Hypospadias and Early Gestation Growth Restriction in Infants
Naveed Hussain, MBBS*; Azhar Chaghtai, MD*; C. D. Anthony Herndon, MD‡; Victor C. Herson, MD*; Ted S. Rosenkrantz, MD*; and Patrick H. McKenna, MD‡
ABSTRACT. Objective. There has been a major in-crease in the incidence of hypospadias in infants in the 1990s, but the risk factors are not known. Although there are scattered reports in the literature regarding the asso-ciation of low birth weight and hypospadias, this has not been systematically studied. The objective of this study was to determine the association between early gestation intrauterine growth and hypospadias.
Methods. A retrospective review of 13 years of admis-sions to 2 tertiary care neonatal intensive care units (NI-CUs) in Connecticut (1987–2000) showed that 112 (1.66%) of 6746 male infants had any degree of hypospadias. Of these, 8 were part of a genetic syndrome and were ex-cluded. A retrospective cohort analysis of these 6738 in-fants was performed. Infant growth parameters at birth (weight, head circumference, and length) were analyzed along with maternal risk factors known to be associated with changes in fetal growth, including maternal age, race, diagnosis of preeclampsia, gestational diabetes, and maternal use of alcohol or tobacco or substance abuse during pregnancy.
Results. The incidence of hypospadias in the NICU population increased 10-fold from 0.4% in 1987 to 4% in the first quarter of 2000. Hypospadias was significantly more common in infants who had uniformly poor intra-uterine growth (<10th percentiles) in the various param-eters measured: birth weight, length, or head circumfer-ence. There were no significant differences in maternal age or race, nor were there differences in the use of alcohol, tobacco, or street drugs by the mother. There were no differences between singletons and multiple-gestation births. However, the frequency of occurrence was significantly higher among first-born infants (1.9%) compared with all other infants (0.9%).
Conclusions. The incidence of hypospadias in our NICU population has increased 10-fold during the 13-year period of study. There was a significant association of hypospadias with poor intrauterine growth. The growth restriction was probably of early gestational cause as there was proportionate involvement of somatic (weight and length) and brain growth (head circumfer-ence). The increasing frequency of hypospadias and its association with poor intrauterine growth originating in early gestation suggests that common environmental fac-tor(s) that have an impact on both conditions may be
involved.Pediatrics2002;109:473– 478;hypospadias, SGA, intrauterine growth, temporal trend, risk factors.
ABBREVIATIONS. SGA, small for gestational age; AGA, appro-priate for gestational age; LGA, large for gestational age; NICU, neonatal intensive care unit; UCHC, University of Connecticut Health Center; GA, gestational age; BW, birth weight; SEM, stan-dard error of the mean; EDC, endocrine disrupter chemicals.
T
he development of a normal urethra in males is a complex process, and factors that lead to its abnormal development, resulting in hypospa-dias, are largely unknown.1Hypospadias is one ofthe most common congenital anomalies in males.2
Reports from Europe and the United States have shown an increasing incidence of hypospadias from 1970 to the present.3,4
The risk factors for hypospadias are not well un-derstood. An association between low birth weight and hypospadias has been made,4 – 6but a systematic
analysis of the association between hypospadias and growth restriction has not been conducted. It is also not clear whether the low birth weight is attributable to a lower gestational age or intrauterine growth restriction.5,6 With currently available norms for
in-trauterine growth to classify even the most immature infants into small, appropriate, or large for gesta-tional age (SGA, AGA, and LGA, respectively), it is possible to delineate whether the intrauterine growth was affected in early or late gestation on the basis of birth weight and corresponding changes in head cir-cumference and length.7,8 An infant who is
propor-tionately affected in all of its growth parameters is more likely to have incurred an insult early in ges-tation, whereas the infant who is disproportionately affected, with sparing of changes in its length and head circumference, is more likely to have incurred an insult later in pregnancy.7,8
We hypothesized that hypospadias is associated with restriction in fetal growth early in pregnancy. The aim of this study was to describe the association between hypospadias and intrauterine growth and to characterize some of the risk factors that may be involved.
