RESEARCH ARTICLE
Heat dissipation does not suppress an immune response in
laboratory mice divergently selected for basal metabolic rate
(BMR)
Aneta Książek* and Marek Konarzewski
ABSTRACT
The capacity for heat dissipation is considered to be one of the most important constraints on rates of energy expenditure in mammals. To date, the significance of this constraint has been tested exclusively under peak metabolic demands, such as during lactation. Here, we used a different set of metabolic stressors, which do not induce maximum energy expenditures and yet are likely to expose the potential constraining effect of heat dissipation. We compared the physiological responses of mice divergently selected for high (H-BMR) and low basal metabolic rate (L-(H-BMR) to simultaneous exposure to the keyhole limpet haemocyanin (KLH) antigen and high ambient temperature (Ta). At 34°C (and at 23°C, used as a control), KLH challenge resulted in a transient increase in core body temperature (Tb) in mice of both line types (by approximately 0.4°C). Warm exposure did not produce line-type-dependent differences in Tb(which was consistently higher by ca. 0.6°C in H-BMR mice across both Ta values), nor did it result in the suppression of antibody synthesis. These findings were also supported by the lack of between-line-type differences in the mass of the thymus, spleen or lymph nodes. Warm exposure induced the downsizing of heat-generating internal organs (small intestine, liver and kidneys) and an increase in intrascapular brown adipose tissue mass. However, these changes were similar in scope in both line types. Mounting a humoral immune response in selected mice was therefore not affected by ambient temperature. Thus, a combined metabolic challenge of high Taand an immune response did not appreciably compromise the capacity to dissipate heat, even in the H-BMR mice.
KEY WORDS: BMR, Blood parameters, Artificial selection, Humoral response, Immunosuppression, Trade-offs
INTRODUCTION
Studies on energy balance are based on the assumption that the available resources, most notably energy, are limited. These limitations are therefore assumed to ultimately lead to trade-offs between competing demands. When conditions deteriorate, the resources are shunted between physiological functions, minimising the allocation of resources to the least essential functions (Norris and Evans, 2000; Ricklefs and Wikelski, 2002; Stearns, 2002; Ewenson et al., 2003; Kilpimaa et al., 2004; Boots, 2008). The heat dissipation limitation (HDL) hypothesis (e.g. Król and Speakman, 2003a,b; Król et al., 2003, 2007) suggests, however, that energy
budgets are limited by the ability to dissipate body heat, rather than by the constraints of acquiring or processing energy, which result in resource allocation. This hypothesis requires reinterpretation of the direct cause of the physiological trade-offs and considers that the trade-offs are determined by the ability to avoid detrimental overheating rather than by the limited energy availability or the ability to assimilate energy.
The HDL hypothesis builds on many studies that have demonstrated that animals simultaneously forced to combine energy-consuming functions elevate food intake to meet the increased demands (e.g. Hammond et al., 1994; Hammond and Kristan, 2000; Johnson and Speakman, 2001; Speakman et al., 2001; Speakman and Król, 2005; Zhang and Wang, 2007). The HDL hypothesis predicts that the level of energy assimilation increases until it is constrained by the capacity to dissipate the body heat generated as a by-product of processing the energy that fuels physiological functions (Król and Speakman, 2003a,b; Król et al., 2003, 2007; Speakman and Król, 2010).
To date, the limiting effect of heat dissipation (HD) has been exclusively tested under peak metabolic demands, mostly in lactating rodents and lagomorphs (Król et al., 2007; Wu et al., 2009; Zhao and Cao, 2009; Valencak et al., 2010; Simons et al., 2011; Gamo et al., 2013a; Valencak et al., 2013; Yang et al., 2013; Zhao, 2011; Zhao et al., 2013a,b). It remains to be seen, however, whether the effect of HD also holds for energy expenditures incurred by physiological processes other than peak metabolic demands induced by reproduction. Energy expenditures should become particularly constrained by HD at high ambient temperatures because the heat flow between an animal and its environment is primarily a function of the temperature gradient (Król et al., 2003, 2007; Speakman and Król, 2010). Most importantly, HD should come into play even at much lower rates of food consumption than those elicited by, for example, lactation, if the applied metabolic stressor elicits substantial generation of body heat and a concomitant increase in body temperature (Barr et al., 1922). An immune challenge is the stressor meeting this condition, as heat generation is part of the immune response itself and is primarily manifested through fever (Klasing and Leshchinsky, 1999; Lee and Klasing, 2004; Lee, 2006).
Furthermore, immune responses require energy to recognise and eliminate pathogens and to fuel cell proliferation and diversification or major histocompatibility complex molecule synthesis during antigen presentation in cell-mediated and humoral responses (Demas et al., 1997; Ots et al., 2001; Książek et al., 2003; Martin et al., 2003; Li et al., 2007; Ilmonen et al., 2008; Cai et al., 2009; Cutrera et al., 2010; Muehlenbein et al., 2010). Because infection-elicited heat generation through fever is compulsory (Kluger et al., 1998; Luheshi, 1998; Leshchinsky and Klasing, 2001), immune responses can be considered to be particularly relevant for testing
Received 30 July 2015; Accepted 1 March 2016
Institute of Biology, University of Białystok, Ciołkowskiego 1J, Białystok 15-245, Poland.
*Author for correspondence (anetak@uwb.edu.pl)
Journal
of
Experimental
HD limitations because an excessive rise in body temperature (i.e. the inability to cope with heat production) leads to the deregulation of physiological processes. The adverse effects of excessive body temperature include apoptosis, hypoxia, oxidative stress, mitochondrial dysfunction, liver damage, DNA damage to germ cells, and cerebral ischemia, among others (e.g. Cui et al., 2004; McAnulty et al., 2005; Chang et al., 2007; Bloomer et al., 2008; Lee et al., 2008; Haak et al., 2009; Paul et al., 2009).
The limitation of energy expenditures by the capacity to dissipate heat should be manifested as an increase in the core body temperature (Tb) and/or the downregulation of heat-generating physiological processes. To test this prediction, we challenged mice divergently selected for high and low basal metabolic rate (BMR) with the keyhole limpet haemocyanin (KLH) antigen and subjected
them immediately (without acclimation) to high ambient
temperature (34°C) for 5 days – the period covering peak
antibody production. Mounting an immune response against KLH is particularly fitting in this context because it triggers a significant
20–30% increase in O2 consumption and therefore increases
metabolic heat production in mice (Demas et al., 1997). Furthermore, a comparison of the physiological responses of the mice selected for high and low BMR provides a robust model for investigating HD-related costs of mounting immune responses for the following reasons: in addition to the genetically based 40% between-line-type difference in BMR (and therefore the heat production rate), the mouse line types differ with respect to theTb recorded at 23°C by 0.6°C (A.K., unpublished data; present study) and by more than 1°C within the thermoneutral zone at 30°C (Gębczyński, 2005). Throughout the selection experiment, body mass (BM) and thermal conductance of the mouse line types did not appreciably differ (Gębczyński, 2005; Konarzewski and Książek, 2013); therefore, any between-line-type differences inTbare likely to stem from differences in the rate of heat generation rather than from the insulating properties of the pelage. Furthermore, mice of the high-BMR line type (H-BMR) have 20 to 30% larger and more metabolically active internal organs (small intestine, liver, kidneys and heart; Książek et al., 2004) whose metabolic rates contribute disproportionately to BMR (Konarzewski and Diamond, 1995). In contrast, the low-BMR line type (L-BMR) is characterised by a significantly larger intrascapular brown adipose tissue (IBAT; for
detailed review of the between-line-type differences, see
Konarzewski and Książek, 2013). Additionally, we have
demonstrated previously that our mice are a suitable model for investigating the relationship between the immune response and the function of the internal organs and components of the energy budget (Książek et al., 2003, 2009; Książek and Konarzewski, 2012).
We expected that if the ability to dissipate excessive heat is a factor limiting energy turnover rate, then the combined challenge of high ambient temperature and an increase in compulsory heat generation incurred by mounting an immune response should result in a rise inTb. Because mice of both line types do not differ with respect to thermal conductance and BM, we expected that their maximum rate of HD related to the physical properties of the pelage should be comparable. Therefore, despite a conspicuous between-line-type difference inTbat room temperature, theirTbunder heat stress should be similar and should be imposed by the maximum rate of heat loss elicited by the combined effect of both applied stressors. We also expected line-type-dependent responses to high ambient temperature to be in terms of a reduction of the mass/size of heat-generating organs (i.e. pronounced reduction of internal organs in the H-BMR line type and, conversely, a pronounced reduction of IBAT in mice of the L-BMR line type). Furthermore, we expected a greater decrease in the immune response to the KLH antigen (expressed as the synthesis of specific antibodies) in the mice selected for high BMR. This decrease is expected because the HD capacity-related trade-offs involving the immune response would be more readily detectable in these mice because of their higher Tb already manifested at room temperature.
