EUR 4SSI
e
European Atomic Energy Community - EURATOM FIAT S.p.A., Sezione Energia Nuoleare - Torino
. Societa
ANLTWJky
GenovaNUCLEAR
1FUEL· WITH BURNABLE POISON
Part 2;" Preparation ·and out•of•pile evaluation of
Gd
20
3 poisoned U02 pelletsby
G. ABATE-DAGA, I. AMATO and G. GRAPPIOLO (FIAT)
Topical
Rewrt
January 1, 1969 - May 31, 1970
1970
@
This document was prepared under the sponsorship of the Commission of the European Communities.
Neither the Commission of the European Communities, its contractors nor any person acting on their behalf:
make any warranty or representation, express or implied, with respect
to the accuracy, completeness or usefulness of the information contained
in this document, or that the use of any information, apparatus, method or process disclosed in this document may not infringe privately owned rights; or
assume any liability with respect to the use of, or for damages resulting
from the use of any information, apparatus, method or process disclosed in this document.
This report is on sale at the addresses listed on cover page 4
at the price of FF 6.65 FB 60.- DM 4.40 Lit. 750 Fl. 4.30
When ordering, please quote the EUR number and the title which are indicatec;l on the cover of each report.
Printed by Van Muysewinkel, Brussels Luxembourg, November 1970
EUR 4SSI
e
European Atomic Energy Community - EURATOM
FIAT S.p.A., Sezione Energia Nucleare - Torino
Societa ANSALDO S.p.A. - Genova
EUR 4551 e
NUCLEAR FUEL WITH BURNABLE POISON
Part 2. Preparation and out-of-pile evaluation of Gd,O, poisoned UO, pellets by G. ABATE-DAGA, I. AMATO and G. GRAPPIOLO (FIAT)
Topical Report January 1, 1969 - May 31, 1970
European Atomic Energy Community - EURATOM FIAT S.p.A., Sezione Energia Nucleate - Torino Societa ANSALDO S.p.A. - Genova
Contract No. 008-61-12 PNII
Luxembourg, November 1970 - 40 Pages - 20 Figures - FB
60.-Gd,O, microspheres about 500 microns diameter, have been molybdenum coated through metal vapor deposition in fluidized bed. The coating thick-ness has been maintained in the range 25-30 microns.
The optimum density of gadolinia microspheres in order to maintain coating integrity at high temperature is in the range 85-90 % of theoretical value
EUR 4551 e
NUCLEAR FUEL WITH BURNABLE POISON
Part 2. Preparation and out-of-pile evaluation of Gd,O, poisoned UO, pellets by G. ABATE-DAGA, I. AMATO and G. GRAPPIOLO (FIAT)
Topical Report January 1, 1969 - May 31, 1970
European Atomic Energy Community - EURATOM FIAT S.p.A., Sezione Energia Nucleare - Torino Societa ANSALDO S.p.A. - Genova
Contract No. 008-61-12 PNII
Luxembourg, November 1970 - 40 Pages - 20 Figures - FB
60.-Gd,O, microspheres about 500 microns diameter, have been molybdenum coated through metal vapor deposition in fluidized bed. The coating thick-ness has been maintained in the range 25-30 microns.
The optimum density of gadolinia microspheres in order to maintain coating integrity at high temperature is in the range 85-90 % of theoretical value
POISON
uation
ness has been maintained in the range 25-30 microns.
The optimum density of galodinia microspheres in order to maintain coating integrity at high temperature is in the range 85-90 % of theoretical value ( T.D.). UO, pellets poisoned with 1200 ppm of Gd,O, were obtained at about 95 % T.D.
Tests performed at high temperature both under gradient and isothermal conditions showed an acceptable behaviour of molybdenum coated gadolinia microspheres in UO, pellets.
KEYWORDS
MOLYBDENUM FUELS
PARTICLES METALS VAPORS COATING FLUIDIZATION SUSPENSIONS GADOLINIUM URANIUM OXIDES PELLETS
3
-I
lfD
E X1. Ill'J.'BOMC'.l'Elf
5
2. EVALUATION OF HIGH TDPEBJ.TURE Gd
2
o
3 - JloCOKPA!Il3ILITY 6
3.
OPl'IMIZ.l'l'IOlf OF Mo-COATING PROCEDURE 64.
DCRAIIICJ.L BERA VIOUR OF Mo-CO.l'.l'llG 75.
Sil'l'ERll'G All' CRAIU.CTERIZA'l'IOlf OF POISODD PELL!1fS 106. EVALU.A.'.l'IOlJ OF POISONED
uo2
PELLnS A.'f HIGH TEIPERA.TURE 116 .1 • Thermal 61'4dient experiments 11
6.2. Isothermal experiments 12
1.