METHODS
We undertook a retrospective cohort study of a total of 6746 male admissions to the neonatal intensive care units (NICUs) at University of Connecticut Health Center (UCHC; 3157) and the Connecticut Children’s Medical Center (3589) between January 1987 and April 2000. These NICUs take care of infants from the central and northern regions of the state of Connecticut. It is important to note that the reason for NICU admission was not From the Division of Neonatology, *Department of Pediatrics, University
of Connecticut Health Center, Farmington, Connecticut; and ‡Division of Urology, Department of Surgery, Connecticut Children’s Medical Center, Hartford, Connecticut.
This study was presented, in part, as a platform presentation at the Society for Pediatric Research Meeting; May 12–16, 2000; Boston, MA.
Received for publication Sep 29, 2000; accepted Aug 23, 2001.
Reprint requests to (N.H.) University of Connecticut School of Medicine, Farmington, CT 06030-2948. E-mail: hussain@nso1.uchc.edu
hypospadias in any of these infants. Diagnosis was based on physical examination by an essentially constant group of caregiv-ers over the entire period of the study. Confirmation of the diag-nosis and grading of severity were done by consultant urologists. In cases in which ambiguous genitalia were noted, other causes, such as congenital adrenal hyperplasia, were ruled out by labora-tory testing before male gender was ascribed to the infant.9
Eight infants with multiple anomalies or recognized genetic syndromes associated with hypospadias were excluded from the analysis. Presence of cryptorchidism was not used as a criterion for exclusion. Additional analyses were done on the remaining 6738 infants.
Data were collected prospectively on all admissions, based on a standard questionnaire or data sheet, and entered into computer-ized databases. From these fields of data sets, queries for infant data included information on gestational age (GA), birth weight (BW), birth length, occipitofrontal head circumference at birth, and singleton versus multiple births. Queries for maternal data included information on maternal age, race, gravidity, use of alcohol, use of tobacco, substance abuse, maternal diabetes, hy-pertension, or preeclampsia. For the purpose of this analysis, infants were classified on weight percentiles based on the Con-necticut standards published in 1996.10Infants less than the 10th percentile were considered SGA, whereas those greater than 90th percentile were LGA; the rest were considered AGA. For head circumference and length, current growth percentile standards by Britton et al,11compiled from a large US population, were used.
Statistical analyses of data included regression analyses of hy-pospadias frequency in relation to the growth parameters studied. Univariate analyses of risk factors were done using t test for continuous variables and 2test for nominal variables. Signifi-cance was determined byP⬍.05. The statistical package StatView 4.0 (Abacus Concepts, Inc, Sunnyvale, CA) was used for these analyses.
RESULTS
Temporal Trends: Increasing Frequency of Occurrence of Hypospadias
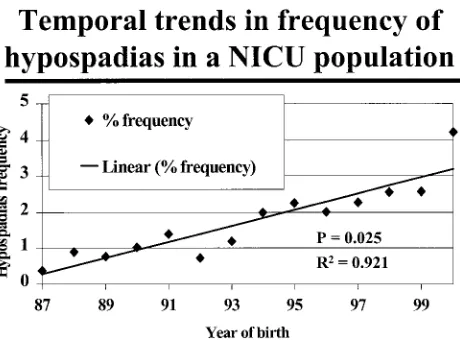
Figure 1 shows the frequency of occurrence of hypospadias in the 2 NICUs for the 13 years of study from 1987 to 2000. Hypospadias frequency in both NICUs increased from 0.4% in 1987 to 4% in the first quarter of 2000. This was a significant increase with time (P⫽.025), and the relationship of hypospadias frequency to time was linear (R2 ⫽ 0.921) by a
re-gression analysis. The referral patterns and popula-tion distribupopula-tion (GA, BW, proporpopula-tion of SGA in-fants, race, gender, and mortality rate) of infants did
not change significantly during the period of study (data not shown).
Hypospadias and Prematurity
As this study was done in a NICU population, we investigated whether the change in frequency was related to prematurity. Figure 2 shows that there was no relationship of the frequency of hypospadias to the gestational age (at birth) of an infant. The fre-quency of hypospadias ranged between 0% and 3.5% across the 23- to 43-week range of gestational ages.