To analyse these trade-offs, we compared the between-line-type, warm-elicited changes in BM,Tb, anti-KLH immunoglobulin M (IgM) production, haematological parameters (leucocyte counts and neutrophil/lymphocyte ratio), mass of the lymphatic organs (as the source of the immune cells involved in antigen recognition and elimination), and mass of internal organs involved in energy processing. Our study adds to the still small number of studies on the HDL hypothesis testing the effect of applied metabolic challenges on
Tb(e.g. Zhao et al., 2010; Valencak et al., 2013; Gamo et al., 2013a,b versus Zhao et al., 2013a,b; Yang et al., 2013; Duah et al., 2013; van der Vinne et al., 2014 and many others published before 2012), as well as numerous studies that have demonstrated only the effect of high ambient temperatures on an immune response without discussing constraints imposed by the ability of animals to lose heat (e.g. Bartlett and Smith, 2003; Mashaly et al., 2004; Niu et al., 2009; Jin et al., 2011).
MATERIALS AND METHODS Animals
We used Swiss-Webster mice (Mus musculus Linnaeus 1758)
subjected to long-term artificial selection protocols to generate high and low BM-corrected BMR line types, as described previously (Książek et al., 2004). Briefly, animals were maintained on a 12 h:12 h light:dark schedule and at ambient temperature of 23°C. The BMR of 12- to 16-week-old mice was measured for 3 h in an open-circuit respirometry system. The males and females with the highest and lowest mass-corrected BMR (over 4 min minimum) were chosen as the progenitors of the H-BMR and L-BMR selection line types, respectively. A similar procedure was used to produce subsequent offspring generations and resulted in a greater than 40% differentiation of the two line types with respect to BMR. Because of the logistic constraints stemming from the difficulties associated with BMR measurements, our line types were not replicated. However, we have demonstrated repeatedly that the between-line-type differences in BMR and several other traits were large enough to claim that they reflect genuine change in the frequencies of genes related to BMR rather than genetic drift (Książek et al., 2004; Brzęk et al., 2007, 2012, 2014; Gębczyński and Konarzewski, 2009; Książek and Konarzewski, 2012). Our studies have shown that the between-line-type separation in BMR is consistently retained over time, which suggests that artificial selection for metabolic traits List of symbols and abbreviations
BM body mass BMR basal metabolic rate
H-BMR mice selected for high basal metabolic rate HD heat dissipation
HDL heat dissipation limit
IBAT interscapular brown adipose tissue IgM immunoglobulin M
KLH keyhole limpet haemocyanin
L-BMR mice selected for low basal metabolic rate N/L ratio neutrophil/lymphocyte ratio
Ta ambient temperature Tb core body temperature WBC total white blood cells
Journal
of
Experimental
applied early in life generates a long-term genetic response (Brzęk et al., 2012, 2014; Sadowska et al., 2013, 2015).
In this study, we used males from generation F33. The experiment
was performed using 55 H-BMR and 55 L-BMR males (aged 16–
18 weeks, 34–45 g BM), and the measurements of BMR were a part of the selection procedure. Body-mass-corrected BMR differed considerably between the L-BMR (43.1±0.6 ml O2 h−1) and H-BMR individuals (64.4±0.8 ml O2 h−1) at the beginning of the experiment (ANCOVA;F1,107=791.08,P<0.0001). The day before the experiment, the animals were placed into individual plastic cages. The mice had free access to food (murine laboratory chow with a caloric content of 12.96 kJ; Wytwórnia Pasz Labofeed, Kcynia, Poland) and water.
Experimental procedures
Because of logistic constraints, the experiment was conducted in two successive batches, within which mice from selected line types were randomly assigned to experimental groups (Table S1). The immunised mice received a single subcutaneous injection of the
novel KLH antigen (150μg, Calbiochem, San Diego, CA, USA)
suspended in 0.1 ml sterile saline (Klein and Nelson, 1998; Klein et al., 1999). KLH is an innocuous respiratory protein derived from the giant keyhole limpet (Megathura crenulata) that generates a robust, non-replicating antigenic response in rodents without making the animals sick (e.g. Drazen et al., 2002; Demas et al., 2004). We used KLH for the following reasons: (1) to avoid the unpredictable mortality of the experimental animals, (2) to ensure that the individual mice were forced to mount a primary humoral response (KLH is an‘exotic’antigen that had not been encountered by the mice previously) and (3) to trigger an immune response similar to live pathogens. Most importantly, the KLH-induced immune response triggers a rise in Tb ( present study), so it constitutes an effective metabolic challenge generating a calorigenic effect during immune stimulation.
The control groups were immunised with an equal amount of sterile saline. Following KLH challenge, half of the mice from the immunised and non-immunised groups were assigned to two temperature treatments: the mice were maintained at 23°C or exposed immediately to heat stress at 34°C. We exposed the mice to temperatures exceeding room temperature to reduce the gradient between the ambient and core body temperature (decreasing the heat loss capacity; Speakman and Król, 2010) and to determine the line-type-dependent changes in the costs of mounting an immune response under heat stress. We have chosen 34°C following a pilot study in which we initially exposed immunised mice to 36°C. However, such exposure resulted in the overheating of the sentinel animals, which may compromise their welfare. The heat stress of 34°C was close to, but did not exceed, the capacity of our mice to dissipate heat. Therefore, the application of this temperature allowed us to assume that an immune challenge superimposed on the heat stress would create conditions revealing HDL.
Following immunisation, the animals were maintained under their respective thermal conditions for 5 days. This sampling period was chosen to capture the peak phase of IgM production (the primary immunoglobulin in the blood during an initial humoral response) during the course of the immune response to KLH (Demas et al., 2004). The core body temperature of each individual was recorded once a day at 12:00 h to an accuracy of 0.1°C using a
digital thermocouple thermometer (BAT-12, Physitemp
Instruments, Clifton, NJ, USA). The thermocouple was inserted 2 cm into the colon. An increase in body temperature is equivalent to an increase in oxygen consumption (Barr et al., 1922). We
therefore used Tb measurement as a reliable and direct proxy of metabolic costs incurred by an immune challenge in favour of food intake, which typically decreases in warm-exposed mice (e.g. Król et al., 2003; Wu et al., 2009; Zhao, 2011). A reduction of food intake is difficult to interpret, as it may induce the re-allocation of resources between the reduced costs of maintenance of digestive organs and the increased need for an immune response (Książek et al., 2003). Following the temperature measurement, the mice were weighed to an accuracy of 0.1 g.
On day 5 after immunisation with KLH or saline, the animals were killed by cervical dislocation. The organ masses, anti-KLH IgM production in the serum and leucocyte counts were examined. The procedures were performed in accordance with the guidelines proposed by the Local Ethical Committee on Testing Animals at the Medical University of Białystok ( permit no. 2007/50).
Detection of anti-KLH IgM in serum
The blood samples were allowed to clot and were centrifuged at 448gfor 30 min. The serum was removed and stored at−80°C for later analysis (Klein and Nelson, 1998; Klein et al., 1999). Serum IgM concentrations were assayed using the specific
Mouse Anti-KLH IgM ELISA kit per the manufacturer’s
protocol (Life Diagnostics, West Chester, PA, USA). The optical density of each sample was determined using an automated microplate reader (BioTek, Winooski, VT, USA) equipped with a 450-nm wavelength filter. The serum samples were run in duplicate. The anti-KLH IgM concentration in the serum was expressed in units ml−1.
Leucocyte counts and neutrophil/lymphocyte ratio
To evaluate the relative number of white blood cells, we stained
blood smears using the May–Grünwald–Giemsa method. The
stained smears were scanned under 1000× magnification, and the total white blood cell (WBC) count was estimated as the number of leucocytes per 10,000 erythrocytes. To investigate the neutrophil/ lymphocyte (N/L) ratio, we counted 100 leucocytes and classified them as neutrophils or lymphocytes (Ots et al., 1998; Saks et al., 2003). These simple measures provided robust and repeatable data on blood cell numbers (Saino et al., 1995).
