ACICijOWLEDGEMEijT§
The author ia mo't grateful to Dr
J
.Fergusson for his \.l.Lu.au"'"" and encouragement., thank&w·.
T. Robina on for hi$ help in the oryetallography seation.Ph
en
dto
R
n: ...
cpdiphO$
diars
ear
Methyl (CH3-)
Phenyl
(c
6a
5-)ethylenediamine (NH
2
-caf ..
ca
2- NH2)diethyld:tthiocarbamato
I0
2H5)2Ncs ... 2)
alkyl or aryl group cyolopentadianyl (C
0
Hs)a bidentate phosphine
a bidentate arsine
ABS.TRACT
The work reported in this thesis is divided into three parte .. Part A ia concerned with etudies on stable oomplexes of dinitrogen, and related lig-ands ~ Part B detail$ studies on the rhenium (V) oxo ... oomplex a~
2
:Re0015
, and part C outlines general expert mental details ., ·Part A.. Some reactions of the dinitrogen oomplex [Ru(NH3)5(N2)] 01 2
have been investigated and the oXidation potential of the [Ru(NH
3)5(N2
)J
:"&+ ion in neutral conditions bas been found to be approximately •0.95 volts. The reactions have demonstrated that [Ru(NH3)5(N2)
J
012 prepared using hydradne hydrate is impure,the impurity is probably [ Ru(NH
3)5 (N2H4)
1
012• The dinitrogenof purified
l
Ru(NH3)5 (N2) ] 012 has not yet been reduced in
aqueous conditions , oontJ;ary to a previous report.
The reactions of some ruthenium, oJilmlum ~ iddium and rhodium complexes with hydrazine hydrate and with metal reduotants
(e.g. Zn) in concentrated ammonia were investigated. A number of products including [M(NH
3)5 (N2)
J
2
+, [
M(mi3)4 (N2)2
J
2+,lM(NH
3)5 (CO)]
z+
(M= Os, Ru)1 \Jr(NH3)4 (CO)Cl] :11+and [Rb(NH
3)5H
J a+,
together with hydrazine containing produots,belongs to the orthorhombic space group Pnma with a = 1351.5, b • 1046 ..
s,
o •
681 .. 5pm and ~ = 4~~ The cations oncrystallographic mirror planes and the interatomic distances within the cation are os-N
2 (1$4pm); N:N (l12pm) 11nd Os•NH3 (212.,.215pm).
The ruthenium complex (Ru(NH3)5(N
2) ] c12 was found to form solid
solution• with (NH3)
5ol] Cl2 whioh are ilomorphou.a with the above osmium complex.
system for the ruthenium diniU'oqen complex. The enetgy of the \>(NN) absorption in the solid solutions is up to 50 om ... l lower than fot pure [Ru(NH3)5(N2) 1
,
andit
is suggested that thisintetaotions in the solid state.
also that the two metals; osmium iridium, form strong-er bonds to halide and amJm.OJrthl, than do
the
were studied in this section. This complex ion is reported being different from most rhenium (V) complexes which
study demonstrated that
hi9h paramagnetism reported preViously has been 1hown to due to th$ presence paramagnetic Os
1 lntroduotion
Nitrogen
Flxatton
Stable Dinitrogen Complexes · summary of Present Work
2 The Purity and Reaotlons of lRu(l{R
3)5(N2)] Xntroduot!on
Purification of [Ru(N:a
3)5(N2)
1
012 Reactions ExPerimental 1 1 8 17 012 21 22 27 31 of Metal ...u.., ...
"'~""'., under Reducing ConditionsIntroduot!on 34
Discussion (a) Compounds prepared 3 6
(b) Reactions 48
(c) Comparison Metal Ions 51
Experimental
lntroduotion
Collection and Reduction of Data Discussion
5 Solid Solutions Involving ( Ru(NH3)
rage,.~o
6 0 Infrared Spectra of soma Ammine Complexes
Introduction
82
Results and Discussion Experimental
·'1 Discussion of Dinitrogen as a Ligand
PARTS The Rhenium (Y.) Complex ReOCl;-Introduction
Review of Ra (V)
SummarY' work in PreGent Study Re•ulta
Preparation of
cs
2Re0015 96
Properties of Cs2
102
PART 0 Expedmenta.i 106
6 1
s
9 10u.
