, namely, GR, MD, SD, and PVS, were scored 80, 60, 40, and 20, respectively).
RESULTS
Correlations Between Initial Clinical Findings and Outcome
Fourteen (61%) of the 23 patients evaluated at a minimum age of 3 years had a poor neurodevelop-mental outcome (SD, PVS), 8 (35%) had MD, and 1 (4%) was normal (GR). Thirteen (78%) of the 18 chil-dren with low initial GCS scores (⬍9) had SD or PVS, as compared with only 1 of 5 children with higher initial GCS scores (P⫽0.04). Of the 20 children (20 of 23 [87%]) who had bilateral retinal hemorrhage ini-tially, 12 had retinal detachment or vitreous hemor-rhage; 9 of these 12 had poor outcome (SD or PVS), as compared with 5 of the 11 patients with less severe or no ocular damage (P⫽ .04). Of the 7 children with skull fractures, 6 had SD or PVS, and outcome was significantly worse among the children with skull fractures (P⫽.01).Twelve of the 14 children with a slowing in head circumference growth (⬎2 standard
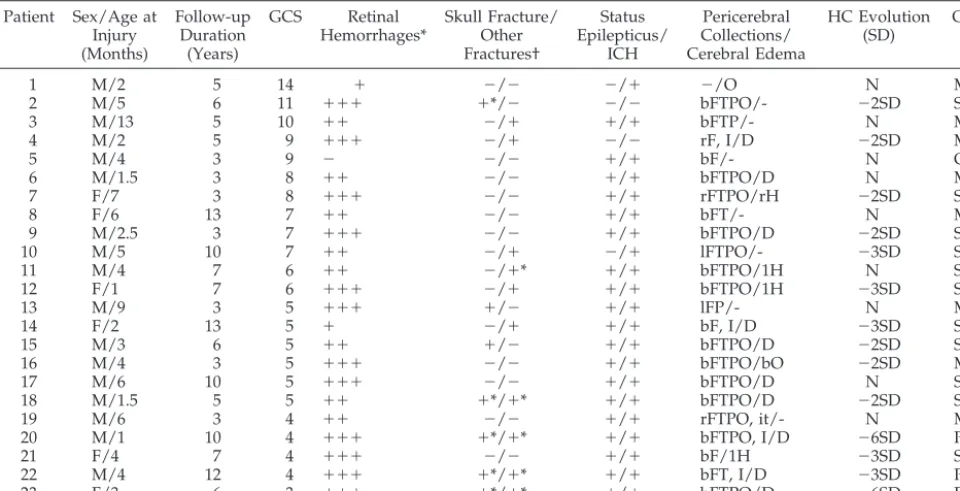
TABLE 1. Comparison Between “Initial” Clinical and Radiologic Data With Long-Term Outcome Patient Sex/Age at
Injury (Months)
Follow-up Duration
(Years)
GCS Retinal Hemorrhages*
Skull Fracture/ Other Fractures†
Status Epilepticus/
ICH
Pericerebral Collections/ Cerebral Edema
HC Evolution (SD)
GOS
1 M/2 5 14 ⫹ ⫺/⫺ ⫺/⫹ ⫺/O N MD
2 M/5 6 11 ⫹⫹⫹ ⫹*/⫺ ⫺/⫺ bFTPO/- ⫺2SD SD
3 M/13 5 10 ⫹⫹ ⫺/⫹ ⫹/⫹ bFTP/- N MD
4 M/2 5 9 ⫹⫹⫹ ⫺/⫹ ⫺/⫺ rF, I/D ⫺2SD MD
5 M/4 3 9 ⫺ ⫺/⫺ ⫹/⫹ bF/- N GR
6 M/1.5 3 8 ⫹⫹ ⫺/⫺ ⫹/⫹ bFTPO/D N MD
7 F/7 3 8 ⫹⫹⫹ ⫺/⫺ ⫹/⫹ rFTPO/rH ⫺2SD SD
8 F/6 13 7 ⫹⫹ ⫺/⫺ ⫹/⫹ bFT/- N MD
9 M/2.5 3 7 ⫹⫹⫹ ⫺/⫺ ⫹/⫹ bFTPO/D ⫺2SD SD
10 M/5 10 7 ⫹⫹ ⫺/⫹ ⫺/⫹ lFTPO/- ⫺3SD SD
11 M/4 7 6 ⫹⫹ ⫺/⫹* ⫹/⫹ bFTPO/1H N SD
12 F/1 7 6 ⫹⫹⫹ ⫺/⫹ ⫹/⫹ bFTPO/1H ⫺3SD SD
13 M/9 3 5 ⫹⫹⫹ ⫹/⫺ ⫹/⫹ lFP/- N MD
14 F/2 13 5 ⫹ ⫺/⫹ ⫹/⫹ bF, I/D ⫺3SD SD
15 M/3 6 5 ⫹⫹ ⫹/⫺ ⫹/⫹ bFTPO/D ⫺2SD SD
16 M/4 3 5 ⫹⫹⫹ ⫺/⫺ ⫹/⫹ bFTPO/bO ⫺2SD MD
17 M/6 10 5 ⫹⫹⫹ ⫺/⫺ ⫹/⫹ bFTPO/D N SD
18 M/1.5 5 5 ⫹⫹ ⫹*/⫹* ⫹/⫹ bFTPO/D ⫺2SD SD
19 M/6 3 4 ⫹⫹ ⫺/⫺ ⫹/⫹ rFTPO, it/- N MD
20 M/1 10 4 ⫹⫹⫹ ⫹*/⫹* ⫹/⫹ bFTPO, I/D ⫺6SD PVS
21 F/4 7 4 ⫹⫹⫹ ⫺/⫺ ⫹/⫹ bF/1H ⫺3SD SD
22 M/4 12 4 ⫹⫹⫹ ⫹*/⫹* ⫹/⫹ bFT, I/D ⫺3SD PVS
23 F/3 6 3 ⫹⫹⫹ ⫹*/⫹* ⫹/⫹ bFTPO/D ⫺6SD PVS
ICH indicates intracranial hypertension; F, frontal; T, temporal; P, parietal; O, occipital; I, interhemispheric; b, bilateral; r, right; l, left; it, infratentorial; D, diffuse; H, hemispheric; HC: head circumference; N, normal; SD, standard deviation.
