Equilibrium Studies on Binary and Ternary Complexes of
Transition Metal Ions with Thiouracil as Binary and
some N-Donor Ligands as Ternary Ligands
V.Ravinder Reddy*, A.Rajeshwar Rao† and K.Venugopal Reddy#
*Department of Chemistry,
Govt. Degree & P.G. College, (Osmania University), Siddipet, Telangana, India. e-mail: ravinviru@gmail.com
†Department of Chemistry, Narsimha Reddy Engineering College,
Jawaharlal Nehru Technological University,Hyderabad Telangana, India. #
Department of Chemistry,
Osmania University, Hyderabad, Telangana, India.
(Received on: May 18, 2015)
ABSTRACT
Equilibrium studies have been carried out on the formation of binary MA and ternary MAL complexes [where M = Co(II), Ni(II), Cu(II) and Zn(II); A=2 – thiouracil (TURA); L = alanine (Ala), phenylalanine (Phala), tryptophan (Trypt), 2, 2 – bipyridyl (bipy) and o –phenanthroline (o –phen)] by pH metric technique in aqueous medium at 25, 30, 45 oC ± 1 oC and 0.1M KNO3 ionic strength. All metal
ions were formed 1:1 and 1:1:1 ternary complexes. The thermodynamic parameters,
∆
,
∆
and ∆
associated with binary and ternary complexes systems were
calculated and discussed. The relative stabilities of ternary complexes are quantitatively expressed in terms of the statistical parameter ∆logK. The results were discussed in the light of the basicity of ligands, statistical aspects, electrostatic interactions, density, stacking interactions, nature of donor sites and stereo chemical aspects. The concentration profiles have indicated the favorability of the formation of binary and ternary complexes in general as reflected in ∆logK values.
Key Words: Equilibrium Studies, Binary, Ternary and Transition Metal,
Complexes, Pyrimidines.
INTRODUCTION
reactions in biological systems. Metal ions also play an important role in the initiation and propagation of polymerization reactions of RNA. Hence, the study of interaction of metal ions with several purine and pyrimidine analogs gained much attention1-5and their biological role in many living systems6-8. As reported earlier on stability of mono and disubstituted purines9,10 and pyrimidines11-15 with transition metal ions in aqueous medium at 45oC and µ = 0.1 M (KNO3). In continuation of previous work, we present here the formation constants of binary and ternary complexes of 2 – thiouracil with bivalent transition metal ions in the presence of other ligands (L) containing N-N and N-O donor sites in aqueous medium. The required dissociation constants and formation constants of ligands (L) with Co(II), Ni(II), Cu(II) and Zn(II) were determined under the present experimental conditions and values thus obtained were presented.
EXPERIMENTAL
The ligand, 2–thiouracil was purchased from Sigma Aldrich Chemical Co., USA. Alanine (Ala), phenylalanine (Phala), tryptophan (Trypt), 2, 2’–bipyridyl (bipy) and o– phenanthroline (o–phen) were procured from E. Merck while the metal salts were of AnalaR grade obtained from E. Merck. For binary complexes, the metal–ligand ratios were kept at 1:1 and 1:2. For study of mixed ligand complexes, the primary ligand, secondary ligand and metal ion ratios were kept 1:1:1. The acid dissociation constants of ligands, stability constants of binary and ternary complexes were calculated by direct algebraic method16by known equations. The results obtained with an accuracy of ± 0.06 log units and listed in Tables 1 and 2. The distribution of various species as a function of pH was calculated by using BEST17 computer program for binary and ternary complexes.