12 1314
15
16
Properties of the Nitrogenase
Proteint
Bttuotura1 ·
on 'I:>initro;en
Complexes Propert!e$ the Productsfrom
thereaction of
Properties ·of (Rh(NH
3)
1
Br2.;) (00) Absorption Frequencies for Ru (U) and lr(Ul)
Oomplexel
Properties of {Os(NH
3) 5 (CO)] of (dto)2 (OO)n
Reaction Products
Calculated
Reactions Metal.
Hydrogen
Atoms
Structure Factors
4
16
2337
4044
4'148
65
65tnteratomio lllstanoes and Angles in (Os(NH
3) 5(N2)
J
012 66 Route""Mean""Square Amplitudes of Vibration ofAnisotropic Atoms
Changes in Solid Solution Unit Cell Sizes with Composition
66
26
( <
Low-frequency Infrared Spectra of
Rhenium (IV) and (V) Chloro-complexes
11
100
Number 1 2 3 4 5 6 7 8 9 10 11
Transition Metals which with Dinitrogen
Two Possible Modea of Dinitrogen Co .... ordination Reactions of Ruthenium Complexes with Hydrazine Infrared Spectrum of [Ru(NH3)
5 (N2) ] ZnC14
infrared Spectrum of {Ru(NH3) 5 (co)
J
012Infrared Spectrum of [ lr(NH3) 4 (CO) Cl) 01 2 Inftared Spectrum of oi$ [ Ru(NH3)
4 (N2) 2] Bt2
Reaction. Sequence to
Infrared Spectrum of [Os(NH3) 5 (CO)) 012
6
a
15
1822
31 38 42 45 4612 Infrared Spectrum of the Product from the Reaction of 50 lOs(NH3)5Cl) 012 with Mg/Hg
in·
NH313 Contents the lOs(NH3)5(N2)] 012 Unit Cell
14 The Cation ( (NH
3) 5(N2)
J
Z+ 6715 Variation of Force Constante with Bond Lengths for 68
N.,..N Systems
P~M!o
94
100
Introduotion lt{itroSJen fixat&on
One of the most important ptocet~~~ses for Ute, as we know it, is the biological :fixation of atmospheric dinitrogen.
It
been variously estimated 1 '2 that in the order of 100 to 1000 million toni of dinitrogen, of which man about one tenth, ate fixed annually on of dinitrogen fixation probably the main limitation agricultural production in many less developed countries 1• ln developed countries, biological fixation still accounts for about two thirds of the annually fixed dinitrogen.The chemical problem, in increasing the amount of nonbiologioal dinitrogen, stability the
d:l.nitrogen
~
is;~40
l<J'. mole""l(S),
but the chemical the high energy
(52 4 kl mole -l) 3 to ... ""''" K. the first bond ..
This the cotteeponding bond dis ...
sociation isoelectronio ion (2
k1
mole -1)
3 acetylene molecule (252 kJ, mole "" 1) 3 and carbon monoxide molecule (298 kJ.mole- 1) 3 •(A more ext.e:mn
Monoxide is shown in Table 1).
Propert_y Dinitrogeh* Carbon Monoxide*
Bond Dissociation
Energy 945 kJ/mole 1073 kJ/mole
Dissociation Energy Corres p. Double
Bond 421 kJ/mole 775 kJ/mole (3)
Difference 5 24 kJ/mole 298 kJ/mole (3)
lst Ionisation
Potential 15.51 volts 14 .1 volts
Bond Stretching
2330cm- 1 (125) 2160cm-1(112)
Frequency
Force Constand 2 2. 2xl 05dynes/cm(l25) 18. 6x1 05 dynes/ cm(ll2)
Bond length 108.9 pm (58) 112.8 pm (143)
Dipole Moment 0 esu O.llxlO -18 esu
2
Dinitrowen
before any reaction can oceur.
ttu:~tt';ll11Cfl'lllll $UOb
as
thealkali "",..",. ... ""
1 bUt '~>A"''"'•""The •n~~~>U'-'""'
by and Sm1th4,
1 • Interaction
3 • Interaction
4 •
various
methods5 •
v.atton will be
... u ... !n all
au:l1tr,09E~n with metal
A few
orararu.e compounds
6 '1 as thealso
with butresultinS1
formulation. Compounds which the d1nitrogen
t or
ion, of unknown
sulphur
bet!Ul nrnnn~
co•lve,nt1on~~~ dia=onium salt, w1U
not
further.