deviations) had SD or PVS, and there was a signifi-cant difference in outcome between children with head growth slowing and those with unaffected head growth (P⫽ .003; Tables 1 and 2).
Correlations Between “Early” (Within the First 3 Months After Injury) Neuroradiologic Findings and Outcome
The initial CT scan showed subdural or subarach-noidal hemorrhage in all but 1 of the children (22 [96%] of 23). The hemorrhages were bilateral in 17 children, unilateral in 5, and interhemispheric in 4. Sixteen of the 23 patients had parenchymal edema; the edema was confined to 1 lobe in 1 patient (patient 1), to a vascular territory in 1 patient (patient 16), and to 1 hemisphere in 4 children (patients 7, 11, 12, and 21); the 10 remaining patients had bilateral parenchy-mal edema. Two of the 16 patients had intraparen-chymal hematoma (patients 15 and 18). MRI was performed between day 1 and day 15 (mean: day 4) in 7 patients (patients 3, 5, 7, 13, 15, 19, and 23). MRI provided no additional information as compared with CT during the first 15 days, except in patient 19, who had lesions on the MRI (performed at day 1) that were not detected by the initial CT scan on day 0. These lesions consisted of a subdural hematoma in the posterior fossa and a focal lesion in the corpus callosum. In contrast, in all 9 patients who had an MRI between day 15 and day 90 (mean: day 33), this investigation provided additional information on in-traparenchymal lesions as compared with the initial CT scans. Three types of intraparenchymal lesions were seen on MRI done during this period: 1) lobar atrophy of the gray and white matter suggesting residual postcontusion damage (patient 4); 2) arterial infarcts (n ⫽ 4) either at a single site (anterior cere-bral artery, patient 19) or at multiple sites (posterior cerebral arteries, patient 16; carotid artery, patients 7
and 21); and 3) white matter lesions suggesting gli-otic scars (n⫽7), with predominant involvement of 1 hemisphere in 2 patients (patients 7 and 21) and bilateral involvement in 5 patients (patients 9, 16, 17, 19, and 23). The atrophic areas and white matter scars exactly matched the sites of edema seen on the initial CT scans (Tables 1 and 2). Comparison of patients with and without intraparenchymal lesions (Table 2) showed that detection of such lesions by MRI and/or CT during the first 3 months was sig-nificantly associated with severe developmental out-come (P ⫽ .04), particularly when the lesions were diffuse (P⫽.025).