Hypospadias Is Associated With Poor Intrauterine Growth
Intrauterine growth was assessed with 3 parame-ters: BW, length, and head circumference. Figure 3 shows the relationship of hypospadias to intrauter-ine growth. Of the total number (6738 infants) of male admissions, 1122 were SGA. Hypospadias was found in 43 (3.83%) of the 1122 SGA infants com-pared with 59 (1.27%) of the 4626 AGA infants and 2 of the 505 LGA infants (0.39%;P⬍.0001). A total of 487 infants did not have complete growth data for evaluation.
Hypospadias and BW Percentiles
To eliminate the impact of GA on BW, we corre-lated the percentiles of BW for each GA category with the occurrence of hypospadias. As shown in Fig 4, when infants were stratified by the BW percen-tile,10we found that there was a significant
associa-tion (P⬍ .0001) of increased frequency of hypospa-dias in the lowest weight (3rd–10th) centiles and conversely the least frequency in the highest weight (90th–97th) centiles. There was a logarithmic rela-tionship as shown by the best-fit curve, with R2 ⫽
0.98.
Hypospadias and Birth Length Percentiles
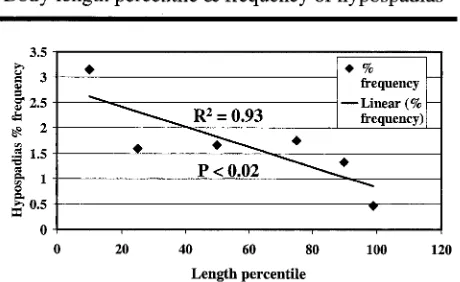
Change in longitudinal growth follows changes in weight when there are chronic problems of fetal growth. When hypospadias frequency was plotted (Fig 5) with birth length percentiles,11we found that
there was a significant association (P ⬍ .02) of in-creased frequency of hypospadias in the lowest length (3rd–25th) centiles and, conversely, the least
Fig 1. Temporal trends in the frequency of occurrence of hypos-padias in 2 NICUs in Connecticut (N⫽6738 male infants). Data from January 1987 to April 2000. Significant trends in increase in frequency shown by linear regression analysis.
frequency within the highest length (75th–97th) cen-tiles. There was a linear relationship withR2⫽0.93.
Hypospadias and Head Circumference Percentiles
In problems of fetal growth, head circumference is the last to be affected among the 3 parameters stud-ied. In Fig 6, hypospadias frequency was plotted with head circumference percentiles,11 and, again,
there was a significant association (P ⬍ .0004) of increased frequency of hypospadias in the lowest
head circumference (3rd–25th) centiles and, con-versely, the least frequency within the highest head circumference (75th–97th) centiles. There was a lin-ear relationship as shown by the best-fit curve with
R2⫽0.98.
Combination of Growth Parameters and Hypospadias Frequency
To add support to our hypothesis, we found that when infant growth was affected in all 3 parameters, (weight, length, and head circumference), the maxi-mum occurrence of hypospadias (4.5%) was found. In contrast, when none of these growth parameters were⬍10th percentile, the frequency of hypospadias was markedly lower (1.1%) and this difference was statistically significant (P ⬍.05).
Maternal Factors and Hypospadias
We investigated whether any of the maternal fac-tors that are known to affect fetal growth had an impact on the occurrence of hypospadias. Infants with hypospadias were born to mothers of a more advanced age (29.0 ⫾ 0.6 years; mean ⫾ standard error of the mean [SEM]) compared with mothers of nonaffected infants (27.6⫾0.8 years; mean⫾SEM), but the difference did not reach statistical signifi-cance (P⫽ .057). However, the frequency of occur-rence of hypospadias was higher among first-born infants (1.9%) compared with all other infants (0.9%), and this difference was statistically significant (P ⬍
.001). There were no differences in the occurrence of hypospadias associated with differences in maternal race or diagnosis of gestational diabetes, hyperten-sion, or preeclampsia. There were also no significant differences between singletons and multiple births (data not shown). The information about the mater-nal use of alcohol, tobacco, or illicit drugs was avail-able only from 1 center (UCHC). None of these 3 factors was significantly associated with the inci-dence of hypospadias (data not shown).