COlfCLUSIOl'S12
5
-I11 a previous paper (1) the aavantagea of the use of nuclear f'Uel poisoned with gadolinia mioroapherea ha.Te been shown; in the aame
paper the preparation and characterisation of
0420
3~aiorospherea had been reportei. Soope of this paJ>e ia a a.eaoription of thee:xperillenta oarried out in order to obtain~) poisoned U02 pelleta suitable aa nuclear tuel.
The teohaolo17 of fabrication of U02 pellets poisoned with 04203
micro-spheres involTea the problea of oompatibilit7 between the two materials. illia Chalmers (2) studied this problea fro• a phenomenological point of
view and oonolude4 that
-04203
ud U02 ata:t't to react at1500°c
and the reaction is oom;lete at1705°c
for soaking time of two hours. Beals and Handwerk(3,4)
studied byX-ray•
and chemical analyses theBJ'&tem U02 - Od.2')3 by coainteriag of a mi:l:ture of components at the temperature of
1700°0
and ooaoludecl that a face-oentered-oubic solid solution exists over the entire compositional range between U02 ~nd~03.
On these basea it is obvious that at t7Pioal reactor operating conditions it is not possible to maintain the disorete distribution of poison and that it ia necesa&r7 to find a -4..iftuaior.aal laalrrier between
04203
anduo
2 • This barrier ought to have the following properties,1) to be compatible chemicall7 with- ain;terillg atmosphere of pellets;
2) to be compatible chemioall7 with U02 and ~ 3 both in isothermal and gradient temperature conditions;
3) to have thermal upansion coefficient veq- close to that of GcJ.
2
o
3 anduo2,
4) to be low-neutron-absorber,
5)
to be stable under irradiation.These requirements are satisfied OJU.1' by some of the so called refractory metals such as tungsten, molybdenum, ·niobium and. tantalum.
These latter are to be excluded because the atmosphere of sintering is lqdrogen and niobium and tantalum have a strong trend to hydriding
(5,6).
!oth tungs..iten and molybdeDWD aatiaf)' to miolear requirements also it molybdenum. :i.s the lowest neutron absorber. The molybdelltlll seems 'to bethe mQst suitable material. Revertneleaa his thermal expansion coefficient is somewhat different from that
04203
anduo
2 ~'!'he compatibility between stoiohiometrio
uo
2 and molybdenum has been studied upto 2000°0 by Sanderson et others (7) and their,results show that no reaction occurs up to 2000°0.
On the oontrary no published data are available for the system <Jc1i).,-Mo and at oonsequenoe it was neoess&r7 to perform some experi•n,es on the compati-bility between the two materials.
On these bases, the problem of preparation of
uo
2 poisoned with Gd203
has been affronted in the following wa.,:a) evaluation ot ~igh temperature oompatibilit7 between Gd203 and Mo;
b) optimization ot Mo-coating prooe~e;
o) studJ' Jf the thermomeohanioal beha~iour ot poisoned pellets and of the criteria to avoid oracles in the Mo-coating
d) sintering and characterization of poisoned pellets;
e) high temperature evaluation of poisoned pellets both in isothermal and in gradient temperature conditions.
2. EVALUATION OF HIGH TEMPERATURE Od203-Mo COMPATIBILITY
In order to evaluate the compatibility 'between molybdenum and gadolinia at hig temperature, pellets of molybdenum powder with dense gadolinia miorospheres have been attained through current powder metallurgy prooedurea. Molybdenum pellets were hydrogen sintered and a densit7,of about
9~
of theoretical value was oqtained.The denee molybdenum pellets gadolini,e,,d.oped were undergone th•rmar treatment of2000°C up to 20 hour. lio reaction at the interface between molybdenum and gadolinia microsphere j.s evi,dent (fig.1). In consideration of this observation it has been assumed that the compatibility of the molybdenum with urania and gadolinia is good enough to recommend the mol7bd.ertUID as reaction barrier between the ura'ni• and gadolinia.
3.
OPrIMIZATIOlf OF Mo-COATING PROOEJ)URJVapour deposition process in fluidized bed is the best methol tor coating urania miorospheres.'l'he apparatus used for ooatirl6 the gadolinia microspherea with molybdenum layers is the sQJDe which is desori'becl elsewhere (8).
The molybdenum coating methods availableare the thermal decomposition ot
-7-Jlol7bdenum exaoarbonyl thermally decomposes at temperatures in the order of 200°0 • .ls carrier gas it has been used argon. This tne of ooat,ing gives l&7ers little compact and adherent -to the gadolinta.
'l'he coating t7Pe based on reduct1.on of moly'bdel1'WD pentachloride involves the following reaction
2 Mo + 10 HCl (1)
'l'he process is oariied out as :followss qdrogen (and an inert gas carrier) is passed ove~ or tnrougb the solid or liquid metal halide housed in a boat and heated in order to obtll.in the 20 or
3°"
(1>7
volume) of metal halide in the vapour phase. Then the gas mixture flows through thecoating chamber, reacting at the heated surface and depositing an adherent 1-,er of non-volatile reaction products. 'l'he spent gases pass on out of the oh.amber and are either suitably disposed off.