Correlation Between “Late” (>3 Months After Injury) Neuroradiologic Findings and Outcome
Twenty-one children had neuroimaging studies (MRI in 20 patients and CT in 1) ⬎3 months after injury (range: 3 months to 13 years; mean: 5.6 years). Chronic subdural hygroma was found in 4 of these children (patients 9, 15, 17, and 21). Sixteen of the 21 patients displayed parenchymal lesions that were also detectable on the initial CT scans or early MRI. The remaining 5 children (patients 3, 5, 8, 10, and 13) had normal initial and late cerebral imaging. Ten children (patients 3, 5, 7, 9, 13, 15, 16, 19, 21, and 23) had both early (before 3 months) and late (after 3 months) MRI. Comparison of these 2 time points showed that the 3 types of intraparenchymal lesions detected on early MRI scans persisted on late MRI and consisted of the following: 1) atrophy involving temporal and parieto-occipital lobes (patients 4 and 12) indicating residual damage after contusion by direct impact or “contre-coup” injury; 2) arterial in-farcts detected in 9 patients, either at a single site (middle and anterior cerebral arteries in patients 11 and 19, respectively) or at multiple sites (anterior
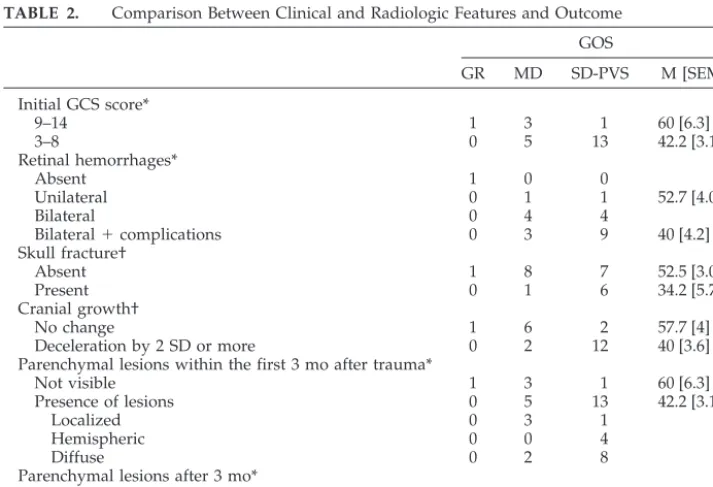
TABLE 2. Comparison Between Clinical and Radiologic Features and Outcome GOS
GR MD SD-PVS M [SEM]
Initial GCS score*
9–14 1 3 1 60 [6.3]
3–8 0 5 13 42.2 [3.1]
Retinal hemorrhages*
Absent 1 0 0
Unilateral 0 1 1 52.7 [4.06]
Bilateral 0 4 4
Bilateral⫹complications 0 3 9 40 [4.2]
Skull fracture†
Absent 1 8 7 52.5 [3.09]
Present 0 1 6 34.2 [5.7]
Cranial growth†
No change 1 6 2 57.7 [4]
Deceleration by 2 SD or more 0 2 12 40 [3.6]
Parenchymal lesions within the first 3 mo after trauma*
Not visible 1 3 1 60 [6.3]
Presence of lesions 0 5 13 42.2 [3.1]
Localized 0 3 1
Hemispheric 0 0 4
Diffuse 0 2 8
Parenchymal lesions after 3 mo*
Not visible 1 3 1 60 [6.3]
Presence of lesions 0 3 13 40 [3.1]
M indicates mean; SEM, standard error of the mean. *P⬎.01⬍.04.
cerebral arteries, patient 18; posterior cerebral arter-ies, patient 16; carotid artery, patients 7 and 21; other multifocal patterns, patients 20, 22, and 23); and 3) subcortical gliotic scars in the cerebral white matter detected in 14 patients, usually in both hemispheres (13 of 14 patients), with no preferential distribution pattern. Eleven patients had focal (n⫽ 3) or diffuse (n ⫽ 8) lesions of the corpus callosum; 4 of these patients (patients 16, 20, 22, and 23) also had lesions in the cerebellar hemispheric white matter. Finally, comparison of patients with and without intraparen-chymal lesions visualized on MRI performed ⬎3 months after injury showed that the presence of such lesions was significantly associated with a poor neu-rodevelopmental outcome (P⫽.02).