Morphometrics
Following the collection of blood samples, the lymphatic organs (thymus, lymph nodes and spleen) and the metabolically active internal organs (small intestine, liver, kidneys, heart and IBAT) were excised, cleared of blood, food stuffs and adherent fat, and weighed to an accuracy of 0.001 g.
Statistics
Changes in BM and Tb were analysed using repeated-measures
ANOVA, in which line type (L-BMR or H-BMR), ambient temperature (23 or 34°C) and immunisation (KLH or saline) were the main fixed effects, and batch affiliation was a random effect. We also conducted a four-way ANOVA with the above main factors to test for differences in Tb separately within each day of the experiment. All models also included the respective first- and second-order interactions. The batch effect and second-order interactions were not significant in any of the analyses (P>0.5) and therefore were not reported throughout the paper.
The anti-KLH IgM concentrations, WBC counts and N/L ratio were arcsine transformed to meet the assumption of normality and analysed by ANOVA with the main effects described above. The same ANOVAs were used to analyse changes in the total mass of the
Journal
of
Experimental
examined organs. To distinguish between changes in total and BM-corrected organ masses, we used ANCOVA by including the total BM (minus the mass of the examined organ) as a covariate. The significance was tested atP=0.05. The tests were performed using the STATISTICA 6.0 statistical package.
RESULTS
Body mass and body temperature changes
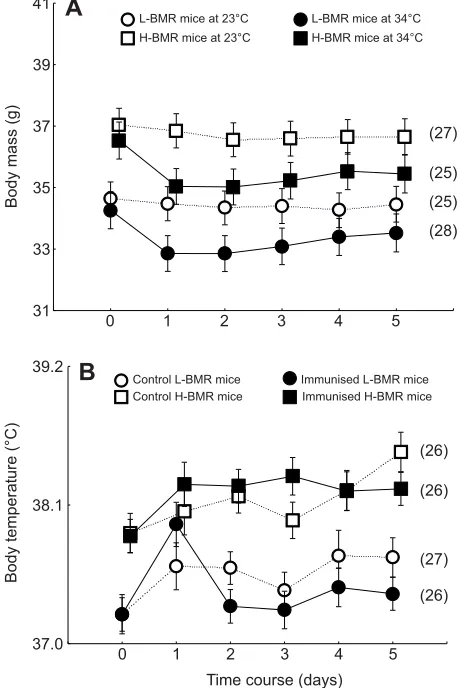
The four-way repeated-measures ANOVA demonstrated that there were highly significant changes in BM during the course of the experiment (F5,445=54.90, P<0.0001; Fig. 1A). The BM of the warm-exposed animals was lower than that of animals maintained at 23°C by approximately 3% (F1,89=4.11,P=0.04). This difference most likely stemmed from the reduction of the mass of the internal organs (see below). The significant interaction between the time
course and the ambient temperature (F5,445=41.32,P<0.0001) was also an indication of between-temperature differences in BM changes. The interaction likely stemmed from the drop in BM during the first day of warm exposure (Fig. 1A). Throughout this experiment, mice from the H-BMR line type consistently maintained a slightly higher BM than mice from the L-BMR line type (by approximately 2.2 g, i.e. 6%; F1,89=17.49, P<0.0001). Immunisation with the KLH antigen itself did not affect BM (F1,89=0.36,P=0.5).
At the conclusion of the experiment, there was no difference (ANOVA;F1,89=0.46,P=0.5) in carcass mass (calculated as total BM minus the mass of the internal organs and digesta), which was used as a proxy for the difference in the carcass fat content in selected mice (28.8±0.4 and 29.2±0.4 g for the L-BMR and H-BMR mice, respectively).
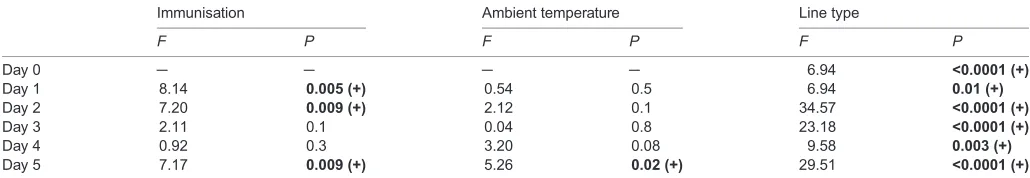
Immediately before the experiment, mice from the H-BMR line type exhibited a significantly higher core body temperature than the L-BMR mice (ANOVA; F1,103=17.23, P<0.0001; 37.9±0.1 and 37.3±0.1°C for the L-BMR and H-BMR mice, respectively). This difference persisted throughout the experiment, as demonstrated by the four-way repeated-measures ANOVA (F1,89=27.90,P<0.0001; Fig. 1B) as well as the ANOVAs conducted for each day of the experiment separately (Table 1, Table S2). Tb was affected by immunisation with the KLH antigen (four-way repeated-measures
ANOVA; F1,89=5.6, P=0.02): immunisation increased Tb by
approximately 0.4°C in the first, second and fifth days following immunisation (Table 1, Table S2). Throughout the experiment, the exposure to high ambient temperature itself did not affectTb (four-way repeated-measures ANOVA; F1,89=1.3, P=0.2). Significant interactions between the time course and line type as well as the time
course and ambient temperature (F5,445=4.0, P=0.001 and
F5,445=3.9, P=0.002, respectively) reflected fluctuations in the core body temperature in warm-exposed L-BMR mice but not in the warm-exposed H-BMR mice (Fig. 1B).
Humoral response, leucocyte counts, and N/L ratio
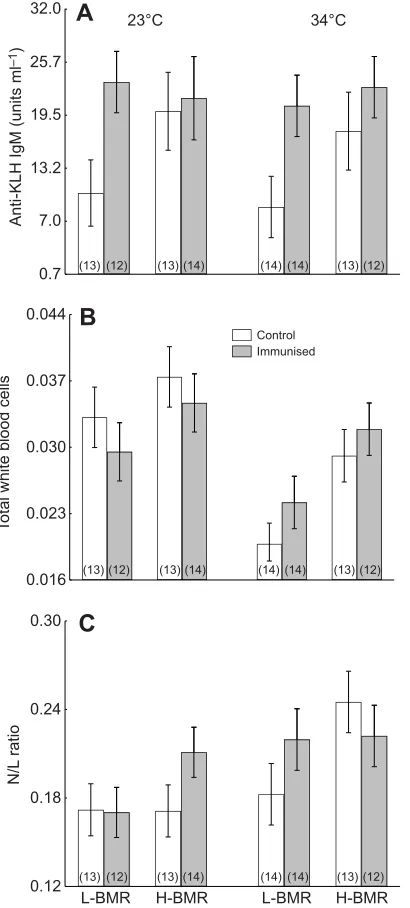
The production of IgM specific for the KLH antigen was affected by
immunisation (30% increment; ANOVA; F1,89=11.32, P=0.001;
Fig. 2A) but not by ambient temperature or line type (ANOVA;
F1,89=0.11, P=0.7 and F1,89=2.91, P=0.08, respectively). The interactions between the main factors were not statistically significant. The lack of significant differences in the humoral response between mice maintained at 23 and 34°C suggests that exposure to warm conditions was not immunosuppressive.
Leucocyte counts (WBC) differed between temperatures
(ANOVA; F1,89=13.05, P=0.0005) and line types (ANOVA;
F1,89=8.54,P=0.004) but were not affected by immunisation with the KLH antigen (ANOVA;F1,89=0.009,P=0.9). The leucocyte level was lower by approximately 21% in the warm-exposed animals relative to the animals maintained at 23°C (Fig. 2B). The H-BMR mice exhibited 22% higher WBC counts than the L-BMR mice, and this difference was independent of ambient temperature (0.033±0.001 and 0.027±0.001, respectively). The interactions between ambient temperature, line type and immunisation were not significant.