~clrogen used in the process must have a dew-point of
55°0,
sotraps of moleculare sieve were used. 'l'he carrier gas flow was
2,5
1/min. '1'he optimum evaporation temperature resulted to be190•c,
because at lower temperatures the chloride condensed in the colder part of the apparatus and obstructed it. J'or ihis reason lqdrogen and oarrier gas (argon) were preheated before being flown through the evaporator.Fig. 2 eh.owe some polished- oross-seotion of llo-ooated 042')3 miorospheres~ 'l'he mol7bdemua lqer clensit7 is about 8<>% of the theoretical valwa and the total chloriae pick up about 800 PP••
4
.omunc.u, :BEili
V!OURo,:
Ko-00.A.'l'IlfO. I o
Because of the different thermal expansion ooeffioients of uraa.ia,
mol7bdenwu a~ gadolinia (fig.)) (8-19)Jdepar.t.ing from the deposition
tempera~e, i.e. equilibrium temperature, (null stresses), there is the rising of some atreasea; the related study is oomplioated b7 the creep
phenomena in all the materials involved and b7 the faot that is difficult to foreoaat quantitativel7 the effect of the present porosities.
It has been calculated.
(20-27)
the stress in the Jlo-coating b7 taking up the elastic •ehaviour of Mo- and ~3 b7 considering a simple surface contact be1ween the two materials.
2ti..B
..J..
r 2 B01 2 BC2 1r
=-~
T r d r+
-
-1 - 'f' 3 1 - 2v 1
+
'f' • 3r
r r
(2)
.zl,,
B 1 r 2 B01 BC2 1"<
E'l'<r
s-j
'l'r dr+ , - 2v +--t 1 - V 3 1 + 'f' r3 1 - .,.
r
(3)
where
d"
r and.a-
t are reapeotiTel7 raclial and ciroUDlferential stress, a and r ue rea;veotivel.7 the outer aad. the inner radius, .L the thermal expansion ooeffioient, Eis YouDB'• modul1ta, vis Poisson•• ratio, T is the absolute temperature andc
1 and~ are integration constants.The solution
ot
these equations is performed by assuming the oongruenoe and the equilibrium between •o and 0d203.Since the o1rcwnf'erential stress ia predomiaant on the radial stress, the values of the oiroumterential stress at different temperatures are shown in fig.4 for mioroapb.erea diameter of
JOO,
500
andTOOjtl
andJlo-ooat1AB thickness of
5, 10
and25
Ju
J the equilibrim temperature has been undertaken once 300°0 (lo deposition fro• Jlo (00)6)and once 900°c(Ko cleposition from lo 015). It oan be seen that at temperatures relat1vel.7 low, the value of
CT't
is higher than the ;yield pointu-7 •
A comparison between the two llo-cleposition processes abowa that lower
stresses arise in Ko-coating obtaiJled thrpugh the h;ydrogen reduction of ohlorldeJ for this reason the chloride reduction baa been the process followed tor Ko-coating
at
gadolinia miorospheres.As a consequence of the stress results the-0&1.0lf,la..tion of t~e 'bursting
pressure of the Jlo-ooating baa been i,erforaed. !he bursting preaaure for a ,hin spherical shell (29) is givea b7 t~e relatioaa
...
2n+ 1
»
•o;
T (:
t
3n (4)where t aad Rare reapeotivel7 the th~okness and the radius ot the shell,
e ia the basis of natural lo,arithma,
cr'
0 a.nd nare constant deducedfrom the diagram
d -
tin the simplified. forms· •
d
O f, n • The variation ot the bursting pressure with the geometrical parameter& of the ~03 and the Jro-ooating is shown in fig.5 tor a temeperatureot about
1300°01the • i n oonoluaioa is that an ia.crease in the ahell thickness baa a a .oonse
-quence an inore&aeot
the 'buretins pressure of the molybdenum shells for this reason a mol.7bderwm shell thiolcnese in the range 25 - 30 microns haa been adopted for coating the gadolinia miorospheres.9
-miorospheres in U02• It is
veryhard
to
describe the mechanical phenomena which occur on the miorospheres during the densifioation stage of U02 athe most realistic assumption is to consider thatuo
2 sinters with null gap on thegadolinia mol7bdell\JJll coated inclusion. In this case it is neoessa17 to perfol"Jll the calculation of the-pressure exercised b7 the mol7bde11WD coating on the Uo
2 matrix iuring the cooling from the temperature Ti (aull stress) to the room temperatureJ for this calculation the
miorospherea are considered as molJbdenum inclusions. In the elastic field, the U02 matrix is subjected to a pressure (30,31) given b7 the following relation:
P (T) • 12 G IC A
tJ::.dJ
(5)31:+40
0
Go• 0,29
J\ro '
IC • 1l\.o
A
T • ! - ! (6)where
- 2T
'
i2 Ko
B is the Young•s modulue, v the Poisson•s ratio and A J., the difference
between thermal expansion coefficients.