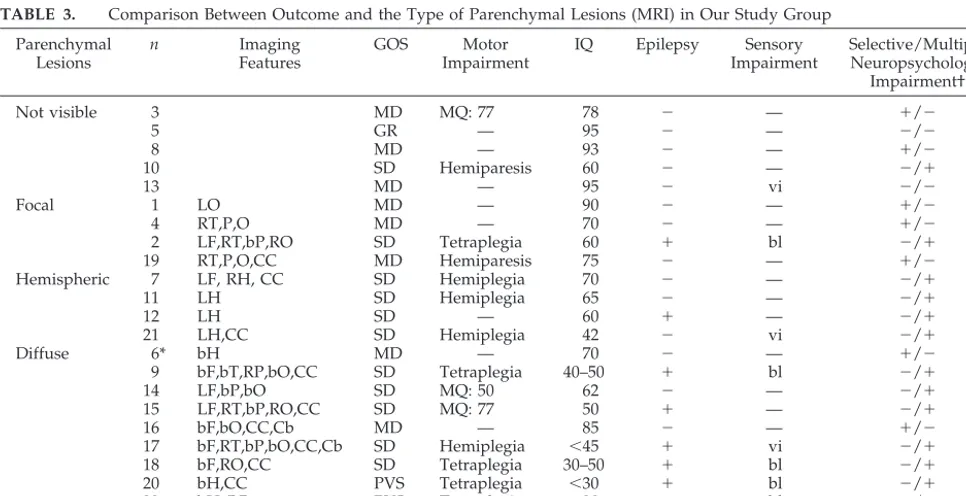
Recovery seemed to be related to the extent of lesions seen by late MRI (Tables 2 and 3). Patients (n⫽10) with diffuse lesions had more severe motor and intellectual impairments and were more likely to have blindness and epilepsy than patients with focal (n⫽4) or hemispheric (n⫽4) lesions. Seven of the 9 patients (Table 3) who had epilepsy belong to the subgroup of “diffuse lesions.” Of these 7 patients with epilepsy, 2 had West syndrome and 5 had poly-morphic seizures. In these patients, epilepsy started immediately after injury in 3 patients and within the first 2 years in 4. Seizures were refractory to treat-ment in 6 of the 7 patients. Among the patients who had no visible lesions on imaging studies (n⫽ 5), 4 had residual disabilities, namely, hemiparesis and mental deficiency (patient 10), visual sensory defect (patient 13), or visuospatial impairment and atten-tion deficit (patients 3 and 8).
DISCUSSION
Results from our series of patients with NAHI underline the prognostic value of bilateral retinal and vitreous hemorrhages, initial low GCS score, presence of skull fractures, cranial growth decelera-tion, and intraparenchymal brain abnormalities de-tected by neuroimaging during the first 3 months. These clinical and radiologic findings were signifi-cantly associated with poor long-term neurodevelop-mental outcome.
After 3 years, 96% of our patients had disabilities, which were severe (61%) or moderate (35%). This is in agreement with the main reported series13,14,24 in
which 45% to 69% of children had poor outcome. Bilateral retinal and vitreous hemorrhage were asso-ciated in our series with poor neurodevelopmental outcome as that reported in earlier studies.7,16,17The
depth and duration of coma have been shown to predict the outcome of brain injury in older chil-dren.30 This was also the case in our younger
pa-tients. This point is also in agreement with a recent study showing that neurologic outcome at 3 and 12 months was correlated with initial GCS and duration of impaired consciousness.22 The presence of skull
fractures, which indicates shaken impact syndrome, was also associated with poor neurodevelopmental outcome. Shaking without head impact has been reported to cause brain lesions in infants.6,8 –10,31,32
The presence of skull fractures seems to be associated with a worse neurodevelopmental outcome. This was inferred from our results and was pointed out in the literature.2Cranial growth deceleration was
an-TABLE 3. Comparison Between Outcome and the Type of Parenchymal Lesions (MRI) in Our Study Group Parenchymal
Lesions
n Imaging
Features
GOS Motor
Impairment
IQ Epilepsy Sensory Impairment
Selective/Multiple Neuropsychologic
Impairment†
Not visible 3 MD MQ: 77 78 ⫺ — ⫹/⫺
5 GR — 95 ⫺ — ⫺/⫺
8 MD — 93 ⫺ — ⫹/⫺
10 SD Hemiparesis 60 ⫺ — ⫺/⫹
13 MD — 95 ⫺ vi ⫺/⫺
Focal 1 LO MD — 90 ⫺ — ⫹/⫺
4 RT,P,O MD — 70 ⫺ — ⫹/⫺
2 LF,RT,bP,RO SD Tetraplegia 60 ⫹ bl ⫺/⫹
19 RT,P,O,CC MD Hemiparesis 75 ⫺ — ⫹/⫺
Hemispheric 7 LF, RH, CC SD Hemiplegia 70 ⫺ — ⫺/⫹
11 LH SD Hemiplegia 65 ⫺ — ⫺/⫹
12 LH SD — 60 ⫹ — ⫺/⫹
21 LH,CC SD Hemiplegia 42 ⫺ vi ⫺/⫹