(1)
This high
of aD&Iotiil~
(2)
!n
Until
very :recel\ilYun111:1lle ln th!flt
nVAI'"'H teaotiO;n (N
2
3
S!lzrm3)
PfO'OeSUB, but
in an
dtmttogen
fixation
dinttrogen
known• The
100,000
,000
40,000
as non ... heme
Metall,lO
atoms
=
1:6Stable 1 or 2
•
a mbctute two
""a«J,E!11.ltU'lnt!m
••llt'tr<~:~rf!'lt!:e14
r
Nb
Tc
Hf
T
FIGURE 1
_ a) Metals that act as fixation catalysts
{ 1
H
b) Metals that react with (Ar-N;) in modelreactions of Nitrogenase
atoms definitely to involved.
'(3)
to reproduce biological dinitrogen fixation
been investigated with a number of transition metal
'rhe significant findings
are:-(a) catalytic reduction of dinitrogen with -~., ... ----·~
early transition metals,
•
(b) preparation then reduction of model compounds,
(c)
such as Ar-
N;,
using intermediate transition metalcomplexes of
thf;l
molecules.
relatively stable transition metal
, but in this case reduction of
not
Figure 1 , reproduced from Murray and Smith and updated, shows
. the transition which have been investigated for the three
types reaction above • reactions will now be
in more detail.
(a) most successful reactions in reproducing biological
nitrogen f!Jcation under mild conditions have involved
the early transition metals a. .... -. .... ~~''"' as reduction •
In
, the final '~"0'"11"~""~"~ nitro9en product to be a metal
nitride which is readily hydrolysed to ammonia. This reaction
biological systems 17• of the
•
6
are more similar to those involving dinitrogen interactions with
metal surfaces.
The most thoroughly investigated involves
( -cp)
2 TiC12
18
' 1 probably as this complex gives the
relative ytelds of nitrogen. schools of thought
as to the intermediate complexes involved in this titanium
Much of this work
Olivl:l 18•
recently reviewed by Henrtoi-Oliv~ and
The authors above 18 consider that the reaction involves a
dimeric hybride (III) - (II) , but the
bonding of the di.nitrogen molecule, proposed to co-ordinated
to titanium (II) , could not determined from the esr s
they obtained •
Van Tamelen disagree suggest that· no hydride
intermediate is involved. that in the ( 7( -cp)
2 TiC12
system, the initial reaction involves co ... o:rdination dinitrogen
to { Ti( 1r -cp)
2
l ,
with the formation of the intermediate[ Ti(N
2) ( -cp)2
J
2 • Van et al.20
that dimeric
such as by
Henrioi-Oliv~
Olive 18 areonly formed in the d:Lnitrogen, but these dimers must
dissociate back into titanocene before ~;:my reaction with dinitrogen
occurs.
Franklin and
Byrd~
1 have interpreted potentiometricstudies of the T1Cl
d:lnitrogen: titanium complexes possible, depending on the solvent used. Possibly this explains some of the problems in interpretating the titanium cyclopentadianyl system above.
Yamamoto et al. 22 have isolated a series of nitrogen containing complexes from reducing (TiC1
3 (THF)3
J
withmagnesium at room temperature in the presence of dinitroqen. The initial black product analy:;;ed as {TiNMg2c1
2 (THF)) n. Five
moles of reductant are required (2. 5 gm. atoms I\l.fg/mole T1) and the product gives no dinitrogen on pyrolysis at 200°C. Part of the magnesium chloride can be exchanged for aromatic amines such as pyridine.. The authors consider the complex to be either a
dinitrogen bridged system with a low NN bond order, or a nitride with a low TiN bond order ..
Further work on the above titanium nitrogen complexes may positively identify the intermediates involved in the reduction of dinitrogen with the early transition metal complexes •
(b) Parshall23 and others 24 have investigated the reduction of molecules, analogues
to
dinitrogen, suchas
Ar-N;. These mole-cules are co-ordinated to transition metal ions, than the triple bondin the ligand is reduced.
From information at present available the requirements to
1. The metal th<Ute with the
to in
low
oxidation states , es pee! ally d8 electronaonfif'Ut'atio~l
•with the complexes should not be strong 1( •aoofa!ptor ligands*. The other ligands present in the aomplexe• ate predominantly aminea and phospbines together with
hydtide,
or
halide~~S .. The :~~ource of dinittogen either dinitrogen (the metal
The
most
oommon nitrogenoontllintng
molecules used in reactions to• The unus 'Ltal
reaction in Which ammonia to dinitrogen in the presence
ruthenium (lli) (IV) and a reducing aoe:m:
*
A number. of impure diruttogen complexes oontaintnv 1( bonded ligands have been proposedrecently
butformulatlon.s
such ar&ide$, also seem oonsi•tent with the evidence
by of the techniques listed below.