J'ig.6 shows the variation of p versus T for two different values of Ti. The tensile strenght of
uo
2 matrix deduced on the basis of 'bending test(32) is in the range of
10-15
kg lmm2; at consequence during the coolingstep there will be the formation of cracks in
uo
2a experiments performed in order to eDmine this behaviour showed that the cracks originated during the cooling of U02 sintered pellets concern a radial zone about 200microns deepJ generall7 the cracks are stopped 'b7 porosities or grain boundaries (fig.7).
In all the above speoulatiTe remarks, the relationship and. the phenomena in the plastic field are not considered.
Unfortupatel.7 the calculation of the deformatiou and the creep behaviour of molybdenum o<ated gadolinia inclusions is ve17 difficult to perform
also in 00111tideration of the poor creep
aata
of the material involved in the analy•ia. From a qualitative ~oint of view, it is eas7 to forecast that the beat performance of gadolinia miorospheres occurs whenthe miorospheres have porosities that ma7 be absorbed b7 creep in order to oompensate the thermal expansion diff'erenoe between the mol7bdenum and. the gadolinia.
The 8Dmination of the influence of the gadolinia porosities on the
'.l'he pertonano• teate haTe been done,thro~h heat treatment in qdrogen atmosphere
at
1700°0 up toSO
houra,on theuo
2 pellets poisoned with25
miorone mol7bdenva coated -500
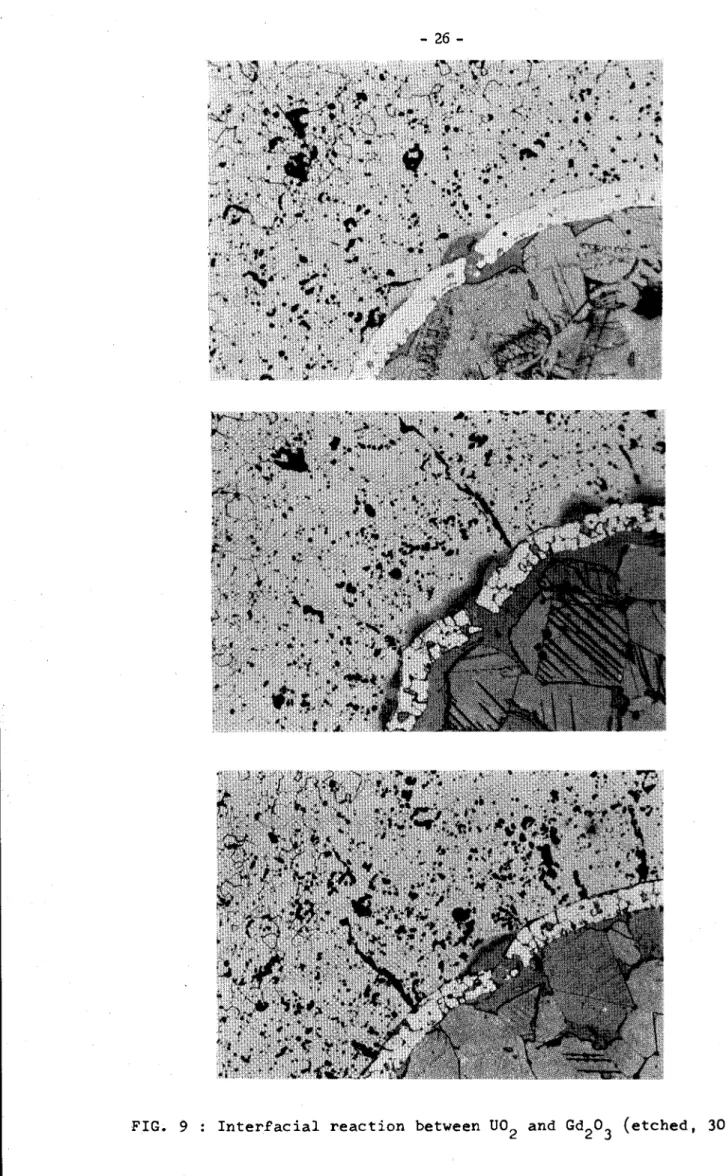
microns diameter gadolillia miorospheree at the a!)ove reported densities. The result• obtained are SUDUD&rised in fig.Sa it oan be seen that the mioroapherea with poor densit7 undergoa grain groY'th )Z'ooesa with the ooaleaoeaoe ot tine porosities in lar.ge poroeitieas it can be aeen that the final 4ell&it7 of the mioroapherea iS quite different troa 'Wte initial va.lueJ it can l»• seen that after 10 hour• the miQroapheres with the crenait7 ot
94~
of theoretical value(!.D.)