Diffuse 6* bH MD — 70 ⫺ — ⫹/⫺
9 bF,bT,RP,bO,CC SD Tetraplegia 40–50 ⫹ bl ⫺/⫹
14 LF,bP,bO SD MQ: 50 62 ⫺ — ⫺/⫹
15 LF,RT,bP,RO,CC SD MQ: 77 50 ⫹ — ⫺/⫹
16 bF,bO,CC,Cb MD — 85 ⫺ — ⫹/⫺
17 bF,RT,bP,bO,CC,Cb SD Hemiplegia ⬍45 ⫹ vi ⫺/⫹
18 bF,RO,CC SD Tetraplegia 30–50 ⫹ bl ⫺/⫹
20 bH,CC PVS Tetraplegia ⬍30 ⫹ bl ⫺/⫹
22 bH,CC PVS Tetraplegia ⬍30 ⫹ bl ⫺/⫹
23 bH,CC,Cb,BG PVS Tetraplegia ⬍30 ⫹ bl ⫺/⫹
* Patient 6 underwent CT only.
† Selective neuropsychologic problems included visuospatial and attention deficiencies in patients 3 and 8; slowness and attention deficiency in patients 1, 4, 6, and 16; and speech retardation without mental retardation in patient 19. Multiple neuropsychologic impairment indicates mental retardation and psychiatric symptoms.
CC, indicates corpus callosum; Cb, cerebellum; BG, basal ganglia; MQ, Motor Quotient, according to Bayley Scale54; vi, visual impairment;
other sign of adverse prognostic significance in our study. This is in keeping with earlier data.11,12 It is
noteworthy that in the series studied by Barlow, severe initial seizures and presence of intracranial hypertension predicted poor neurodevelopmental outcome.18,19Our results are in agreement with this
findings.
MRI is recognized as the most sensitive tool for detecting brain lesions in NAHI.33–37 Previous
pro-spective studies showed a correlation between intra-parenchymal lesions visualized on brain imaging and short-term outcome.21,22Our retrospective study
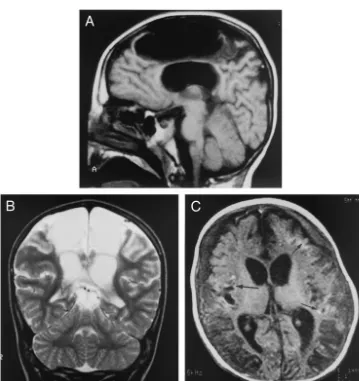
is in line with these results. Our results show, in addition, that the reported poor neurodevelopmental outcome in NAHI persists in the long-term. To our knowledge, our study is the first to link early and late MRI findings to long-term neurodevelopmental outcome. Our results showed that MRI done 15 days or more after injury disclosed evidence of 3 etio-pathogenic mechanisms leading to atrophy, namely, contusions, infarcts/stroke, and white matter scars (Fig 1). Infarcts were detected in 50% of our patients.
In another series, similar lesions (called “infarcts/ edema”) were reported in one third of 15 patients with NAHI.22The pathophysiologic mechanisms
un-derlying stroke in NAHI remain unclear. Strangula-tion has been suggested as a possible cause of infarc-tion in the distribuinfarc-tion of the carotid artery.38,39
However, arterial wall dissection, which was long underrecognized in children, is now known to be among the leading causes of arterial ischemic stroke in the pediatric age group.25,40Injuries such as direct
cervical blow or stretching of the neck may be re-sponsible for most cases of arterial dissection. These mechanisms may be implicated in NAHI. Another plausible mechanism for stroke may be fat embolism, a classic complication of long-bone fractures. Such fractures were present in 5 of our patients (patients 11, 18, 20, 22, and 23). Direct intracranial damage (tearing, thrombosis) to the arterial wall could also be another underlying mechanism. Additional neu-roradiologic studies, for instance, using MR angiog-raphy, may provide more insights into the mecha-nism of infarcts/stroke in NAHI.