(1) lnftarettl sp$otra by identiftcation26 the
~
(NN)iilit!JOI'Ption around
2000om""'t. If
the15N15N
illotopeused a !n energy the ~ (NN) absorption
60w70 om*1 whloh is good evidence for the
~.~,""lfi!:;IJ.!>vw of dlnlt.rogea !I
Pteouation•
have to be taken~tu>slenc:'e of d1n:Ltrogen, e ~ (NH
3) 10
CN
2)J ,
2 000 om ·l may du4!1 to
the evolved , by
in!lrate~d $peotral and
t1A1~At1 for most
A brief survey wUl
cUlJ::tea•·anc~e of th•
have been repOrted and •. .u~.,'il>
tbeit Pl'Ql)el'ties) are listed as
(together with
ll
Ptobably
the
most·tntetesUnq
newthe
bridgeddinitroten
prepared
. ' . by Ohatt•• . vroup80 ,31 , Item the:published but the btldted complexes do have e~remely law hqueno!es (i-e down 1630 om•1 with Nbdl5) for the
~
'ttN) infrared al::u~orption., in ~ (NN) absotptionenergy
tcompared with
or
4-1,3
0 fJ,•::a;:~.~.AQ
comPlexes
mayboth prepared
M(N2)it
3(PMe2Ph)3
34 and
(where M • ~au,Ofll). frequency the ~ (NN) (!absorption in
<
ltu but the stab:Utty ofin the
dlnitrot~u:'t,
~
In theoomplexes
38 , viz13
the first complex, the use of 15N16N oonf1tml the existenoe of a dinitr~gen · oomplex. These two (X) carbonyl products in whichabwe
aU.lO
om'""1
I'
show the ird1u~noa of 1f bonding ligands
on the '"""'"''"" .. (i) dinitto~l). Vlillllj;loi.'IZII~ID whioh not contain carbon. the ll (NN) absorption
app~Ximataly
.cnn~
1 in their inftllfad spectra.ftl• oomplexea
OOtll)et (I) "" and
( lu(d1ars)2 (N3)
1
•
However
1 the(d1m)2(N
2)olJ can he prepared (rom thil
az~A:t.e
,
a
reacucJninvolving
NO+.et al,
44"""'''"u,,.(N2)
2)
(1Ph4)2 trennn~Etn by
probably
·~l'<'IIIIU"""' OXJ.aa1:1on
states,
probablYdetected'U)
(W), (ita oase the •etra.phenylbcrate
talth
ll$o~
et a1.
41have
~nftrmed
the
poeJd.bUity
<»d.dat1onof
llmmirte
•
'-'bey1howed
that
both
[oa(»m
3) 2
>J
a+
and [Ru2CN:a:
1
>
10tN1
)]
4+
wtU
unaerg()
ayoltc,
one electron.,
oxidation then r.duotion raaotione 1fteuaueou1
(25°C)• H2S04 I01utton. fb.e
te.te
donflt&ntl for the deoontPOsitio:n of these ox.td!.sed complexe1 Z x1(t'•a
fU\!I0•1 and 1 xut•l
sec""lre•pecUvelv47 •
monomer
[Ru(NH3
>1
a+
oould not
oxtdl$ed
W•
compound g1V'Il!tlan tmmedlat€!1
evolution
dtru.troge:n 47 •
bean
develo,.a
40• A mtxture of the two ruthenium dinttrogen001tlli1lfl:H! ions [
1\u(~a>&
(N
all
l+
~d
(
) to(N2)]
4
*'
Il)fe!oar•:td by the decomposition [ ku(NH
3)8(:N3)] z+. that acid rea,Ctl
wt'b
the u:t<le ttarting mateHalto firat gf.'Ve
a nltrene
intemedtate, wbtah
the dlnlttot«!ln product&.
tufther
react•
form(d)
J!nu&oa&.
Pf.Q~Jf!A•e, ft~. ~amto1Jiaiaf
•l•
41
others
49,so
have
addittoaalN
Ill
N
'
End on co.-ordination (A)
..