~ow some ore.aka in the $"ting an.a. some reaction 'bf.irtwee11uo
2 and.04203
oo~a; it is interesting to observe that also after a treatment of
50
~ours at 1700°0 these mivroapherea do not show a very extensive reaction
(ttg.9).
The ooatiDBS ot all the other mioroepheres are Dot injurei.
'.l'he ntaia oonolusiou are that mio::r:oapheres with jlensity in the range
85-901'
ot
theoretical T&lue are the beat solution to be ac\opted'in orderto produoe U02 pellets poisoned. with mol7bclenum coating Gd.
2
o
3 miorospherea.iI• o~dei- to enluate the influence of the gad.olinia miorospherea inolusion
on the ainteJ:'ing rate of U02 pellet•, aintering experimeata have been carried out on pressed pellet, dopel nth 600 and 1200 ppa of gadolinia aioroephvea,400-500 m.iorou of tiaaeter,
23-30
miorou of coating'tihiolmese. '!he ur&J'lia, oeraaio grad.e powderr used tor pellets preparatiqn
-.a
supplied b7 lfukem Wolfgang(Oe:rmaJJT).
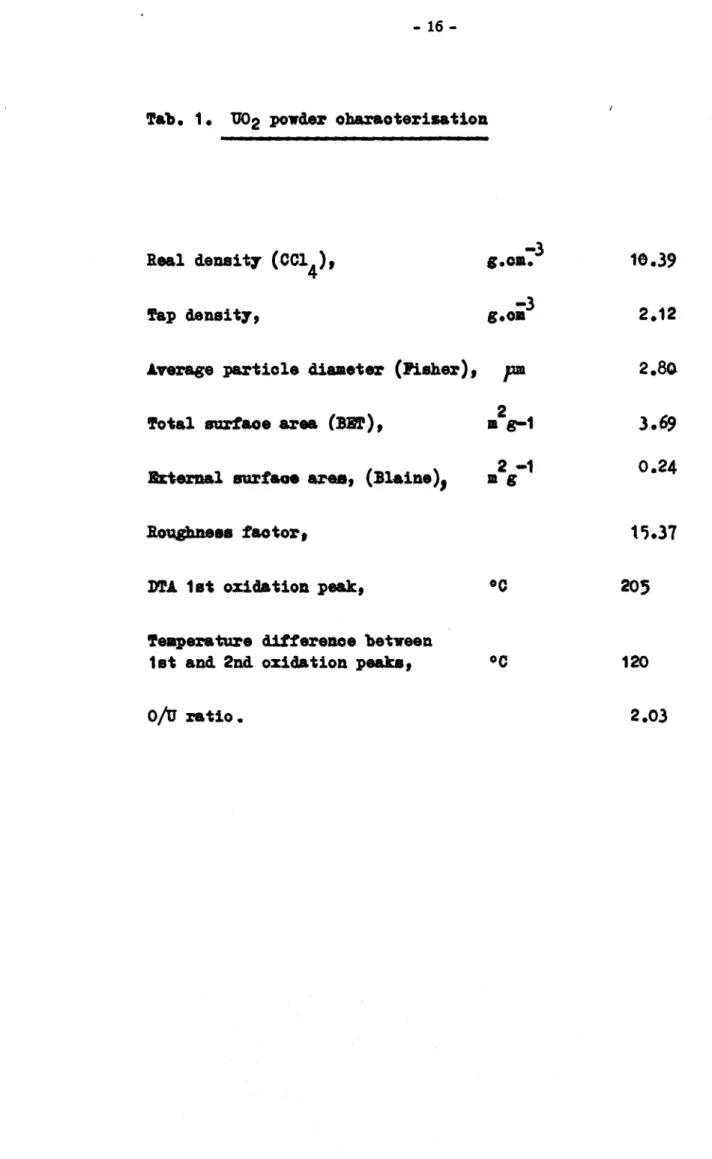
The powder propertiea are 8WIUD&rizecl in Tab.1.
The mixture
waa
pressed into pellets up to green denait7 of50-55%
T.D. The green pellets were hq.tecl in hydrogen to a maximum temperature of1700° at a rate of 300° 0/h. The sintered densities o)tainecl are shown in fig. 10.
Fig. 11 abows an isothermal sintering curve for the same pellets. In both cases no appreciable behaviour differences between pure and poisoned U02 baa been observed.
The poisoned
uo
2 pellets wore characterized b7 the following teatss 1) porosimetr7;
11
-In tig. 12 it is represented the pore distribution tor unpoisoned and poisoned U~ and in tig. 13 there are represented the amounts of open and closed porosity, al~a for unpoisoned and poisoned UO;!. In both
cases no a~preciable differences exist among the pellets.