Severity of Hypospadias
The severity of hypospadias, based on meatal opening, was available from all 52 infants from 1 of the sites (UCHC). An anterior hypospadias (glan-dular and distal penile) was seen in 65%, mid-hypo-spadias was seen in 12%, and posterior hypomid-hypo-spadias
Fig 3. Flow diagram showing the distribution of infants with hypospadias in relation to their intrauterine growth status. Statis-tical analysis by the2test.
Fig 4. Relationship of hypospadias frequency to percentiles of BW in newborn infants (based on local Connecticut norms10). There was a significant inverse relationship.
Fig 5. Relationship of hypospadias frequency to percentiles of birth length in newborn infants (based on current US norms11). There was a significant inverse relationship.
was seen in 23% of the infants. The BW percentile of infants with anterior hypospadias was 24⫾4 centile (mean⫾SEM) and those with posterior hypospadias was 13⫾7 centile (mean⫾SEM); however, because of relatively small numbers, a relationship of severity of hypospadias to severity of growth restriction could not be made definitively. Of note were 4 in-fants with severe symmetric growth retardation whose hypospadias was so severe that gender as-signment initially was not possible at birth. Only after investigations for ambiguous genitalia were completed was it confirmed that these were male infants with severe hypospadias. Cryptorchidism was present as a related diagnosis with hypospadias in 27% of infants.
DISCUSSION
We found a trend of increasing incidence of hypo-spadias between January 1987 and April 2000 in a selected population of infants who were admitted to tertiary-level NICUs in Connecticut. This 10-fold in-crease was not explained by changes in referral pat-terns or criteria for diagnosis in these centers and probably reflects a true overall increase in incidence. These data agree with the trends suggested by Pau-lozzi et al3 and, more recently, by Riley et al,12 but
the magnitude of change is much greater. It is ex-tremely difficult to obtain retrospective population-based data on the true state or nationwide incidence of hypospadias because congenital defect registries do not usually track this malformation. We believe that the current report reinforces the need for pro-spective tracking of the incidence of hypospadias at the state and national levels.
A significant association between hypospadias and intrauterine growth restriction is demonstrated in this report. This association with low BW has been suggested6,13,14 but has not previously been well
characterized. A recent report reaffirmed the associ-ation and speculated on factors involved in critical periods early in gestation.15It is widely known that
factors that inhibit intrauterine growth of the infant cause a proportionate decrease in somatic (weight and length) and brain (head circumference) growth if these factors are active early in gestation.7,8If growth
inhibition is caused by nutritional and other factors in the second and third trimesters of gestation, then there is relative sparing of brain growth (head cir-cumference) with a predominant effect on weight initially and changes in length occurring after a more prolonged insult.7,8 In our analyses of intrauterine
growth parameters, we found that there was a con-sistent involvement of growth parameters, ie, weight, length, and head circumference, in infants with hypospadias, and this finding is highly sugges-tive of an effect in early gestation on growth—per-haps early enough to affect adversely urethral devel-opment.
There was a higher incidence (35%) of the more severe forms of hypospadias, ie, mid- and posterior, in our study. This represents a notable increase in the severity of hypospadias compared with a previous study, which reported that only approximately 13% of the cases were of the severe type.16The reason for
this is not clear, but with the increase in incidence of hypospadias that has been noted, the increase in severity is not unexpected and strengthens the argu-ment that external factors may be involved.
The causes for poor intrauterine growth are many. A myriad of factors, from genetic to placental to maternal conditions and environmental changes, have been studied. Some of these factors, such as maternal preeclampsia,17 gestational diabetes, and
the use of alcohol or tobacco or substance abuse by the mother,4,18 were investigated in our study and
were not found to be important in relation to the occurrence of hypospadias in the infant. A higher incidence of hypospadias was seen in first-born in-fants in our study. Although this has been noted in a previous report,19no explanation has been
forthcom-ing. It is known that first-born infants are more likely to have growth restriction,20 and the relationship to
hypospadias may be linked to this observation. Ad-vanced maternal age was found to be a risk factor by Fisch et al.21In our study, there was a similar trend,
but no significant relationship could be demon-strated.