Similar to the leucocyte counts, the N/L ratio was affected by
ambient temperature (ANOVA; F1,89=8.55, P=0.004) and line
type (ANOVA; F1,89=5.08, P=0.02) but not by immunisation
(ANOVA; F1,89=0.58, P=0.4). The interactions between these factors were not statistically significant. In contrast to the effect of ambient temperature on WBC counts, animals maintained
at 34°C exhibited 20% higher N/L ratios than animals
maintained at 23°C (Fig. 2C). The H-BMR mice exhibited 14%
Body mass (g)
0 1 2 3 4 5
31 33 35 37 39 41
A
L-BMR mice at 23°C L-BMR mice at 34°C
H-BMR mice at 23°C H-BMR mice at 34°C
(27)
(25)
(28) (25)
Body temperature (°C)
0 1 2 3 4 5
Time course (days) 37.0
38.1 39.2
B
(26) Control L-BMR mice Immunised L-BMR mice Control H-BMR mice Immunised H-BMR mice
(26)
(27)
[image:4.612.59.289.244.588.2](26)
Fig. 1. Changes in body mass (BM) and body temperature (Tb) in mice immunised with keyhole limpet haemocyanin (KLH; at day 0) and immediately subjected to 5-day exposure to one of two ambient temperatures.(A) BM of animals exposed to 34°C was lower than in animals maintained at 23°C (P=0.04), while immunisation with the KLH antigen itself did not affect BM (P=0.5; for clarity, groups of immunised and non-immunised animals within each line type and day of experiment were pooled). (B) In contrast to BM, the increase inTbwas affected by KLH challenge (P=0.02) but not by exposure to 34°C (P=0.2, thus the respective temperature groups were accordingly pooled). Throughout the experiment, mice from the high basal metabolic rate (H-BMR) line type maintained a higher BM (A) andTb(B) than mice from the low basal metabolic rate (L-BMR) line type (four-way repeated-measures ANOVA;P<0.0001 for both effects). In this and in subsequent figures, values are raw (untransformed) means (±s.e.m.), and sample sizes are
provided in parentheses.
Journal
of
Experimental
higher N/L ratios than L-BMR mice, and this difference was independent of ambient temperature (0.220±0.008 and 0.192± 0.008, respectively).
Mass of lymphatic organs
Table 2 shows a summary of the analyses of total and BM-corrected masses of lymphatic organs. The masses of these organs were not affected by ambient temperature. Immunisation with KLH affected the mass of the lymph nodes but not the masses of the spleen or thymus. Independent of immunisation, the masses of the lymph nodes and spleen were higher in H-BMR mice (by approximately 14% and 46%, respectively), whereas the mass of the thymus was higher in L-BMR mice (by approximately 21%; Table S3).
The lymph node mass ANOVAs/ANCOVAs demonstrated
significant ambient temperature×immunisation and ambient
temperature×line type interactions (Table 2). These interactions resulted from the substantial increase in lymph node mass following KLH challenge in mice from both line types
experiencing the 23°C treatment (Fig. 3). The separate
ANCOVA analysis within each temperature treatment also demonstrated a significant interaction between immunisation and
line type at 34°C (F1,49=4.65, P=0.03) but not at 23°C
(F1,45=0.05, P=0.8). Whereas the mass of the lymph nodes
increased significantly in the immunised L-BMR mice
(F1,25=9.23,P=0.005), there was no change in lymph node size between the immunised and non-immunised animals from the H-BMR line type (F1,23=0.008,P=0.9; Fig. 3).
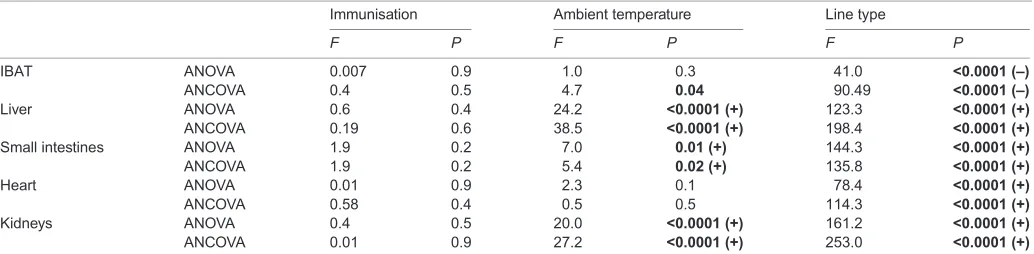
Metabolically active internal organs
The exposure to 34°C resulted in a decrease in the mass of the small intestine, liver and kidneys (by 9%, 11% and 10%, respectively) and a slight but statistically significant increase in IBAT mass (by approximately 2%; Table 3, Table S4). Immunisation with the KLH antigen did not affect the mass of metabolically active internal organs (Table 3). The ambient temperature×line type and immunisation×line type interactions were not significant.
Independent of the effect of ambient temperature and immunisation, line type affected the mass of the metabolically active internal organs (Table 3, Table S4). With the exception of IBAT mass, which was 24% larger in the L-BMR mice, the masses of the small intestine, liver, kidneys and heart were significantly higher in the H-BMR line type (by approximately 45%, 28%, 34% and 21%, respectively).
DISCUSSION
Heat dissipation as a factor limiting energy turnover rate Earlier studies (e.g. Król and Speakman, 2003a,b; Król et al., 2003; Zhao, 2011, 2013b; Valencak et al., 2010, 2013; Yang et al., 2013)
have tested HDL by comparing maximum energy intake elicited by metabolic stress (typically lactation) at low or high ambient temperatures and have indirectly inferred the significance of HD as a factor limiting the rate of energy expenditure. Our approach was different in two important aspects. First, we exposed our experimental animals to ambient temperature higher than those used in other studies (34°C in the present study versus 30°C, for example, in Król and Speakman, 2003a,b; Król et al., 2003; Wu et al., 2009; Zhao, 2011). We thus created conditions in which the effect of overheating should be particularly discernible. We paid special attention to monitoring changes in core body temperature, which, surprisingly, has been rarely reported in studies on HD limitation, except some of the most recent papers (Zhao et al., 2010; Gamo et al., 2013a,b; Valencak et al., 2013). We did so because elevation of Tb should be expected when animals are unable to dissipate excess heat generated as a by-product of compulsory metabolic processes.
Second, to increase compulsory heat generation, we imposed an immune challenge rather than stressors known to increase energy assimilation rates (e.g. lactation in Wu et al., 2009; Valencak et al., 2010; Zhao, 2011; Yang et al., 2013), thereby triggering compulsory co-generation of body heat. In this context, unlike when mice experience lactation, immune stress is likely to elicit an increase in compulsory heat generation (Demas et al., 1997; Prendergast et al., 2004), even in the absence of a concurrent increase in the energy assimilation rate. Therefore, unlike studies that have aimed to test the significance of HD for maximum sustained metabolic rates (reviewed in Speakman and Król, 2010), we tested the potential effect of HD, which was expected to come into play at the rates of energy assimilation that were lower than during lactation.
Because of the higher rate of energy turnover manifested by the elevatedTbat room temperature, we expected that mice from the H-BMR line type were more likely to be limited by HD at 34°C. We therefore surmised that if HD limits the rate of energy expenditure, rates of L-BMR mice will‘catch-up’with those of H-BMR mice, and both line types will experience similar degrees of overheating. We also expected that mounting an immune response would result in a line-type-dependent suppression of antibody production and a reduction of the internal organs and IBAT to prevent overheating. Our more specific hypothesis predicted that the immune response to the KLH antigen would be suppressed to a greater extent in the animals characterised by the high BMR because of the elevated heat production rate, which would be increased further by the heat-generating humoral response.
[image:5.612.47.562.80.169.2]KLH challenge resulted in a transient increase ofTbin mice of both line types (Fig. 1B, Table 1, Table S2), which testifies to its calorigenic effect. The meanTbof immunised mice from H-BMR Table 1. Summary of ANOVA results of core body temperature (Tb) measured each day following simultaneous KLH challenge and exposure to 34°C in laboratory mice
Immunisation Ambient temperature Line type
F P F P F P
Day 0 ─ ─ ─ ─ 6.94 <0.0001 (+)
Day 1 8.14 0.005 (+) 0.54 0.5 6.94 0.01 (+)
Day 2 7.20 0.009 (+) 2.12 0.1 34.57 <0.0001 (+)
Day 3 2.11 0.1 0.04 0.8 23.18 <0.0001 (+)
Day 4 0.92 0.3 3.20 0.08 9.58 0.003 (+)
Day 5 7.17 0.009 (+) 5.26 0.02 (+) 29.51 <0.0001 (+)
Interactions between the main effects were not significant.P-values <0.05 are inbold. Positive signs followingP-values indicate immunised>non-immunised, kept at 23°C>warm-exposed or H-BMR>L-BMR. Day 0 d.f.=1,103; Day 1–5 d.f.=1,89.