The main conclusions are that the sintering mechanism ot the urania is not influenced by the gadolinia microsphere additions at the foreseen
con~entratioru so· no difterence in open-closed porosity distribution and
in ~pen porosity diameter has been observed tor poisoned.and unl)Oisoned pelle1a,.
loreover, corrosion teats pertormed in pressurized water at 160 atla ~d
340°C
tor50
hrs did not show differences in behaviour of poisoned and unpoisoned pellets.6.
EVALUATION OF POISOIEDuo2
PELLE'l'S AT HIGH 'l'DIPERATUBEThe poisoned U~ pellets were evaluated from the compatibility point of
view under high temperature gradient conditions and through high
tanpe-rat,uoe long time treatments.
6.1 Dermal gradient experiments
'l'he thermal gradient experiments were carried out with an apparatus described elsewhere (33).
Tiie heating is performed through an electrically heated tungsten rod; the •kin of pellets stack is cooled through a water jacket; the controlled atmosphere was obtained through an argon flow.
'l'he following ex;eriments were performed:
i. temperature of the core of pellets stack:
2300°C;
temperature of the skin of pellets stack:650°c;
for 1 hour and 3 hours (fig. 14)1
ii. temperature ot the core of pellets stack:
2500°c1
temperature of the skin of pellets stack: 670°C;for
20
min. and 1 hour (fig. 15);iii. temperature of the core of pellets stack:
27oo•c;
temperature of the skin of pellets stack: 700°C;for
5 min. and
20
min. (fig.16);
'l'he results obtained by t~ performed experiments showed that the behaviour
ot
the gadolinia poisoned pellets is quite aatistaotor,y under high thermal padient; ~.erall7,the Mo-coating gadolinia miorospheres were not damaged in all the tests.6.2. Isothermal e%1)8riments
'l'he isothermal experiments have been carried out on poisoned pellets h•~ted through a tungsten induction heated container. 'l'he experiments were performed at 2200°C for 10 hours. As it can be seen in fig-.18, no damage on the coating layers has been obtained, but generally the porosity is displaced in a particular area of the interface between the gadolinia microsphere and the mol7bdenum layer. Generall7, this
area was located where microoracks between the mol7bdenum coating and the urania are, presents a possible explanation
of
this phenomenon oan be given assuming that the microcraoks aot as discontirmitesregions regarding the thermal behaviour and so the porosities migratio~ occur preferentially in this area.
At this temperature for long time or at higher temperature an
intergranular diffusion of the molybdenum into the gadolinia oan be observed (fig.19) and in this case the process produces oraoka in the ooatiag with consequent
uo
2 - 04203 reaction (fig.20).7.
CONCLUSIONSThe following main conclusions oan be drawri from the performed research work:
1) a molybdenum coating on the gadolinia miorospheres avoids the U02 - GdQ03 reaction;
2) in order to have a coating which maintains its integrity in spite of the different thermal expansion of the molybdenum, gadolinia and urania miorospherea with a density in ~he range 85-9Q% of the
theoretical density and molybdenum coating thickness in the range 25-30 microns must be used;
3) the sintering behaviour of the UO? powder is n(\+ affected by additions up to 1200 ppm of 500 microns diameter gadolinia microspheres coated with
JO
microns of molybdenum;4) the perfomance of the poisoned pellets under out of pile thermal gradient up to 2900°c is aoceptableJ
13
-6.
isothermal test showed. that at high temperat,u,e (more than 2200•0) an intergranular diftusioa of the mol7bdellUII b7 the coating into the gadolinia occurs and this phenomenon carries to a dist~otion of the ban'itr.8. REJ'ERDOES
1)
Abate-Dag&, a.,.Amato, I.1and Grappiolo,a.,
l'uolear tuel with burnable poison., part.1. G~03 mj..croaplJeres preparation from the sol-gel method, J'iat Report;. Gil - 53 (1969),. EOR 4550,e.2) Joint US/liDRA'l'OM,R& D Program AT (11-1) 1229, Final Report, Fabrication Development .AOJP -
64
,S5 (
1964).
l)
Beals, R.J.,and Handwerk, J.R.,J.
Jm. Cer. Soo.,48,
271 (1965).4)
»eals, R.J., Bandverk, J.H.,and. Wrona, B.J., J.Am.
Cer. Sop.,52, 1,
578 (1969).
5)
Amato, I., Energia Nu.cl.,14, 12, 730
(1967).6)
Amato, I.1and Negro, A., J. Less-Common Meta.la,16, 468 (1968).
7) Sanderson, M.I., Groa•an, L.B., Hill, R.W • .,and Iramnoff, A.I., C ompatibil1t7 ot nuclear tuel and thermionic materials, '!hermionio
Conversion Specialist Oontereaoe, Gatlinburg, 'l'ennessee, October 1963.
8) Amato, I.,and Ardizzone, L., Rev. Int. Bautea Temper.et Retract., t.4 297 (1967).
9)
Kempter, C.P.Jand Elliot, R.o., J. Chem; Phys., 30,1524 (1959).
