Controlling Oxygen Content by Varying Oxygen Partial Pressure in Chromium
Oxynitride Thin Films Prepared by Pulsed Laser Deposition
Kazuma Suzuki
+1, Toshiyuki Endo
+2, Teruhisa Fukushima
+2, Aoi Sato
+2, Tsuneo Suzuki,
Tadachika Nakayama, Hisayuki Suematsu and Koichi Niihara
Extreme Energy-Density Research Institute, Nagaoka University of Technology, Nagaoka 940-2188, Japan
Chromium oxynitride Cr(N,O) thinfilms were prepared by pulsed laser deposition in a highly reactive atmosphere, which consisted of pure oxygen gas and nitrogen plasma from a radio-frequency radical source. In order to control the oxygen content in the thinfilms, oxygen partial pressure (PO2) in the chamber was varied from 5 to 10©10¹5Pa under afixed total pressure of 1.5©10¹2Pa. The thin films were then
characterized by X-ray diffraction, infrared spectroscopy, electron energy loss spectroscopy and nano-indentation testing. It was found that the oxygen content of the thinfilms changed from 0 to 62 mol%with increasingPO2. The thinfilms with only the NaCl-type (B1) Cr(N,O) phase
were prepared under PO2<7.5©10¹5Pa. The chromium content of the B1 phase decreased from 47 mol%, which was close to the
stoichiometric composition of CrN, to 40 mol%when the oxygen content was increased. The hardness increased up to 32 GPa with increasing
PO2up to 7.5©10¹5Pa. [doi:10.2320/matertrans.M2013047]
(Received February 4, 2013; Accepted April 2, 2013; Published June 25, 2013)
Keywords: chromium, nitride, oxynitride, hardness, laser ablation
1. Introduction
Hard coatings have been applied on cutting tools, auto parts and mold tools to improve their characteristics which include high hardness, wear resistivity, chemical stability, oxidation resistivity and so on. Diamond-like carbon (DLC) and transition metal nitride have been widely used as the hard coating materials. In particular, chromium nitride (CrN) has excellent properties such as oxidation resistivity and chemical stability.1,2) However, CrN thin films typically have low hardness compared to other materials such as titanium nitride (TiN) and titanium aluminum nitride ((Ti,Al)N), which is limiting the use of CrN coatings.
In our previous work, chromium oxynitride (Cr(N,O)) thin
films were prepared by pulsed laser deposition (PLD).3,4) PLD is a deposition method excelling sputtering and arc ion plating in composition control of thin films. Inumaru et al. reported that PLD could prepare epitaxial thin films to evaluate the characteristics which include the electronic and the magnetic properties.5) Cr(N,O) had a NaCl-type (B1) structure similar to that of CrN. The hardness of Cr(N,O) thin
films increase with increasing the oxygen content and the maximum value exceed 30 GPa.4) Materials with added fourth elements to Cr(N,O) for improving hardness have also been studied.6,7) For example, chromium magnesium oxy-nitride ((Cr,Mg)(N,O)) thin films exhibit hardness values as high as 35 GPa.6)In addition, Urgen et al.has described the effect of oxygen content on the tribological behaviour of Cr NO coatings,8)and then I. M. Rosset al.has used CrNO layers in multilayer films for enhancement of the oxidation and tribological performance.9)
Since the hardness of Cr(N,O) increases with increasing the oxygen content, precise oxygen content control and its measurement are required for identification of the solubility limit and elucidation of the hardening mechanism. Until now,
Cr(N,O) thinfilms were prepared by depositing Cr vapor in N2 or NH3 ambient gas with residual oxygen.3,4) However, the oxygen content control was difficult in this method. In the current work, an intuitive method using modification of the oxygen partial pressure (PO2) for the precise oxygen content control is proposed. A highly reactive atmosphere of O2 mixed with N radicals is used in this method. Chromium oxynitiride thin films prepared by reactive magnetron spattering in N2/O2 reactive gases and effect of PO2 on chromium oxynitride thin films were studied.1012)However, preparation by PLD using O2 reactive gas has not been reported. Our goal in this study is to develop a highly precise control method of oxygen content and to obtain the maximum hardness in Cr(N,O) thin films.