There is controversy regarding the pathogenesis of hypospadias.1,22,23Heinonen et al24 and Kallen and
colleagues4,13explored various maternal risk factors,
but no definite conclusions were drawn. Many are of the opinion that it is unlikely that genetic factors play a role.25–28Recent evidence from animal29,30and
hu-man studies31,32has suggested a relationship of male
genital abnormalities with environmental factors. Hypospadias is more commonly seen in infants of infertile couples who conceived by in vitro fertiliza-tion33 or other assisted reproductive techniques.34
Fertility drugs have been suspected, but a recent meta-analysis failed to show an effect.35
Anti-andro-gen compounds have been implicated36; especially
the environmental anti-androgens or “endocrine dis-rupter chemicals” (EDCs) have been implicated in causing male genital abnormalities, including hypo-spadias in humans,37,38 and there seems to be
in-creasing evidence for this from animal studies.39 – 41
Conversely, in case reports of overdosage with me-droxyprogesterone in early pregnancy, there seemed to be no adverse effects on the fetus.42However, it is
important to bear in mind that the exposure of the EDCs, albeit in low concentrations, is continual and starts from conception through the most critical pe-riods of early embryogenesis. Moreover, the pres-ence of a combination of these agents may have more than an additive effect.
Because a cause-effect relationship has not been demonstrated between known factors for poor growth and hypospadias, we speculate involvement of other unknown factors. A common theme that may perhaps interrelate the incidence of growth re-striction and hypospadias is the report that some environmental factors, especially “endocrine dis-rupters,” have been shown to be associated with both growth restriction and hypospadias. Heindel et al39 showed that contaminated groundwater can
cause both growth restriction and reproductive abnormalities in mice. Gray et al40,41 demonstrated
anti-androgenic activity, including linuron and poly-chlorinated biphenyls congener 169. In a case-control study, maternal testosterone levels at 6 to 14 weeks’ gestation were significantly lower in pregnancies that resulted in growth restriction and male genital abnormalities.43Because a major part of testosterone
in this period is derived from the placental-fetal unit, it would not be unreasonable to implicate this unit and the effects of anti-androgens on it in the patho-genesis of hypospadias. The alteration of the fetal-placental-maternal unit’s interaction by any factors perhaps also disturbs early pregnancy growth and increases the risk for hypospadias.13
Of additional concern may be that the cumulative toxicities of EDCs, especially polychlorinated biphe-nyls, which have an anti-androgen effect, have al-ready been demonstrated in the aquatic life around Connecticut, from where this study is reported.44,45
The relationship of these chemicals to human effects is still unknown. However, the alarming increase in hypospadias frequency and severity in this region with no reasonable explanation needs a detailed sci-entific investigation. This point was well emphasized by Dolk,46who after a review of current literature on
the incidence of hypospadias commented, “There is enough evidence to take the apparent increase in prevalence of hypospadias seriously,” and suggests that “efforts to investigate the etiology of hypospa-dias, including assessment of the endocrine-dis-rupter hypothesis, must be renewed.”
A major limitation of this study is that it is based on data from a NICU population of infants. How-ever, none of the infants was admitted with the pri-mary diagnosis of hypospadias, and because there is adequate representation of infants of all gestational ages, we believe that the data presented here are still clinically relevant. Because this study was retrospec-tive, our findings would be more useful in the for-mulation of a hypothesis rather than in providing answers; therefore, more population-based prospec-tive studies need to be performed before any final conclusions can be drawn.
ACKNOWLEDGMENTS
We thank Susan Zaremba, Marlene Holman, Marta Baker, and Barbara Westman for invaluable help with data collection and retrieval.
REFERENCES
1. van der Werff JF, Nievelstein RA, Brands E, Luijsterburg AJ, Vermeij-Keers C. Normal development of the male anterior urethra.Teratology. 2000;61:172–183
2. Stock JA, Scherz HC, Kaplan GW. Distal hypospadias.Urol Clin North Am. 1995;22:131–138
3. Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems.Pediatrics.1997;100:831– 834
4. Kallen B, Bertollini R, Castilla E, et al. A joint international study on the epidemiology of hypospadias.Acta Paediatr Scand Suppl. 1986;324:1–52 5. Weidner IS, Moller H, Jensen TK, Skakkebaek NE. Risk factors for
cryptorchidism and hypospadias.J Urol. 1999;161:1606 –1609 6. Calzolari E, Contiero MR, Roncarati E, Mattiuz PL, Volpato S.