Journal
of
Experimental
and L-BMR lines averaged 38.3±0.1 and 37.7±0.1°C, respectively, and was therefore comparable withTbrecorded in dams during peak lactation [38.5±0.5°C in Valencak et al., 2013; 38.3±0.18°C in Gamo et al., 2013a; as well as in physically active, pregnant mice (37.91±0.2°C in Gamo et al., 2013b)]. This comparison is important, because irrespective of the differences in total energy expenditure incurred by the primary metabolic challenge (in this case lactation versus immune response), the rate of co-generation of extra heat, if limiting, should be manifested as a rise inTb. Because
theTbof lactating dams and our mice are comparable, it is likely that the possible limiting effect of compulsory heat co-produced by lactation or mounting an immune response is similar, despite vastly different total energy expenditures.
Contrary to the effect of KLH, the exposure to 34°C itself did not result in elevation ofTbin either of the line types. There was also no synergistic effect of both stressors, as indicated by the lack of a significant interaction between KLH challenge and warm exposure. Furthermore, similar to their behaviour at room temperature, warm-exposed H-BMR mice consistently maintained a higher Tb. This finding is particularly important in light of the lack of between-line type differences in thermal conductivity (Gębczyński, 2005) because it demonstrates that the lowerTbof the L-BMR mice was not attributed to their higher capacity for HD through a less insulated body surface. Furthermore, we did not observe line-type-dependent suppression of specific IgM production (Fig. 2A), and there was no change in the masses of the spleen and thymus (Table 2, Table S3).
Following exposure to high temperature, mice from both line types exhibited a significant decrease in WBC counts (Fig. 2B), mass of the energy-producing organs (Table 3, Table S4) and, consequently, BM (Fig. 1A). We associated this decrease with a decrease in food intake observed in mice exposed to 34°C (A.K., unpublished data). The reduction in food intake in mice exposed to temperatures even lower than those used in the present study (30°C) has also been reported in other studies (Król and Speakman, 2003a, b; Król et al., 2003; Wu et al., 2009; Zhao, 2011). This finding is consistent with the reduction in the mass of the internal heat-generating organs that has been observed in non-reproductive Mongolian gerbils exposed to 30°C (Yang et al., 2013) as well as with the results of our study (Table 3, Table S4). These reductions can be interpreted to be a means of balancing energy turnover with the limitations imposed by HD. However, reductions in the mass of organs reported in the present study were similar in scope in both selected line types, as indicated by the lack of a significant line type×ambient temperature interaction. Thus, a combined metabolic challenge of high ambient temperature and mounting an immune
Anti-KLH IgM (units ml
–1
)
0.7 7.0 13.2 19.5 25.7 32.0
23°C 34°C
A
(13) (13) (14) (13)
T
o
tal white blood cells
0.016 0.023 0.030 0.037 0.044
Control Immunised
B
N/L
ratio
L-BMR 0.12
0.18 0.24 0.30
C
H-BMR
H-BMR L-BMR
(14) (12) (14)
(12)
(13)(12) (13)(14) (14)(14) (13) (12)
[image:6.612.73.278.173.627.2](13)(12) (13)(14) (14)(14) (13) (12)
Fig. 2. Immune response to KLH, white blood cell (WBC) counts and neutrophil/lymphocyte (N/L) ratio in experimental groups.(A) The 5-day exposure to 34°C did not suppress a humoral response against KLH challenge in selected mice (ANOVA;P=0.7). (B) The leucocyte level was lower in warm-exposed animals than in animals maintained at 23°C (ANOVA;P=0.0005). (C) In contrast to the effect of ambient temperature on leucocyte counts, animals maintained at 34°C exhibited a higher N/L ratio than animals maintained at 23°C (ANOVA;P=0.004). In B and C, H-BMR mice exhibited higher WBC counts and N/L ratios than L-BMR mice (ANOVA;P=0.004 and P=0.02, respectively), while immunisation did not affect blood parameters (P=0.9 andP=0.4, respectively).
L
ymph node mass (g)
L-BMR 0.009
0.012 0.015 0.018 0.021
Control Immunised
23°C 34°C
H-BMR L-BMR
H-BMR
(13)(12) (13)(14) (14)(14) (13) (12)
Fig. 3. Mass of lymph nodes in experimental groups.The lymph node mass of immunised mice from both line types changed in an ambient-temperature-dependent manner. The separate ANCOVA analysis within each temperature treatment demonstrated that there was a significant interaction between immunisation and line type at 34°C (P=0.03) but not at 23°C (P=0.8). The mass of the lymph nodes increased significantly in immunised L-BMR mice (P=0.005), whereas there was no change in lymph node size between
immunised and non-immunised animals from the H-BMR line type (P=0.9).
Journal
of
Experimental
[image:6.612.327.545.489.655.2]response did not appreciably compromise the capacity to dissipate
heat, even in H-BMR mice with significantly higher Tb.
Furthermore, we also observed a slight increase in IBAT mass (Table 3, Table S4). The above observations question the significance of HD as a constraint on the rate of energy expenditure in our study system.
To our knowledge, no studies reporting a decrease (Bartlett and Smith, 2003; Khajavi et al., 2003, 2003; Mashaly et al., 2004; Niu et al., 2009; Jin et al., 2011) or the lack of difference in the level of the immune response following exposure to high ambient temperatures (Dabbert et al., 1997; Mashaly et al., 2004; Sutherland et al., 2006; Pamok et al., 2009; Star et al., 2009) associated this response with the capacity to dissipate excess heat. Instead, these results have been discussed primarily in the context of physiological responses to environmental stress (e.g. as consequences of the reduction in food intake for the development of immune responses in Bartlett and Smith, 2003 and Niu et al., 2009), biochemical damage (e.g. an increase in the production of reactive oxygen species; Lin et al., 2006; Mujahid et al., 2007; Pamok et al., 2009) or dysregulation of the expression of specific molecules, cells and/or pathways underlying innate and acquired immunity itself (e.g. major histocompatibility complex molecules, antigen-presenting cells or the synthesis of co-stimulatory cytokines; Jin et al., 2011). Furthermore, the studies cited above differed not only in length (ranging from hours to days; see Sutherland et al., 2006 versus Star et al., 2009) but also in the continuity of the warm exposure (e.g. constant trial in Star et al., 2007 versus cyclic trial in Jin et al., 2011). Thus, these studies bear little direct relevance to the potential
significance of HD limitation, as they have primarily focused on the dysregulation of immune functions incurred by acute hyperthermia.
Immune response under heat stress
Our hypothesis predicting that there should be line-type-dependent immunosuppression in warm-exposed mice was not corroborated by the results. The absence of an effect of exposure to 34°C on a humoral response was accompanied by a decrease in WBC counts and an increase in N/L ratio (Fig. 2). These data are consistent with other studies that have examined the effect of high ambient temperature on WBC counts and N/L ratio (Khajavi et al., 2003; Mashaly et al., 2004; Aengwanich, 2008).
[image:7.612.46.564.72.188.2]Independent of the effect of temperature on WBC counts and N/L ratio, both parameters were significantly higher in H-BMR than in L-BMR mice (Fig. 2B,C). These results suggested that, despite the heat-stress-elicited decrease in WBC counts, mice from the H-BMR line type retained a sufficient pool of B-lymphocytes circulating in the blood. The mice synthesised specific antibodies, which resulted in the lack of immunosuppression. Moreover, the exposure to 34°C forced the H-BMR mice to increase their neutrophil counts (i.e. increase the N/L ratio); that is, higher temperatures made mice invest in non-specific (innate) mechanisms, which determine the first line of immune defence against pathogens (Roitt et al., 2000). This result is consistent with other studies that have demonstrated that heat stress increased, or did not change, parameters of non-specific immunity, such as the percentage of neutrophils, monocytes or eosinophils, the anti-inflammatory effect of neutrophils, the neutrophil response to chemoattractants, respiratory burns of Table 2. Summary of ANOVA/ANCOVA results of total and body-mass-corrected mass of lymphatic organs in laboratory mice
Immunisation (A)
Ambient
temperature (B) Line type (C) Interaction
F P F P F P F P
Spleen ANOVA 0.12 0.7 0.66 0.4 96.0 <0.0001 (+) n.s. n.s. n.s. ANCOVA 0.004 0.9 0.08 0.7 83.23 <0.0001 (+) n.s. n.s. n.s. Lymph nodes ANOVA 18.7 <0.0001 (+) 2.4 0.1 9.71 0.002 (+) B×C
B×A
7.6 7.7
0.008 0.005
ANCOVA 23.3 <0.0001 (+) 0.7 0.4 5.2 0.02 (+) B×C B×A
7.5 8.1
0.007 0.004
Thymus ANOVA 0.63 0.4 0.001 0.9 11.86 0.0008 (–) n.s. n.s. n.s. ANCOVA 1.10 0.3 0.20 0.6 18.35 <0.0001 (–) n.s. n.s. n.s.