2. Experimental
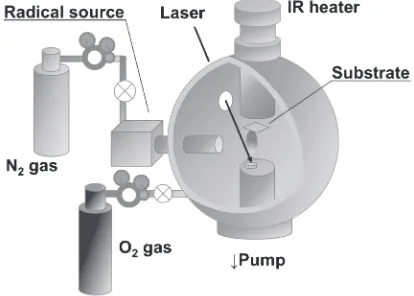
Figure 1 shows a schematic illustration of an apparatus used for preparing thinfilms. Ablation plasma was produced by irradiating an Nd: yttrium aluminum garnet laser (355 nm) onto a Cr target (99.9% purity). The laser was electro-optically Q-switched by a Pockels cell to produce intense pulses of a short duration (7 ns). The laser energy density was
Fig. 1 Schematic illustration of the apparatus used for preparing thinfilms.
+1Corresponding author, E-mail: paper-craft@etigo.nagaokaut.ac.jp.
Grad-uate Student, Nagaoka University of Technology
+2Graduate Student, Nagaoka University of Technology
[image:1.595.323.530.622.770.2]1.7 J cm¹2 and the deposition time was 5 h at a laser-pulse repetition rate of 10 Hz. The deposition surface area was 1 cm2 on a single-crystal (100)-oriented silicon substrate placed at a distance of 45 mm from the target. The substrate temperature was controlled at 973 K using an infrared lamp heater. The deposited film thickness was approximately 100 nm in this condition.
The chamber was first evacuated to a pressure of 2.5©10¹5Pa using a rotary pump and a turbo molecular pump, and the chamber was then filled with oxygen gas (>99.99995 vol% purity). The value of the vacuum gauge at this time was defined as PO2. After O2 gas introduction, nitrogen plasma (>99.99995 vol%purity) from an RF radical source was supplied. Both continuous pumping and intro-duction of O2gas and N plasma were carried out during the deposition. In order to change the oxygen content of the thin
films,PO2 was varied with changing the oxygen gasflow rate using a variable leak valve. The thin films were prepared under a fixed total pressure of 1.5©10¹2Pa.
For composition analysis of the thin films, Rutherford backscattering spectroscopy (RBS) and electron energy loss spectroscopy (EELS) were utilized. In the EELS spectra, since OK-edge and Cr L-edge are very close, only oxygen and nitrogen contents were measured by EELS. Cation and anion contents were calculated from RBS spectra. By these two spectroscopy results, compositions of thin films were precisely determined. The oxygen content was defined as x. The crystal structures of the thinfilms were studied by X-ray diffraction (XRD) using Cu K¡ radiation (0.154 nm) in the BraggBrentano configuration. The chemical bonding state was estimated by Fourier transform infrared spectroscopy (FT-IR). The FT-IR spectrum for each sample was obtained after taking into account of the absorbance of the Si substrate. The film thickness measured in a scanning electron spectroscope (SEM) was approximately 100 nm. The hard-ness of the thinfilms (HIT) was measured by nano-indentation testing under a load of 0.07 mN using a Berkovich indenter. The indentation depth was around 10 nm. The load was determined not to exceed the indentation depth more than 1/8 of thefilms thickness. The microstructures of the thinfilms were observed using a field emission transmission electron microscope (FE-TEM) with a 200 kV acceleration voltage. The TEM samples were made two methods, i.e., thinning by a focused ion beam (FIB) apparatus and by scratching with a diamond pen. The former was used for cross sectional observations in wide area tofind initial growth layer among the thin films. The latter was chosen for plan view observations and compositional analysis to prevent the ion beam damage.
3. Results and Discussion
3.1 Oxygen content
From the results of RBS and EELS measurements, it was found that the thin films contained chromium, nitrogen and oxygen. Figure 2 shows the oxygen content of the thin
films as plotted against PO2. As PO2 increased, xincreased monotonically from 0 to 62 mol%. The oxygen content of the Cr(N,O) thin film was thus successfully controlled by appropriately adjustingPO2. Figure 3 shows the composition
of the thin films. In the thin film without oxygen, the chromium content was 47 mol%, which was close to the stoichiometric composition of CrN. In contrast, the chromi-um content of the thinfilms became closer to that of Cr2O3 when the oxygen content was increased. As far as we know, there is no report on the chromium content in the Cr(N,O) thinfilms due to the increasing of the oxygen content. As we will describe later, the thinfilms have a B1 structure. Hence it is indicated that vacancy was formed in the Cr site by the replacement of N with O.