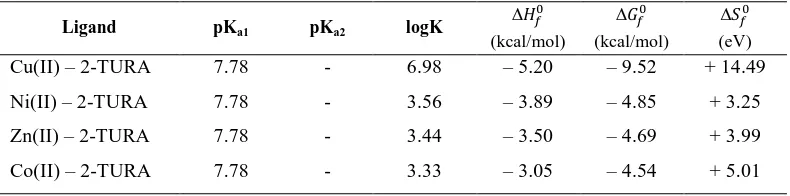
Table 1. Stability Constants and Thermodynamic Parameters of Binary 1:1 Metal(Ii) 2-Thiouracil At 0.1 M Kno3 (25
O
c)
Ligand pKa1 pKa2 logK
∆ܪ (kcal/mol)
∆ܩ (kcal/mol)
∆ܵ (eV) Cu(II) – 2-TURA 7.78 - 6.98 – 5.20 – 9.52 + 14.49
Ni(II) – 2-TURA 7.78 - 3.56 – 3.89 – 4.85 + 3.25
Zn(II) – 2-TURA 7.78 - 3.44 – 3.50 – 4.69 + 3.99
Co(II) – 2-TURA 7.78 - 3.33 – 3.05 – 4.54 + 5.01
Values are accurate to ± 0.06 log K
Table – 2. Stability Constants and ∆ Log K Values At 0.1 M Kno3 (25 O
c)
Ligand logK ∆logK
Co(II) Ni(II) Cu(II) Zn(II) Co(II) Ni(II) Cu(II) Zn(II)
M(II) – 2-TURA Ala 4.21 4.65 7.78 5.05 –0.36 – .68 – 0.44 – 0.07
M(II) – 2-TURA Phala 3.81 4.26 7.72 4.27 –0.40 – .90 – 0.21 – 0.46
M(II) – 2-TURA Trypt 4.09 4.57 7.70 4.75 – .43 – .96 – 0.42 – 0.42
M(II) – 2-TURA bipy 3.72 3.25 5.73 3.42 –0.39 –0.31 – 0.02 – 0.02
M(II) – 2-TURA o – phen 3.47 3.13 6.01 3.58 +0.14 –0.43 – 0.97 +0.14
RESULTS AND DISCUSSION
In aqueous solution proton of alanine (Ala), phenyl alanine (Phala) and Tryptophan (Trypt) dissociates completely and amino group gets protonated to form zwitter ion (diagram-1). Dissociation constant (pKa) corresponding to the protonated amino group was calculated. Ala, Phala and Trypt forms five membered chelate rings with metal ions through oxygen of carboxylate group and nitrogen of amino group. The pyrrolic nitrogen atom of indole ring of Trypt is weakly basic hence, does not participate in coordination with metal ion18– 21.
Ka
(-H+ )
(+H+ )
COO
CH
NH2
R
R = - CH3 = Alanine
= = Phenylalanine
= Tryptophan
N H
= COO
CH
NH3
R
Diagram-1
BINARY SYSTEMS
Neutral 2-thiouracil was taken for the determination of dissociation and stability constant of M – 2-thiouracil. However, potentiometric titrations of 2-thiouracil reveals that it cannot be protonated since it exists in keto form in alkaline solution. N3 atom of 2–thiouracil has greater electron density than N1 due to its location in between thio and keto groups. This is observable from its resonance structures. Hence there is no probability for the N3 atom to gain more electron density than N1. Therefore ionization may be from N3 – H as shown in Fig. 1. Since thiouracil has a thiocarbonyl and carbonyl groups with two potential nitrogen donors hence, it may form very interesting complexes with various metal ions. 2-thiouracil in copper(II) and nickel(II) complexes is bonded through nitrogen atoms after deprotonation and thiocarbonyl sulphur atoms22,23 and in other complexes the bonding may be nitrogen and carbonyl oxygen atom. The stabilities of the 1:1 metal (II) – 2-thiouracil follows the neutral Irving William’s order and decreases in the order Cu(II) > Ni(II) > Zn(II) > Co(II) . Our studies reveal that 1:1 metal – 2-thiouracil complexes are comparatively less stable than 1:1 metal – 2, 4-diamino 6-hydroxy pyrimidine complexes due to basicity difference. In general five membered ring chelates are more stable than six membered rings24. Further interaction of thiouracil group of 2-thiouracil with Co(II), Ni(II), Cu(II) and Zn(II) is expected to be weaken since it involves the combination of soft sulphur donor with border line metal ions . The positive enthalpy and entropy values of metal (II) – 2-thiouracil indicates the complexes formation ofhigh enthalpy values reflects higher stability of 1:1 metal (II) – 2-thiouracil. The effect of temperature on stability of binary complexes of 2-thiouracil, the metal – ligand equilibria were studied at different temperatures, viz, 25, 35 and 45oC, ± 0.1oC. The stability of 1:1 metal – 2–thiouracil complexes were increased in order Co(II) < Ni(II) < Cu(II) < Zn(II) in accord with the Irving - William’s order. An increase in the stability constant of metal – ligand complex with decrease in temperature is observed for all the ligands studied. The potentiometric titration curves for 2-thiouracil in 1:1 ratio of ligand to metal ion at 35oC were formed between a = 0 and a =1 and similar curves were also obtained for Cu(II), Zn(II) and Co(II).