P-values <0.05 are inbold. n.s., not significant. Positive signs followingP-values indicate immunised>non-immunised or H-BMR>L-BMR, while negative signs indicate L-BMR>H-BMR. ANOVA d.f.=1,89; ANCOVA d.f.=1,88. ANCOVAs were run with total body mass as a covariate, which was always statistically significant atα=0.01 or smaller.
Table 3. Summary of ANOVA/ANCOVA results of total and body-mass-corrected mass of metabolically active internal organs in laboratory mice
Immunisation Ambient temperature Line type
F P F P F P
IBAT ANOVA 0.007 0.9 1.0 0.3 41.0 <0.0001 (–)
ANCOVA 0.4 0.5 4.7 0.04 90.49 <0.0001 (–)
Liver ANOVA 0.6 0.4 24.2 <0.0001 (+) 123.3 <0.0001 (+)
ANCOVA 0.19 0.6 38.5 <0.0001 (+) 198.4 <0.0001 (+)
Small intestines ANOVA 1.9 0.2 7.0 0.01 (+) 144.3 <0.0001 (+)
ANCOVA 1.9 0.2 5.4 0.02 (+) 135.8 <0.0001 (+)
Heart ANOVA 0.01 0.9 2.3 0.1 78.4 <0.0001 (+)
ANCOVA 0.58 0.4 0.5 0.5 114.3 <0.0001 (+)
Kidneys ANOVA 0.4 0.5 20.0 <0.0001 (+) 161.2 <0.0001 (+)
ANCOVA 0.01 0.9 27.2 <0.0001 (+) 253.0 <0.0001 (+)
Interactions between the main effects were not significant.P-values <0.05 are inbold. Positive signs followingP-values indicate mice kept at 23°C>warm-exposed or H-BMR>L-BMR, while negative signs indicate L-BMR>H-BMR. ANOVA d.f.=1,89; ANCOVA d.f.=1,88. ANCOVAs were run with total body mass as a covariate, which was always statistically significant atα=0.01 or smaller.
Journal
of
Experimental
[image:7.612.48.565.579.706.2]neutrophils and natural killer cell cytotoxicity (e.g. Ostberg et al., 2005; Sutherland et al., 2006; Bzowska et al., 2011).
In addition, changes in blood parameters were similar to those observed in our previous study, in which mice from selected line types were subjected to another metabolic challenge: a 30% restriction in food intake (Książek and Konarzewski, 2012). Independent of the suppressive effect of food deprivation, H-BMR mice maintained high WBC counts and N/L ratio. Therefore, we suggest that, under metabolic stresses incurring trade-offs involving an immune response, mice selected for a high rate of metabolism tend to invest in the pool of circulating, non-specific blood cells (e.g. neutrophils) and develop innate immunity. This observation is interesting, especially in light of the prediction that non-specific immunity is more energetically costly than specific (humoral) responses (Lee and Klasing, 2004; Lee, 2006).
The synthesis of anti-KLH IgM in immunised L-BMR mice was accompanied by a substantial increase in the mass of the lymph nodes (significant line type×ambient temperature interaction at 34°C). Therefore, KLH challenge most likely resulted in an increase of the numbers of competent lymphatic papules containing numerous lymphocytes, including B-cells that participated in antigen recognition and generated the signal for cell proliferation and the production of specific KLH antibodies (Ostberg et al., 2005). The supposition that the size and function of the lymph nodes are upregulated is supported by the substantial transient increase inTbin L-BMR mice during the first 24 h of the response to the KLH antigen (significant interactions of time course and line type as well as time course and ambient temperature; Fig. 1B).
To summarise, 5-day exposure to 34°C did not equalise theTbof mice of either selected line types, nor did it suppress the humoral response in a line-type-dependent manner. The response of mice of both lines to KLH challenge was similar in scope, although their specific responses were different. Warm-exposed H-BMR mice were able to maintain high WBC counts and aTbthat did not differ from that recorded at room temperature. In contrast, L-BMR mice increased the mass of their lymph nodes, which was accompanied by a transient increase in their core body temperature. In addition, changes in the mass of internal organs (small intestines, liver and kidneys), IBAT and decreases in BM were also similar in both selected line types. We therefore conclude that heat dissipation was unlikely to constrain the responses of our animals to a combined stress of an immune challenge and high ambient temperature, even though their body temperature was equal to or higher than that recorded in lactating mother mice (e.g. Gamo et al., 2013a,b; Valencak et al., 2013). This conclusion is particularly reinforced by the lack of line-type-dependent responses in mice otherwise differing with respect to Tb (but not isolative properties of the pelage). Thus, at least in our study system, heat dissipation is unlikely to be a constraint that leads to an increase inTbthat impinges on vital physiological processes at lower ambient temperatures.
Acknowledgements
We are greatly indebted to M. Lewoc, B. Lewończuk, S. Płonowski and our numerous students for their skilful technical assistance and patience in performing our experiments. We are also grateful to Kim Hammond, Daniel Naya and an anonymous reviewer for their constructive comments.
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.K. and M.K. developed the concept of the study. A.K. performed the experiment, and conducted biochemical and microscopic analyses. A.K. and M.K. conducted statistical analyses of results and prepared the paper.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Supplementary information
Supplementary information available online at
http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.129312/-/DC1
References
Aengwanich, W.(2008). Pathological changes and the effects of ascorbic acid on lesion scores of bursa of fabricius in broilers under chronic heat stress.Res. J. Vet. Sci.1, 62-66.
Barr, D. P., Russell, M. D., Cecil, L. and Du Boise, E. F.(1922). Clinical calorimetry XXXII: temperature regulation after the intravenous injections of protease and typhoid vaccine.Arch. Int. Med.29, 608-634.
Bartlett, J. R. and Smith, M. O.(2003). Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress.Poult. Sci.82, 1580-1588.
Bloomer, S. A., Brown, K. E., Buettner, G. R. and Kregel, K. C. (2008). Dysregulation of hepatic iron with aging: implications for heat stress-induced oxidative liver injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1165-R1174.
Boots, M.(2008). Fight or learn to live with the consequences?Trends Ecol. Evol. 23, 248-250.
Brzęk, P., Bielawska, K., Książek, A. and Konarzewski, M.(2007). Anatomic and molecular correlates of divergent selection for basal metabolic rate in laboratory mice.Physiol. Biochem. Zool.80, 491-499.
Brzęk, P., Książek, A., Dobrzyń, A. and Konarzewski, M.(2012). Effect of dietary restriction on metabolic, anatomic and molecular traits in mice depends on the initial level of basal metabolic rate.J. Exp. Biol.215, 3191-3199.
Brzęk, P., Książek, A., Ołdakowski,Ł. and Konarzewski, M.(2014). High basal metabolic rate does not elevate oxidative stress during reproduction in laboratory mice.J. Exp. Biol.217, 1504-1509.
Bzowska, M. Hamczyk, M., Skalniak, A. and Guzik, K.(2011). Rapid decrease of CD16 (FcγRIII) expression on heat-shocked neutrophils and their recognition by macrophages.J. Biomed. Biotech.2011, 1-14.
Cai, X.-Q., Yang, M., Zhong, W.-Q. and Wang, D.-H.(2009). Humoral immune response suppresses reproductive physiology in male Brandt’s voles (Lasiopodomys brandtii).Zoology112, 69-75.
Chang, C.-K., Chang, C.-P., Liu, S.-Y. and Lin, M.-T.(2007). Oxidative stress and ischemic injuries in heat stroke.Prog. Brain Res.162, 525-546.
Cui, Z.-G., Kondo, T., Feri, L. B., Jr., Waki, K., Inanami, O. and Kuwabara, M.