3.2 Phase identification
Figure 4 shows XRD patterns of the thin films. Peak positions and relative intensities for CrN and Cr2O3 in International Centre for Diffraction Data (ICDD) are also included for comparison. It was found that all samples included a B1 phase based on CrN. In addition, only the sample formed withPO2=10©10¹5Pa included the Cr
2O3 phase. The peaks due to the B1 structure became broad with increasing PO2. Crystallite size effect and lattice strain are considered as a major cause for broadening of XRD peaks. However, as we will describe in the next paragraph,
Fig. 2 The oxygen content in the thinfilms as a function of oxygen partial pressure.
Fig. 3 The composition of the thinfilms.
[image:2.595.331.519.71.235.2] [image:2.595.317.531.290.480.2]crystallite size of the Cr(N,O) thinfilms did not change with increasingPO2. Hence it is suggested that the cause for this broadening is variations in the oxygen content in each crystallite. Figure 5 shows the lattice constants of the B1 phase of each thin film calculated from the peak positions from the (111) and the (200) reflection. The lattice constant decreased with increasing PO2. The decrease in the lattice constant indicates solution of oxygen in the B1 phase. Transition metal oxides and nitrides which have the total valence similar to that of CrN include a lot of vacancies (ex. TiO,13) VO13) and TiN14)). These lattice constants change with increasing number of vacancies in the cation site. In Fig. 3, it is confirmed that the number of chromium vacancies increase with increasing the oxygen content. Hence
it is considered that the cause for the decreasing of the lattice constant is formation of vacancies in the Cr site. Figure 6 shows the FT-IR spectra of the thinfilms. Reference data for CrN and Cr2O3 are also included.15)The absorption spectra of the samples formed with PO2 less than or equal to 7.5©10¹5Pa showed a broad peak mainly at 390430 cm¹1 due to the CrN bond. Absorption peaks due to Cr2O3were not observed. On the other hand, the absorption spectra of the samples formed with PO2 greater than or equal to 8.5©10¹5Pa show sharp peaks at 550 cm¹1, which is due to the CrO bond in the Cr2O3 crystal. From the results in Figs. 4 and 6, it was found that the thinfilms with only the B1-Cr(N,O) phase were prepared under PO2 less than or equal to 7.5©10¹5Pa.
3.3 Microstructure and hardness
Figure 7 shows a bright field image (BFI) of the TEM sample prepared by focused ion beam (FIB) processing to keep the TEM sample thickness being less than 100 nm. According to this image, an initial growth layer was not observed. Figure 8 shows BFI, darkfield images (DFI) and selected area diffraction patterns (SAD) for the prepared thin
films. It was observed that all spots in the SAD pattern from all samples can be indexed for reflection from the B1 structure. The DFI were taken from the 200 diffraction of the SAD. From these DFI, it was found that crystallite size of the thinfilms was approximately 100 nm and did not change with increasingPO2. Rawalet al.12)reported that crystallite size of Cr(N,O) thinfilms prepared by reactive magnetron spattering decreased with increasing oxygen partial pressure in the atmosphere. In our method, variation ofPO2 (10¹5Pa order) is far lower than the total pressure (1.5©10¹2Pa). Hence it is considered that varying PO2 do not affect crystal growth configuration of the Cr(N,O). Figure 9 shows the indentation hardness data for the thin films as a function of PO2. The
Fig. 4 XRD patterns of the thinfilms prepared at different oxygen partial pressures.
Fig. 5 The lattice constants of thinfilms calculated from the XRD patterns. The solid and hollow symbols indicate the lattice constants calculated from the diffraction of 111 and 200, respectively. These values were calibrated from the diffraction of Si substrate.
[image:3.595.323.531.67.313.2] [image:3.595.75.263.383.547.2]results of phase identification by XRD and FT-IR are also shown at the top of Fig. 9. The hardness of the thin films increased with increasing PO2 up to 7.5©10¹5Pa. Above 7.5©10¹5Pa, the hardness decreased. The thinfilms showed a maximum hardness value of 32 GPa, which was similar to the previous results.4) The maximum value was found around the solubility limit.