Subcortical gliotic scars were the most common intraparenchymal lesions in our series. We sug-gest that these lesions result from the shearing and tearing injuries described in association with accel-eration-deceleration effects in both children and adults41– 49 and/or from hypoxic axonal damage as
suggested by recent neuropathologic studies tar-geted on patients who presented a very rapid fatal evolution.50,51 In our series, scarring was diffuse; it
involved the white matter subjacent to the neocortex in all affected patients and the corpus callosum in the majority. Most of our patients had grade 2 axonal injury as defined by Adams et al,52which is
associ-ated with a high likelihood of poor outcome. Our results are consistent with those of the radiologic work by Levin et al,53in which the corpus callosum
was shown to be particularly vulnerable to mechan-ical injury, especially in young children.
Another goal of our study was to determine in which time frame neuroimaging provides the best information in NAHI. Between days 1 and 14, when edema is predominant, both MRI and CT scan de-tected intraparenchymal lesions; MRI provided ad-ditional data on the distribution of the lesions and disclosed a small pericerebral hematoma and a focal lesion in the corpus callosum in 1 patient. Thus, as compared with CT scan, very early MRI (between days 1 and 14) did not provide additional data of crucial importance for evaluating the prognosis. In contrast, when performed between 0.5 and 3 months after injury, MRI yielded important information on the distribution and mechanisms of the lesions in all patients. These findings further confirm that changes on brain imaging evolve over time36 and that early
brain imaging (especially before day 15) does not unravel the full extent of damages. The anomalies that we detected by MRI between 0.5 and 3 months were correlated with neurodevelopmental outcome. For instance, stroke was associated with a poor out-come in 78% of children and white matter injuries in 82%. When done beyond the first 3 months after injury, MRI did not provide additional significant information and did not modify the prognostic con-clusions drawn from earlier imaging data. Some lim-itations of the prognostic value of MRI could come from the retrospective method that we used. How-ever, we do not think that such limitations would influence our results in an important way, because the timing and number of neuroradiologic examina-tions were relatively homogeneous in our popula-tion. In fact, timing and number of MRI in our pa-tients were not different in the clinically well-appearing patients as compared with the others. Thus, patients who looked well clinically did not undergo fewer examinations, and consequently focal lesions would not have been missed.
Finally, in our series, the severity of sequelae was shown to be related to the extent of brain lesions. However, we found no correlation between the to-pography of lesions and outcome. This point re-quires additional exploration involving detailed neu-ropsychologic assessments in larger series of NAHI patients, preferably using prospective studies.
REFERENCES
1. Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children.Lancet. 2000;356:1571–1572
2. Duhaime AC, Christian CW, Rorke LB, Zimmerman RA. Nonaccidental head injury in infants—the shaken-baby syndrome. N Engl J Med. 1999;338:1822–1829
3. Collins KA, Nichols CA. A decade of pediatric homicide: a retrospective study at the Medical University of South Carolina.Am J Forensic Med Pathol.1999;20:169 –172
4. Duhaime AC, Gennarelli TA, Thibault LE, Bruce DA, Margulies SS, Wiser S. The shaken baby syndrome: a clinical, pathological and bio-mechanical study.J Neurosurg. 1987;66:409 – 415