Aetio-logical factors in hypospadias.J Med Genet. 1986;23:333–337
7. Villar J, Belizan JM. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome.Obstet Gynecol Surv. 1982;37: 499 –506
8. Sparks JW, Cetin I. Intrauterine growth and nutrition. In: Polin RA, Fox
WW, eds.Fetal and Neonatal Physiology. Vol. 1. Philadelphia, PA: WB Saunders Company; 1992:191
9. Penny R. Ambiguous genitalia.Am J Dis Child. 1990;144:753 10. Roberts C, Mueller L, Hadler J. Birth-weight percentiles by gestational
age, Connecticut 1988 –1993.Conn Med.1996;60:131–140
11. Britton JR, Britton HL, Jennett R, Gaines J, Daily WJ. Weight, length, head and chest circumference at birth in Phoenix, Arizona.J Reprod Med. 1993;38:215–222
12. Riley MM, Halliday JL, Lumley JM. Congenital malformations in Vic-toria, Australia, 1983–95: an overview of infant characteristics.J Paediatr Child Health.1998;34:233–240
13. Kallen B. Case control study of hypospadias, based on registry infor-mation.Teratology.1988;38:45–50
14. Monteleone Neto R, Castilla EE, Paz JE. Hypospadias: an epidemiolog-ical study in Latin America.Am J Med Genet. 1981;10:5–19
15. Gatti JM, Kirsch AJ, Troyer WA, Perez-Brayfield MR, Smith EA, Scherz HC. Increased incidence of hypospadias in small-for-gestational age infants in a neonatal intensive-care unit.BJU Int. 2001;87:548 –550 16. Avellan L. The incidence of hypospadias in Sweden. Scand J Plast
Reconstr Surg. 1975;9:129 –139
17. Akre O, Lipworth L, Cnattingius S, Sparen P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10: 364 –369
18. Battin M, Albersheim S, Newman D. Congenital genitourinary tract abnormalities following cocaine exposure in utero.Am J Perinatol. 1995; 12:425– 428
19. Angerpointner TA. Hypospadias— genetics, epidemiology and other possible aetiological influences.Z Kinderchir. 1984;39:112–118 20. Boo NY, Lye MS, Ong LC. Intrauterine growth of liveborn Malaysian
infants between gestation of 28 to 42 weeks.Singapore Med J. 1994;35: 163–166
21. Fisch H, Golden RJ, Libersen GL, et al. Maternal age as a risk factor for hypospadias.J Urol. 2001;165:934 –936
22. Kluth D, Lambrecht W, Reich P. Pathogenesis of hypospadias—more questions than answers.J Pediatr Surg. 1988;23:1095–1101
23. Baskin LS. Hypospadias and urethral development.J Urol. 2000;163: 951–956
24. Heinonen OP, Slone D, Shapiro S.Birth Defects and Drugs in Pregnancy. Littleton, MA: Publishing Sciences Group; 1977:189 –199
25. Allera A, Herbst MA, Griffin JE, Wilson JD, Schweikert HU, McPhaul MJ. Mutations of the androgen receptor coding sequence are infrequent in patients with isolated hypospadias.J Clin Endocrinol Metab. 1995;80: 2697–2699
26. Bentvelsen FM, Brinkmann AO, van der Linden JE, Schroder FH, Nij-man JM. Decreased immunoreactive androgen receptor levels are not the cause of isolated hypospadias.Br J Urol. 1995;76:384 –388 27. Sutherland RW, Wiener JS, Hicks JP, et al. Androgen receptor gene
mutations are rarely associated with isolated penile hypospadias.J Urol. 1996;156:828 – 831
28. Mehes K. Isolated hypospadias is not associated with signs of midline closure defects.Am J Med Genet. 1998;75:190 –192
29. Arnold SF, Klotz DM, Collins BM, Vonier PM, Guillette LJ, McLachlan JA. Synergistic activation of estrogen receptor with combinations of environmental chemicals.Science.1996;272:1489 –1492
30. Vonier PM, Crain DA, McLachlan JA, Guillette LJ, Arnold SF. Interac-tion of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator.Environ Health Perspect. 1996;104:1318 –1322
31. Giwercman A, Skakkebaek NE. The human testis—an organ at risk?Int J Androl. 1992;15:373–375
32. Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?Lancet.1993;341: 1392–1395
33. Silver RI, Rodriguez R, Chang TS, Gearhart JP. In vitro fertilization is associated with an increased risk of hypospadias. J Urol. 1999;161: 1954 –1957