In general metallic materials, yield stress is related to crystallite size through HallPetch relationship. This relation-ship serves to demonstrate that the yield stress increases with decreasing crystallite size. In Cr(N,O), the crystallite size did not change with increasing the oxygen content. Hence it was found that high hardness was not achieved by the HallPetch
relationship. The decreasing of hardness for PO2 above 7.5©10¹5Pa related to the crystal structure of the thinfilms. In this zone, Cr2O3, which have the lower hardness value than that of CrN, exist as the second phase. It is considered that the hardness decreased due to increase of the volume fraction of Cr2O3.
4. Conclusions
From the above results, it was found that the oxygen content in the Cr(N,O) thinfilms was successfully controlled by varyingPO2 in a pulsed laser ablation process using a RF radical source. The thin films had oxygen content up to
Fig. 7 Bright field image of the sample prepared by focused ion beam processing. This thinfilm was prepared without O2.
Fig. 8 Brightfield (left) and darkfield (right) under excitation of the fcc (200) reflection of the thinfilms prepared (a) without O2,
(b) atPO2=5.0©10¹5Pa, (c) atP
O2=7.5©10¹5Pa and (d) atPO2=8.5©10¹5Pa.
Fig. 9 Nano-indentation hardness of the thinfilms as a function of oxygen partial pressure.
[image:4.595.62.279.69.248.2] [image:4.595.331.520.71.242.2] [image:4.595.134.464.300.587.2]62 mol%. All the samples contained a phase with a B1 structure. In the thin films deposited under high PO2 (²8.5©10¹5Pa), Cr
2O3 existed as a second phase. The chromium content of the B1 phase decreased from 47 mol%, which was close to the stoichiometric composition of CrN, to 40 mol%when the oxygen content was increased. From TEM observations, it was found that crystallite size of the thin
films did not change with increasingPO2. The hardness of the thinfilms increased with increasingPO2, and then decreased forPO2²8.5©10¹5Pa. The thinfilms showed a maximum hardness value of 32 GPa around the solubility limit.
Acknowledgement
This work was supported by Grant-in-Aid for Scientific Research 22686069.
REFERENCES
1) T. Hurkmans, D. B. Lewis, H. Partiong, J. S. Brooks and W. D. Müntz:
Surf. Coat. Technol.114(1999) 5259.
2) J.-N. Tu, J.-G. Duh and S.-Y. Tsai:Surf. Coat. Technol.133134(2000) 181185.
3) T. Suzuki, H. Saito, M. Hirai, H. Suematsu, W. Jiang and K. Yatsui:
Thin Solid Films407(2002) 118121.
4) T. Suzuki, J. Inoue, H. Saito, M. Hirai, H. Suematsu, W. Jiang and K. Yatsui:Thin Solid Films515(2006) 21612166.
5) K. Inumaru, K. Koyama, N. Imo-oka and S. Yamanaka:Phys. Rev. B
75(2007) 054416.
6) H. Asami, J. Inoue, M. Hirai, T. Suzuki, T. Nakayama, H. Suematsu, W. Jiang and K. Niihara:Adv. Mater. Res.1112(2006) 311314.
7) H. Asami, T. Kamekawa, T. Suzuki, T. Nakayama, H. Suematsu, W. Jiang and K. Niihara:J. Jpn. Inst. Met.71(2007) 704707.
8) M. Urgen, V. Ezirmik, E. Senel, Z. Kahraman and K. Kazmanli:Surf. Coat. Technol.203(2009) 22722277.
9) I. M. Ross, W. M. Rainforth, C. R. Seabourne, A. J. Scott, P. Wang, B. G. Mendis, A. L. Bleloch, C. Reinhard and P. Eh. Hovsepian:Thin Solid Films518(2010) 51215127.
10) R. Mientus, R. Grötschel and K. Ellmer: Surf. Coat. Technol. 200
(2005) 341345.
11) J. Shirahata, T. Ohori, H. Asami, T. Suzuki, T. Nakayama, H. Suematsu, Y. Nakajima and K. Niihara:Thin Solid Films519(2011) 34973500.
12) S. K. Rawal, A. K. Chawla, V. Chawla, R. Jayaganthan and R. Chandra:Thin Solid Films519(2011) 76867693.
13) M. D. Banus, T. B. Reed and A. J. Strauss:Phys. Rev. B5(1972) 2775.
14) E. O. Ristolainen, J. M. Molarius, A. S. Korhonen and V. K. Lindroos:
J. Vac. Sci. Technol. A5(1987) 2184.