5. Ludwig S, Warman M. Shaken baby syndrome: a review of 20 cases. Ann Emerg Med. 1984;13:104 –107
6. Sinal SH, Ball MR. Head trauma due to child abuse: serial computerized tomography in diagnosis and management. South Med J. 1987;80: 1505–1512
7. Wilkinson WS, Han DP, Rappley MD, Owings CL. Retinal hemorrhage predicts neurologic injury in the shaken baby syndrome.Arch Ophthal-mol. 1989;107:1472–1474
8. Frank Y, Zimmerman R, Leeds NMD. Neurological manifestations in abused children who have been shaken.Dev Med Child Neurol. 1985;27: 312–316
9. Brenner SL, Mann-Gray S. Race and the shaken baby syndrome: expe-rience at one hospital.J Natl Med Assoc. 1989;81:183–184
10. Zepp F, Bru¨hl K, Zimmer B, Schumacher S. Battered child syndrome: cerebral ultrasound and CT findings after vigorous shaking. Neuropedi-atrics. 1992;23:188 –219
11. Oliver JE. Microcephaly following baby battering and shaking.BMJ. 1975;2:262–264
12. Bonnier C, Nassogne MC, Evrard P. Outcome and prognosis of whip-lash shaken infant syndrome: late consequences after a symptom-free interval.Dev Med Child Neurol. 1995;37:943–956
13. Duhaime AC, Christian CW, Moss E, Seidl T. Long-term outcome in infants with the shaking impact syndrome.Pediatr Neurosurg. 1996;24: 292–298
14. Haviland J, Ross Russell RI. Outcome after severe non-accidental head injury.Arch Dis Child. 1997;77:504 –507
15. Giangiacomo J, Khan JA, Levine C, Thompson VM. Sequential cranial computed tomography in infants with retinal hemorrhages. Ophthalmol-ogy. 1988;95:295–299
16. Matthews GP, Das A. Dense vitreous hemorrhages predict poor visual and neurological prognosis in infants with shaken baby syndrome.J Ophthalmol Strabismus. 1996;33:260 –265
17. Mills M. Funduscopic lesions associated with mortality in shaken baby syndrome.J Am Assoc Pediatr Ophthalmol Strabismus. 1998;68:117–132 18. Barlow KM, Spowart JJ, Minns RA. Early posttraumatic seizures in
non-accidental head injury: relation to outcome.Dev Med Child Neurol. 2000;42:591–594
19. Barlow KM, Minns RA. The relation between intracranial pressure and outcome in non-accidental head injury.Dev Med Child Neurol. 1999;41: 220 –225
20. Han BK, Towbin RB, De Courten-Myers G, McLaurin RL, Ball WS. Reversal sign on CT: effect of anoxic/ischemic cerebral injury in chil-dren.AJR Am J Roentgenol. 1990;154:361–368
21. Ewing-Cobbs L, Prasad M, Kramer L, Landry S. Inflicted traumatic brain injury: relationship of developmental outcome to severity of in-jury.Pediatr Neurosurg. 1999;31:251–258
22. Prasad M, Ewing-Cobbs L, Swank PR, Kramer L. Predictors of outcome following traumatic brain injury in young children.Pediatr Neurosurg. 2002;36:64 –74
23. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale.Lancet. 1974;2:81– 84
24. Ewing-Cobbs L, Kramer L, Prasad M, et al. Neuroimaging, physical, and developmental findings after inflicted and non-inflicted traumatic brain injury in young children.Pediatrics. 1998;102:300 –337
25. Husson B, Rodesch G, Lasjaunias P, Tardieu M, Se´bire G. Magnetic resonance angiography in childhood arterial brain infarcts: a compara-tive study with contrast angiography.Stroke. 2002;33:1280 –1285 26. Jennet B, Bond M. Assessment of outcome after severe brain damage: a
practical scale.Lancet. 1975;1:480 – 484
27. Wechsler D.Manual for the Wechsler Preschool and Primary Scale of Intel-ligence-Revised.New York, NY: The Psychological Corporation; 1974 28. Kaufman AS, Kaufman NL.K-ABC: The Kaufman Assessment Battery for
Children.Circle Pines, MN: American Guidance Service; 1983 29. Wechsler D.Manual for the Wechsler Intelligence Scale for Children-Revised.