N
N O
S H H
Ka
(- H+ )
(+ H+ )
N
N O
S H
1 2
3 4 5
6
Fig. 1: Dissociation of 2-Thiouracil during titration
TERNARY SYSTEMS
2, 2-bipyridyl (bipy) and o-phenanthroline (o-phen) system, the complex formation is simultaneous as against our earlier reports in which the complex formation was stepwise25. The stabilities follow the order Cu(II) > Ni(II) > Zn(II) > Co(II). The ∆log K values show the metal – 2-thiouracil –L (1:1:1) is less stable than their analog (1:1) binary systems. The temperature dependent ∆log K are negative indicating that these systems are less stable than the binary 1:1 metal – 2- thiouracil complexes which are in accordance of statistical considerations. The tentative structure of mixed ligand complexes of M (II) – 2- thiouracil – L is shown in Fig 2.
The thermodynamic data shows that the enthalpy values are exothermic and entropy changes are positive. The negative delta log values do not mean that the complexes are not formed but may be due to a combination like steric hindrance, electrostatic repulsions and natural secondary ligand (L). The factors operating in the stabilization of ternary system may play an important role in determining the stability of enzyme – metal - substrate complexes formed in metallic enzyme catalyzed reactions in biological systems.
N O O
N
M(II)
H2 H2O
HC
RH2C C O
Fig. 2
N H S
H2
The stability constants of alanine or phenyl alanine or tryptophan 1:1 system follow Irving - William’s order of stability and increase in order Co(II) < Zn(II) < Ni(II) < Cu(II) . The stability decreases for all the three systems as temperature increases. The enthalpy values are positive indicating that both entropy and enthalpy are favoring complex formation. The stability constants of 1:1 metal o- phen or bipy follow the Irving - William’s order of stability and decrease in the order Cu(II) > Ni(II) > Zn(II) > Co(II) . The stability constants of the metal complexes decrease in the order o-phen > bipy. This is in accordance with the basicity of the ligands. The enthalpies of formation of metal – o–phen system is more exothermic than metal bipy system and entropy of formation of 1:1 Metal – bipy is more positive than Metal – o - phen system.
REFERENCES
1. D.J. Hodgson., Prog. Inorg. Chem., 23, 211 (1977). 2. L.G. Marzilli., Prog. Inorg. Chem., 23, 255 (1977). 3. E.S. Raper., Coord. Chem. Rev., 61, 115 (1985).
4. M. Goodgame and D.A. Jakuboric., Coord. Chem. Rev., 79, 97 (1987).
5. S.H. Lavrie, Comprehensive Coordination Chemistry, G. Wilkinson Ed. Pergamon Press, Vol. 2 (1987).
6. A.T. Tu and M.J. Heller., Metal Ions in Biol. Systems., 1, 1 (1973). 7. C.M. Freey and J.E. Stoehr., Metal ions in Biol. Syst., 1, 51 (1973).
8. R.B. Martin and Y.H. Mariam., Metal ions in Biol. Syst., Vol. 8, 57 (1979).
12. M.M. Taqui Khan and S. Satyanarayana., Ind. J. Chem., 20A, 814 (1981). 13. M.M. Taqui Khan and S. Satyanarayana., Ind. J. Chem., 21A, 913 (1982).
14. M.M. Taqui Khan, S. Satyanarayana, M.S. Jyothi and Ch. Abraham Lincoln., Ind. J.
Chem., 22A, 357 (1983).
15. A. Grigoratos and N. Katsaros., Inorg. Chim. Acta., 108, 41 (1985).
16. C.F. Richard, R.L. Gustafson and A.E. Martell., J. Am. Chem. Soc., 81, 1033 (1959). 17. R.J. Motekartis and A.E. Martell., Can. J. Chem., 60, 2403 (1982).
18. K.P. Anderson, W.O. Green Lalgh and R.M. Izzatt., Inorg. Chem., 5, 2106 (1966). 19. K.P. Anderson, W.O. Green Lalgh and E.A. Butter., Inorg. Chem., 6, 1056 (1967). 20. D.J. Perkin., Biochem. J., 55, 649 (1953).
21. O.A. Weber and V. Simen., Biochim. Biophys. Acta., 94, 244 (1971).
22. Thomas, WolfHans, Weser, Ulrich., Hoppe-Seyler's Z. Physiol. Chem., 358(1), 47 (1977).
23. Juan F. Villa and Harold C. Nelson., J. Ind. Chem. Soc., 15, 631-636 (1978). 24. R. Cadlin and M.M. Harding., J. Chem. Soc. (A)., 384 (1970).