34. Macnab AJ, Zouves C. Hypospadias after assisted reproduction incor-porating in vitro fertilization and gamete intrafallopian transfer.Fertil Steril. 1991;56:918 –922
35. Raman-Wilms L, Tseng AL, Wighardt S, Einarson TR, Koren G. Fetal genital effects of first-trimester sex hormone exposure: a meta-analysis. Obstet Gynecol. 1995;85:141–149
36. Farrar DJ, Aromin I, Uvin SC, Flanigan TP, Mileno MD. Hypospadias associated with the use of high dose megestrol acetate in an HIV infected woman.Genitourin Med. 1997;73:226
38. Kristensen P, Irgens LM, Andersen A, Bye AS, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiol-ogy.1997;8:537–544
39. Heindel JJ, Chapin RE, George J, et al. Assessment of the reproductive toxicity of a complex mixture of 25 groundwater contaminants in mice and rats.Fundam Appl Toxicol. 1995;25:9 –19
40. Gray LE, Wolf C, Lambright C, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozoli-nate, p,p⬘-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat.Toxicol Ind Health.1999;15:94 –118 41. Gray LE, Kelce WR. Latent effects of pesticides and toxic substances on
sexual differentiation of rodents.Toxicol Ind Health.1996;12:515–531 42. Yovich JL, Turner SR, Draper R. Medroxyprogesterone acetate therapy
in early pregnancy has no apparent fetal effects.Teratology.1988;38: 135–144
43. Key TJ, Bull D, Ansell P, et al. A case-control study of cryptorchidism and maternal hormone concentrations in early pregnancy.Br J Cancer. 1996;73:698 –701
44. Greig RA, Sennefelder G. PCB concentrations in winter flounder from Long Island Sound, 1984 –1986.Bull Environ Contam Toxicol.1987;39: 863– 868
45. Greig RA, Sennefelder G. Metals and PCB concentrations in mussels from Long Island Sound.Bull Environ Contam Toxicol. 1985;35:331–334 46. Dolk H. Rise in prevalence of hypospadias.Lancet.1998;351:770
IMPACT OF RESEARCH
“The main aim of health research is to improve the health of people. Yet the performance of researchers tends to be measured by the scientific quality of their research rather than by its impact on health. This is unsatisfactory, even nonsen-sical, so a committee on the Royal Netherlands Academy of Arts and Sciences is trying to devise a way of measuring the social impact of applied health research. Its first report was discussed at a recent meeting in Amsterdam,1and the Academy
now plans to experiment with methods of measuring social impact.”
REFERENCE
1. Health Sciences Subcommittee of the Medical Committee of the Royal Netherlands Academy of Arts and Sciences. The Societal Impact of Applied Health Research: Towards a Quality Assessment System.Amsterdam, the Netherlands: KNAW; 2001. www.knaw.nl/cg
Smith R. Measuring the social impact of research.BMJ.2001;323:528
DOI: 10.1542/peds.109.3.473
2002;109;473
Pediatrics
Rosenkrantz and Patrick H. McKenna
Naveed Hussain, Azhar Chaghtai, C. D. Anthony Herndon, Victor C. Herson, Ted S.
Hypospadias and Early Gestation Growth Restriction in Infants
Services
Updated Information &
http://pediatrics.aappublications.org/content/109/3/473 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/109/3/473#BIBL This article cites 45 articles, 3 of which you can access for free at:
Subspecialty Collections
s_sub
http://www.aappublications.org/cgi/collection/genitourinary_disorder Genitourinary Disorders
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.109.3.473
2002;109;473
Pediatrics
Rosenkrantz and Patrick H. McKenna
Naveed Hussain, Azhar Chaghtai, C. D. Anthony Herndon, Victor C. Herson, Ted S.
Hypospadias and Early Gestation Growth Restriction in Infants
http://pediatrics.aappublications.org/content/109/3/473
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.