30. Brink J, Imbus C, Woo-Sam J. Physical recovery after severe closed head trauma in children and adolescents.J Pediatr. 1980;97:721–727 31. Conway EE. Nonaccidental head injury in infants: “the shaken baby
syndrome revisited.”Pediatr Ann. 1998;27:677– 690
32. Alexander RC, Sato Y, Smith W, Bennet T. Incidence of impact trauma with cranial injuries ascribed to shaking.Am J Dis Child. 1990;144: 724 –726
33. Brown JK, Minns RA. Non-accidental head injury, with particular ref-erence to whiplash shaking injury and medico-legal aspects.Dev Med Child Neurol. 1993;35:849 – 869
34. Alexander RC, Schor DP, Smith WL. Magnetic resonance imaging in intracranial injuries from child abuse.J Pediatr. 1986;109:975–979 35. Ball WS. Nonaccidental craniocerebral trauma (child abuse): MR
imag-ing.Radiology. 1989;173:609 – 610
36. Dias MS, Backstrom J, Falk M, Li V. Serial radiography in the infant shaken impact syndrome.Pediatr Neurosurg. 1998;29:77– 85
37. Chabrol B, Decarie JC, Fortin G. The role of cranial MRI in identifying patients suffering from child abuse and presenting with unexplained neurological findings.Child Abuse Negl. 1999;23:217–228
38. Bird R, MacMahan JR, Gilles FH, Senac MO, Apthorp JS. Strangulation in child abuse: CT diagnosis.Radiology. 1987;163:373–375
39. Feldman KW, Simms RJ. Strangulation in childhood: epidemiology and clinical course.Pediatrics. 1980;65:1079 –1085
40. Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children.Neurology. 2001;57:1155–1160
41. Jaspan T, Narborough G, Punt JAG, Lowe J. Cerebral contusional tears as marker of child abuse: detection by cranial sonography. Pediatr Radiol. 1992;22:237–245
42. Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due
to non-missile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563
43. Graham DI, Clark JC, Adams JH, Genarelli TA. Diffuse axonal injury caused by assault.J Clin Pathol. 1992;45:840 – 841
44. Crooks DA. The pathological concept of diffuse axonal injury; its patho-genesis and the assessment of severity.J Pathol. 1991;165:5–10 45. Parizel PM, Ozsarlak O, Van Goethem JW, et al. Imaging findings in
diffuse axonal injury after closed head trauma.Eur Radiol. 1998;8:960–965 46. Graham DI, Ford I, Adams JH, et al. Fatal head injury in children.J Clin
Pathol. 1989;42:18 –22
47. Lindenberg R, Freytag E. Morphology of brain lesions from blunt trauma in early infancy.Arch Pathol. 1967;87:298 –305
48. Calder JM, Hill I, Scholtz CL. Primary brain trauma in non-accidental injury.J Clin Pathol. 1984;37:1095–1100
49. Vowles GH, Scholtz CL, Cameron JM. Diffuse axonal injury in early infancy.J Clin Pathol. 1987;40:185–189
50. Geddes GF, Hackshaw AK, Vowles GH, et al. Neuropathology of in-flicted head injury in children. I. Patterns of brain damage. Brain. 2001;124:1290 –1298
51. Geddes GF, Vowles GH, Hackshaw AK, et al. Neuropathology of in-flicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124:1299 –1306
52. Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury: definition, diagnosis and grading.Histopathology. 1989;15:49 –59
53. Levin HS, Benavidez DA, Verger-Maestre K, et al. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neu-rology. 2000;54:647– 653
54. Bayley N.Bayley Scales of Infant Development.3rd ed. San Antonio, TX: The Psychological Corporation; 1993
CONFLICT OF INTEREST
“Academic institutions do not seem to be doing nearly enough to protect against the risks [of conflict of interest]. . . According to one study published in 2000, only 3 of 250 medical schools and research institutions insisted that investigators dis-close their financial conflicts to patients before enrolling them in clinical experi-ments or drug trials. Only 7% of these institutions required their researchers to disclose such conflicts to journals publishing their research. Only 1 of the 10 leading medical schools receiving the greatest amounts of federal funding flatly prohibited investigators from doing clinical research on products of firms with which they had significant financial ties. Most of these merely required disclosure to university officials.”
Bok D.Universities in the Marketplace. Princeton, NJ: Princeton University Press; 2003
DOI: 10.1542/peds.112.4.808
2003;112;808
Pediatrics
Hazim Kadhim and Guillaume Sébire
Christine Bonnier, Marie-Cécile Nassogne, Christine Saint-Martin, Bettina Mesples,
Syndrome
Neuroimaging of Intraparenchymal Lesions Predicts Outcome in Shaken Baby
Services
Updated Information &
http://pediatrics.aappublications.org/content/112/4/808
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/112/4/808#BIBL
This article cites 50 articles, 11 of which you can access for free at:
Subspecialty Collections
_management_sub
http://www.aappublications.org/cgi/collection/administration:practice
Administration/Practice Management
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.112.4.808
2003;112;808
Pediatrics
Hazim Kadhim and Guillaume Sébire
Christine Bonnier, Marie-Cécile Nassogne, Christine Saint-Martin, Bettina Mesples,
Syndrome
Neuroimaging of Intraparenchymal Lesions Predicts Outcome in Shaken Baby
http://pediatrics.aappublications.org/content/112/4/808
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.