10) Roth, J., l'uolear Materials and J!lluipment Corp., Progress Rept. lumeo,
2389
(1963).
11)
:Battelle Memorial Inst., Quarterl7 Rept.5,
l!llRAEO,317 (196').
12)
Steoura, S.;and Campbell, W.J.tu.s.
!u.r. Mines Rept. Invest. D-ll-5847 ( 1961).13) Ploetz, 0.1., Xra1711iak,
c.v.,ana
Dulles, H.B., tJSAEO UPL-M-OLP-1 (1957).14)
Begb.1, a,,per~oaal communication.16. Fieldhouse, I.B., Hedge, I.e., et al., Armour Res. Foundation, WADC-'l'R 55-495 (1956)
17. Rasor, N'.S., and Mc Clelland, J.D., WADC-TR 56-400, (1956).
18. Lucks, C.F., and Deem H.W., Battelle Memorial Inst. Rept., WADC-TR 55~496 (1956).
19. Goldsmith, A., and Waterman, T.E., WADC-TR 58-476 (1959).
20. Climax Molibdenum Company, Inc., Molibd.enum Metal, p. 23 (1960). 21. Parke, R.M., Metal Progr. 59, 81 (1951).
22. fansteel Metallurgical Co., Data Sheet.
23. Metallwerk Plansee, Aktiengesellscha.ft, Reutte, Tirol, .Molibdenum p. 13. 24. Z-uieva, L.s., Godina, N.A., and Keler, E.K., Refractories, 7 and 8 290
(1960), translation of Ogneupo:ry, 25, 8, 368 (1960).
25. Keler, E.K., and Andreeva, A.B., Ogneupory, 5, 224 (1963).
26. Curtis, C.E •• and Johnson,
J.a.,
J. Am. Cer. Soo., 40, 1, 15 (1957). 27. Shaffer, P.T.B., Material Index, PlenuaPreas, New York (1964) P• 338 28. Timoshenko~ S.P., and. Goodier, J.lf., Theory of Elasticity, 2nd &i.,Mc Graw-Hill (1951), pag. 418.
29. Svensaon, N.L., TransactiC>Jl ASJIE., 80, J. Appl. Mech., 326 (1958). 30. Bonilla, C.F., lfuolear Engineer1ng, lie Graw-Hill ( 1957), pag. 577. 31. Amato, I., Ardizzone, L., Crisa, V. and Dusi, G., Rev. Int. Haut Temp.
Retract., t. 5, 117 (1968).
15
-A
C
JC lfO W
LB D O I
K JC :I 'fS
The authors would like to express their gratitude to Mr.
o.
llorocuttiot
lib.ratom for the enlightening and construotin discussions. !hanks are due to E. v,rona who pertormed the oaloulations on the meohan.ioal behaviour of coatedTab. 1. U02 powder oharaoterisation
Real de11Sit7
(cc1
4),-3
g.oa.
1&.39
!'ap densit7, g.oa
-3
2.12Average particle cliameter (Pisher),
pm
2.80lfotal nrfaoe area (:SIil') ,
• s-1
2J.69
Brlernal aurfaae area, (Blaine), Dl 2 -1 g 0.24
Roqgb.Deea factor, 15.37
D'l'A 1st oxidation peak,
oc
205Temperature difference betveen
1st and 2nd oxidation peaka,
oc
12017
-(100 x)
( 350 x)
FIG 1 Compatibility between Gd
FIG 2 Polished cross-section 0£ Mo-coated Gd2o
Reference
{:
(9)
2.4i UOt (10)
(11)
r
cubic (12)~ o monoclinic (13)
•
Gd.o,
11A monoclinic (14)C 0 monoclinic (15)
.2 2.0
Ill
Ii
l16)C
"
Mo (17)~
(18)
)&
.,
(19)-· I-·
1.6~
s:
-
...
g
1.2 j -~~~ ~~ - " 1111 II
Ill~
I
....
·.:; \0
0,8
0
0 200 400 600 800 1000 1200 1400 1600 1800 2000
Temperature •
oe
FIG. 3 - Li•ear thermal expansion ot
uo
1SO
f'1
Ti= 1700 oC/
.t
so
1700 1SOO
Ti: 1000 •C
1000
Temper~lure, •c
SOO
FIG. 6 - Pressure on the U02 matru: from the molybdenum coating during the pellets cooling
J
200
2
"e 1,S
e "' "" f! ~ .., "' ~ Q. "' .!: ~ ~ m O,S
300 400
- ~
~
t = S.B_
SOO
Gd,O, microspheres di~meter, JJ,
FIG. 5 - Variation of the bursting pressure as a :f'unotion of ~03 miorospheres diameter and molybdenum ooating thiokness
q,j
FIG. 7 Micrographic aspect of
uo
2 pellets
Ga
2o
3 poisonedinitial after 7 hours after 15 hours after 30 hours
Fig. 8 - Micrographic aspect of molibdenum coated Od203 microspheree; initial pellets density:
94-9~ T.D.; Gcl203 microspheres densities: (above to below) 81%, 84%, 8~, 94% T.D.; thermal treatment: 1700°c up to 50 hours (unetched.1 120 x, reduced to 2/3).
after 50 hours
FIG. 9 Interfacial reaction between
uo
2 and Gd20..fOO
..-:
o
95~
-,...,
..,_
. ~ 90
C
.» ~
~--i§--5!