(2004). Effects of antioxidants on X-ray- or hyperthermia-induced apoptosis in human lymphoma U937 cells.Apoptosis9, 757-763.
Cutrera, A. P., Zenuto, R. R., Luna, F. and Antenucci, C. D.(2010). Mounting a specific immune response increases energy expenditure of the subterranean rodent Ctenomys talarum (tuco-tuco): implications for intraspecific and interspecific variation in immunological traits.J. Exp. Biol.213, 715-724.
Dabbert, C. B., Lochmiller, R. L. and Teeter, R. G.(1997). Effects of acute thermal stress on the immune system of the northern bobwhite (Colinus virginianus).Auk 114, 103-109.
Demas, G. E., Chefer, V., Talan, M. I. and Nelson, R. J.(1997). Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice.Am. J. Physiol.273, R1631-R1637.
Demas, G. E., Johnson, C. and Polacek, K. M. (2004). Social interactions differentially affect reproductive and immune responses of Siberian hamsters.
Physiol. Behav.83, 73-79.
Drazen, D. L., Jasnow, A. M., Nelson, R. L. and Demas, G. E.(2002). Exposure to short days, but not short-term melatonin, enhances humoral immunity of male Syrian hamsters (Mesocricetus auratus).J. Pineal Res.33, 118-124.
Duah, O. A., Monney, K. A., Hambly, C., Król, E. and Speakman, J. R.(2013). Limits to sustained energy intake. XVII. Lactation performance in MF1 mice is not programmed by fetal number during pregnancy.J. Exp. Biol.216, 2339-2348.
Ewenson, E., Zann, R. and Flannery, G.(2003). PHA immune response assay in captive zebra finches is modulated by activity prior to testing.Anim. Behav.66, 797-800.
Gamo, Y., Troup, C., Mitchell, S. E., Hambly, C., Vaanholt, L. M. and Speakman, R. J.(2013a). Limits to sustained energy intake. XX. Body temperatures and physical activity of female mice during lactation.J. Exp. Biol.216, 3751-3761.
Gamo, Y., Barnard, A., Mitchell, S. E., Hambly, C., Al Jothery, A., Vaanholt, L. M., Król, E. and Speakman, J. R.(2013b). Limits to sustained energy intake. XVI. Body temperature and physical activity of female mice during pregnancy.
J. Exp. Biol.216, 2328-2338.
Gębczyński, A. K.(2005). Daily variation of thermoregulatory costs in laboratory mice selected for high and low basal metabolic rate.J. Therm. Biol.30, 187-193.
Gębczyński, A. K. and Konarzewski, M. (2009). Locomotor activity of mice divergently selected for basal metabolic rate: a test of hypotheses on the evolution of endothermy.J. Evol. Biol.22, 1212-1220.
Journal
of
Experimental
Haak, J. L., Buettner, G. R., Spitz, D. R. and Kregel, C. K.(2009). Aging augments mitochondrial susceptibility to heat stress.Am. J. Physiol. Regul. Integr. Comp. Physiol.296, R812-R820.
Hammond, K. A. and Kristan, D. M.(2000). Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus).Physiol. Biochem. Zool.73, 547-556.
Hammond, K. A., Konarzewski, M., Torres, R. M. and Diamond, J.(1994). Metabolic ceilings under a combination of peak energy demands.Physiol. Zool. 67, 1479-1506.
Ilmonen, P., Penn, D. J., Damjanovich, K., Clarke, J., Lamborn, D., Morrison, L., Ghotbi, L. and Potts, W. K.(2008). Experimental infection magnifies inbreeding depression in house mice.J. Evol. Biol.21, 834-841.
Jin, Y., Hu, Y., Han, D. and Wang, M.(2011). Chronic heat stress weakened the innate immunity and increased the virulence of highly pathogenic avian influenza virus H5N1 in mice.J. Biomed. Biotech.2011, 367846.
Johnson, M. S. and Speakman, R. J.(2001). Limits to sustained energy intake V. Effect of cold-exposure during lactation in Mus musculus.J. Exp. Biol.204, 1967-1977.
Khajavi, M., Rahimi, S., Hassan, Z. M., Kamali, M. A. and Mousavi, T.(2003). Effect of feed restriction early in life on humoral and cellular immunity of two commercial broiler strains under heat stress conditions. Br. Poult. Sci. 44, 490-497.
Kilpimaa, J., Alatalo, R. V. and Siitari, H.(2004). Trade-offs between sexual advertisement and immune function in the pied flycatcher (Ficedula hypoleuca).
Proc. R. Soc. B Biol. Sci.271, 245-250.
Klasing, K. C. and Leshchinsky, T. V.(1999). Functions, costs and benefits of the immune system during developmental and growth. InProceedings of the 22nd International Ornithological Congress(ed. N. J. Adams and R. H. Slotow), pp. 2817-2831. Johannesburg: BirdLife South Africa.
Klein, S. L. and Nelson, R. J.(1998). Adaptive immune responses are linked to the mating system of Arvicoline rodents.Am. Nat.151, 59-67.
Klein, S. L., Gamble, H. R. and Nelson, R. J.(1999). Role of steroid hormones in
Trichinella spiralisinfection among voles.Am. J. Physiol. Regul. Integr. Comp. Physiol.277, R1362-R1367.
Kluger, M. J., Kozak, W., Conn, C. A., Leon, L. R. and Soszyński, D.(1998). Role of fever in disease.Ann. N. Y. Acad. Sci.856, 224-233.
Konarzewski, M. and Diamond, J.(1995). Evolution of basal metabolic rate and organ masses in laboratory mice.Evolution49, 1239-1248.
Konarzewski, M. and Książek, A.(2013). Determinants of intra-specific variation in basal metabolic rate.J. Comp. Physiol. B183, 27-41.
Król, E. and Speakman, J. R.(2003a). Limits to sustained energy intake VI. Energetics of lactation in laboratory mice at thermoneutrality.J. Exp. Biol.206, 4255-4266.
Król, E. and Speakman, J. R.(2003b). Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality.J. Exp. Biol.206, 4267-4281.
Król, E., Johnson, M. S. and Speakman, J. R.(2003). Limits to sustained energy intake VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality.J. Exp. Biol.206, 4283-4291.
Król, E., Murphy, M. and Speakman, J. R.(2007). Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice.
J. Exp. Biol.210, 4233-4243.
Książek, A. and Konarzewski, M.(2012). Effect of dietary restriction on immune response of laboratory mice divergently selected for basal metabolic rate.Physiol. Biochem. Zool.85, 51-61.
Książek, A., Konarzewski, M., Chadzińska, M. and Cichoń, M.(2003). Costs of immune response in cold-stressed laboratory mice selected for high and low basal metabolism rates.Proc. R. Soc. B Biol. Sci.270, 2025-2031.
Książek, A., Konarzewski, M. andŁapo, I. B.(2004). Anatomic and energetic correlates of divergent selection for basal metabolic rate in laboratory mice.
Physiol. Biochem. Zool.77, 890-899.
Książek, A., Czerniecki, J. and Konarzewski, M.(2009). Phenotypic flexibility of traits related to energy acquisition in mice divergently selected for basal metabolic rate (BMR).J. Exp. Biol.212, 808-814.
Lee, K. A.(2006). Linking immune defenses and life history at the levels of the individual and the species.Integr. Comp. Biol.46, 1000-1015.
Lee, K. A. and Klasing, K. C.(2004). A role for immunology in invasion biology.
Trends Ecol. Evol.19, 523-529.
Lee, F. J., Wang, D., Hsu, Y. H. and Chen, H. I.(2008). Oxidative and nitrosative mediators in hepatic injury caused by whole body hyperthermia in rats.
Chin. J. Physiol.51, 85-93.
Leshchinsky, T. V. and Klasing, K. C.(2001). Divergence of the inflammatory response in two types of chickens.Dev. Comp. Immunol.25, 629-638.
Li, F.-H., Zhong, W.-Q., Wang, Z. and Wang, D.-H. (2007). Rank in a food competition test and humoral immune functions in male Brandt’s voles (Lasiopodomys brandtii).Physiol. Behav.90, 490-495.
Lin, H., Decuypere, E. and Buyse, J.(2006). Acute heat stress induces oxidative stress in broiler chickens.Comp. Biochem. Physiol. A Mol. Integr. Physiol.144, 11-17.