•-/'Ti .
i}}
Srntert~hme : 2 hrs
1S
"
~ 8: l!! C-.J') I
i
80~
I
• U02
a
U02 + 600 Pt,)"'udz
05o
U01 +12ooppmudzO,
75
1200 ~30u .• - - -1400 . ~ - - ~500 .
--1600 -1700
5interini temperature~,
0C..
FIG. 10 - Sintered densit7 versus temperature of poisoned and unpoisoned
uo
2 pellets (Sintering times 2 hrs.)
--="95
.
.,,
C
.,
""1'
-"!
go~
.,
..
C:·-
,J)a,
..----~-
k
/
"1}-lftt
I
Sintering temperature : 11.fOO °C
• U02
a
UC>,.
+600ppmG~
20,.
o
U01+'1200ppm
ua
10
5soL----~~~---~~~---~~~-r~~~-r~~~---r~~~~-.
0 1. 2 3 4 ~
~inter·1ng t,me. ,
hour!>.FIG. 11 - Sintered density-versus time of poisoned and unpoisoned
uo
2 pellets(Sintering temperatures 1400•0)
I
~8 j
~161
,!:' i4
·-
,n0
..
,2
0.0..
10~
tO
....
0 8
~
6
4J
2 I
0
•
U0
2•
U02 • 600ppm ·Gad O .
z 5 mi<:ro)phere~ 0 ~~:7
•
V01. • i200ppm
<":ad ·~ .
, niitrosphere~open
poro!t1ty/~~
c.lo!.ecl
p ~
80 85 ~o
95
100Theore
ti
ea\ clen~~
t
1
1.
PIO. 13 - Open and closed porositJ- aa a f'unotioa of deasitJ- for poisoned an4 unpoisoaed
uo
2pellets
I
c.,.,
....
ll'IG. 19 - 'fn>ioal miorostrqo~e gf Mo-coated.
~o
3 aiorospherea attv heat treatment. Temperature 2300°0; time 10 hrs (unetohed, 100 x)
ll'IO. 20 - '1'7Ptoal miorostruature of llo-ooated Od203 miorospherea after heat treatment. 'l'emperature 2J00°0J time 20 hra
NOTICE TO THE READER
All scientific and technical reports are announced, as and when they arc issued, in the monthly periodical "euro abstracts", edited by the Centre for Information and Documentation (CID). For subscription (1 year: US$ 16.40,
£ 6.17, BF 820) or free specimen copies please write to:
Handelsblatt GmbH
" euro abstracts "
Postfach 1102
D 4 Diisseldorf 1 ( Deutschland)
or
Office de vente des publications officielles
des Communaut& europeennes
3 7, rue Glesener Luxembourg
'i.
~ .
To disseminate knowledge is- to disseminate prosperity - I mean
general prosperity and not individual riches - and with prosperity
disappears the greater part of the evil which is our heritage from
All reports published by the Commission of the European Communities are on sale at the offices listed below, at the prices given on the back of the front cover. When ordering, specify clearly the EUR number and the title of the report which are shown on the &ont cover.
SALES OFFICE FOR OFFICIAL PUBLICATIONS OF THE EUROPEAN COMMUNITIES
37, rue1Glesener, Luxembourg (Compte cheque postal N° 191-90)
BELGIQUE - BELGI:£
MONITEUR BELGE
Rue de Louvain, 4042 - 1000 Bruxelles BELGISCH STAATSBLAD
Leuvenseweg 4042 - 1000 Brussel
DEUTSCHLAND
BUNDESANZEIGER
Postfach -5000 K'oln 1
FRANCE
SERVICE DE VENTE EN FRANCE DES PUBLICATIONS DES
COMMUNAUTES EUROPEENNES
26, rue Desaix -75 Paris 15"
ITALIA
LIBRERIA DEU.O STATO Piazza G; Verdi, 10 - 00198 Roma
LUXEMBOURG
OFFICE DE VENTE
DES PUBLICATIONS OFFICIELLES DES COMMUNAUTES EUROPEENNES
37, rue Glesener -Luxembourg
NEDERLAND
STAATSDRUKKERIJ
Christoffd Plantijnstraat -Den Haag
UNITED KINGDOM
H. M. STATIONERY OFFICE P.O. Box 569 - London S.E.1
Commission of the
European Communities
D.G. XIII - C. I. D.