Luheshi, G. L.(1998). Cytokines and fever: mechanisms and sites of action.
Ann. N. Y. Acad. Sci.856, 83-89.
Martin, L. B., Scheuerlein, A. and Wikelski, M.(2003). Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs?
Proc. R. Soc. B Biol. Sci.270, 153-158.
Mashaly, M. M., Hendricks, G. L., Kalama, M. A., Gehad, A. E., Abbas, A. O. and Patterson, P. H.(2004). Effect of heat stress on production parameters and immune responses of commercial laying hens.Poult. Sci.83, 889-894.
McAnulty, S. R., McAnulty, L., Pascoe, D., Gropper, S. S., Keith, R. E., Morrow, J. D. and Gladden, L. B.(2005). Hyperthermia increases exercise-induced oxidative stress.Int. J. Sports Med.26, 188-192.
Muehlenbein, M. P., Hirschtick, J. L., Bonner, J. Z. and Swartz, A. M.(2010). Toward quantifying the usage costs of human immunity: altered metabolic rates and hormone levels during acute immune activation in men.Am. J. Hum. Biol.22, 546-556.
Mujahid, A., Pumford, N. R., Bottje, W., Nakagawa, K., Miyazawa, T., Akiba, Y. and Toyomizu, M.(2007). Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress.J. Poult. Sci.44, 439-445.
Niu, Z. Y., Liu, F. Z., Yan, Q. L. and Li, W. C.(2009). Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress.Poult. Sci.88, 2101-2107.
Norris, K. and Evans, M.(2000). Ecological immunology: life history trade-offs and immune defense in birds.Behav. Ecol.11, 19-26.
Ostberg, J. R., Ertel, B. R. and Lanphere, J. A.(2005). An important role for granulocytes in the thermal regulation of colon tumor growth.Immunol. Invest.34, 259-272.
Ots, I., Murumägi, A. and Hõrak, P.(1998). Haematological health state indices of reproducing Great Tits: methodology and sources of natural variation.Funct. Ecol. 12, 700-707.
Ots, I., Kerimov, A. B., Ivankina, E. V., Hyina, T. A. and Hõrak, P.(2001). Immune challenge affects basal metabolic activity in wintering great tits.Proc. R. Soc. B Biol. Sci.268, 1175-1181.
Pamok, S., Aengwanich, W. and Komutrin, T.(2009). Adaptation to oxidative stress and impact of chronic oxidative stress on immunity in heat-stressed broilers.
J. Therm. Biol.34, 353-357.
Paul, C., Teng, S. and Saunders, P. T. K.(2009). A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death.Biol. Rep.80, 913-919.
Prendergast, B. J., Hotchkiss, A. K., Bilbo, S. D. and Nelson, R. J.(2004). Peripubertal immune challenges attenuate reproductive development in male Siberian hamsters (Phodopus sungorus).Biol. Reprod.70, 813-820.
Ricklefs, R. E. and Wikelski, M.(2002). The physiology/life-history nexus.Trends Ecol. Evol.17, 462-468.
Roitt, I., Brostoff, J. and Male, D.(2000).Immunology. London: Mosby.
Sadowska, J., Gębczyński, A. K. and Konarzewski, M.(2013). Basal metabolic rate is positively correlated with parental investment in laboratory mice.
Proc. R. Soc. B Biol. Sci.280, 20122576.
Sadowska, J., Gębczyński, A. K. and Konarzewski, M. (2015). Effect of reproduction on the consistency of the between–line type divergence in laboratory mice selected on basal metabolic rate.Physiol. Biochem. Zool.88, 328-335.
Saino, N., Møller, A. P. and Bolzern, A. M.(1995). Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav. Ecol. 6, 397-404.
Saks, L., Ots, I. and Hõrak, P.(2003). Carotenoid-based plumage coloration of male greenfinches reflects health and immunocompetence. Oecologia 134, 301-307.
Simons, M. J. P., Reimert, I., van der Vinne, V., Hambly, C., Vaanholt, L. M., Speakman, J. R. and Gerkama, M. P.(2011). Ambient temperature shapes reproductive output during pregnancy and lactation in the common vole (Microtus arvalis): a test of the heat dissipation limit theory.J. Exp. Biol.214, 38-49.
Speakman, J. R. and Król, E.(2005). Limits to sustained energy intake IX: a review of hypotheses.J. Comp. Physiol. B175, 375-394.
Speakman, J. R. and Król, E.(2010). Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms.J. Anim. Ecol.79, 726-746.
Speakman, J. R., Gidney, A., Bett, J., Mitchel, I. P. and Johnson, M. S.(2001). Limits to sustained energy intake IV. Effect of variation in food quality on lactating miceMus musculus.J. Exp. Biol.204, 1957-1965.
Star, L., Nieuwland, M. G. B., Kemp, B. and Parmentier, H. K.(2007). Effect of single or combined climatic and hygienic stress on natural and specific humoral immune competence in four layer lines.Poult. Sci.86, 1894-1903.
Star, L., Juul-Madsen, H. R., Decuypere, E., Nieuwland, M. G. B., de Vries Reilingh, G., van den Brand, H., Kemp, B. and Parmentier, H. K.(2009). Effect of early life thermal conditioning and immune challenge on thermotolerance and humoral immune competence in adult laying hens.Poult. Sci.88, 2253-2261.
Stearns, S. C.(2002).The Evolution of Life Histories. Oxford: Oxford University Press.
Sutherland, M. A., Niekamp, S. R., Rodriguez-Zas, S. L. and Salak-Johnson, J. L.(2006). Impact of chronic stress and social status on various physiological and performance measures in pigs of different breeds.J. Anim. Sci.84, 588-596.
Journal
of
Experimental
Valencak, T. G., Hackländer, K. and Ruf, T.(2010). Peak energy turnover in lactating European hares: a test of the heat dissipation limitation hypothesis.
J. Exp. Biol.213, 2832-2839.
Valencak, T. G., Wright, P., Weir, A., Mitchell, S. E., Vaanholt, L. B., Hambly, C., Król, E. and Speakman, J. R.(2013). Limits to sustained energy intake. XXI. Effect of exposing the mother, but not her pups, to a cold environment during lactation in mice.J. Exp. Biol.216, 4326-4333.
van der Vinne, V., Simons, M. J. P., Reimert, I. and Gerkema, M. P.(2014). Temporal niche switching and reduced nest attendance in response to heat dissipation limits in lactating common voles (Microtus arvalis).Physiol. Behav. 128, 295-302.
Wu, S.-H., Zhang, L.-N., Speakman, J. R. and Wang, D.-H.(2009). Limits to sustained energy intake. XI. A test of the heat dissipation limitation hypothesis in lactating Brandt’s voles (Lasiopodomys brandtii).J. Exp. Biol.212, 3455-3465.
Yang, D. B., Li, L., Wang, L. P., Chi, Q. S., Hambly, C., Wang, D. H. and Speakman, J. R.(2013). Limits to sustained energy intake. XIX. A test of the heat dissipation limitation hypothesis in Mongolian gerbils (Meriones unguiculatus).
J. Exp. Biol.216, 3358-3368.
Zhang, X.-Y. and Wang, D.-H.(2007). Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles (Lasiopodomys brandtii).J. Exp. Biol. 210, 512-521.
Zhao, Z.-J.(2011). Energy budget during lactation in striped hamsters at different ambient temperatures.J. Exp. Biol.214, 988-995.
Zhao, Z.-J. and Cao, J. (2009). Effect of fur removal on the thermal conductance and energy budget in lactating Swiss mice.J. Exp. Biol. 212, 2541-2549.
Zhao, Z.-J., Chi, Q.-S., Cao, J. and Han, Y.-D. (2010). The energy budget, thermogenic capacity and behavior in Swiss mice exposed to a consecutive decrease in temperatures.J. Exp. Biol.213, 3988-3997.
Zhao, Z.-J., Król, E., Moille, S., Gamo, Y. and Speakman, J. R.(2013a). Limits to sustained energy intake. XV. Effects of wheel running on the energy budget during lactation.J. Exp. Biol.216, 2316-2327.
Zhao, Z.-J., Song, D.-G., Su, Z.-C., Wei, W.-B., Liu, X.-B. and Speakman, J. R.
(2013b). Limits to sustained energy intake. XVIII. Energy intake and reproductive output during lactation in Swiss mice raising small litters.J. Exp. Biol.216, 2349-2358.