.
.
.
.
\;
Ru
I
NH3Edge on co-ordination (B)
F&sut! 3
Posa&bl!
Mml!• gf P2~atd&lgt!op of l)tpitrosusmtQ the [ Ru (Na3)
ion [ Ru
2(NH3) 10(N2)
J
-4+.. is on on the first reaction producing { Ru(NH3) 5 (N2
)j
2
+
as prodll,ct,
but on the second reaction cannot compared because of
variations in ionic strength present.
'l'he enthalpy difference between the two possible modes of
co-ordination of molecular nitrogen to the ruthenium ( II )
pentaammine grouping (Fig .3) estimated51 to be
approxim-ately 92kJ. mole -1 by considering the activation energy for the
reaction 1 15N Ru - 15 N. intermediate
in this reaction , but the
activation energy means that this intermediate is only
slightly more stable, (29! 17 kJ. mole""1), than the nonbonded
system composed free dinitrogen and {Ru(NH
3)5
J
2
+.
(U) .... Gray al. 52 have assigned the intense
absorption at 200 ... 270 nm in the spectra of ruthenium and osmium
dinitrogen complexes to a metal to ligand transfer
transition. For the dime:r, £Ru
2(NH3) 10 (N2)
J
4+, the transition.
*
2+eu ~ e
9 ( N2) while the monomers ( [ l\/I(NJ;13) 5 (N
*
=
Ru,Os) the transition is of a similar type (b2
or
e( N2).Gray52 proposed that the total bonding electrons transferred into the
*
NN
dis:tanoe Comments (pm.)
CoH(N
2)(PPb3) 3
[Ruz(NH3) 10(Nz)
j
(BF 4) 4111.4(11)
191.816)
{ Ru(N3HN2)(en)
1
j
•r
6[ oa(NH3) 5
CN
2)1
1
(I)
'*
(1)
pteaent
work
•
energy end consequently the energy of the Ruthenium-:~~( "'T' N 2) transition 1s lower.
Ohatt et • 53 hal competed the ftequenotes of the
~
(CO)8nd
J
(NN) absorption• 1n a number of isoeleotronio carbonyl and dilliti'OIJ'In OOII!PlexeJ •It
was shownthat
the ratio of;~~~~
t ls identical and equal to 1. 088 for all isoeleetronic pairs
considered*, including the free ;a1eu•• This li interpretated53 in termi of the similarity of the type of bonding of these two molecules
metal
ions.
(e) ~tructuref. of :P&n&trogen . Oomslexe" • At the structures
of &be dlnitrooen eompleltes have been determined by x-ray methods52'
ti-4•S 7(Table 3).
tn
the four struatures theN bond!enwth
ranges from 112.4 pm52for (au
2 (NH3) 10 (N2)J
OaF
4> 4 to 110.6 pm55 for (Ru(N3)(N2) (en)2] PF6 (Table 3), compared with the bond length of dinlttogen in the gateous state of 109.8 pm58 • The metal dinittogen bond lengths ranve from 180"
1
pm54 for(N2) (PPh3}3 to 192.8 pm for
£
Ru2 (NH3) lO (N2)] (BF 4) 4 •tn
each M "" N N ~oup ta linear to within a few..
The data on the two disordered str\lcturea56 ,57' 1sbut
is tn overall agreement with the
reaults of the ordered structures,The sign1f1ctmt observations from these dinitrogen structures
are
abort metal todinitrQQ"en
bonds, thelinear metal...,.dinltroven
* ~ (XY) 1 , is the energy of the observed (XY) absorption for
m-oup and the little changed NN bond length in dinitrogen. Simiiat Qb~t~ervationa apply to carbon monoxide complexes • Probably the mo•t stvnificant ob~t~etvaUon 18 that the mea1ured
meta1•din1ttoven bonds length• we almo1t ident1oi!U. to reported
11
The aim of the prel)ent work wars to add
te
our undet~JtancUngof the chemistry of c:U.nltrogen a ligand.
P!ft!
(a)Wh$n
this presentwork was started it
wa1. thought that dinltrogen 1n the QomplexJion ( Ru (NH3) 5 (N2)
1
a+
could teduoed to yield one mole of ammonia60,Gl,
while the othernttrogen atom was unaoaounted for. The diW.trogen in the few other know!\ complexes could not be reducea11.. The complex OoH
(N1(P~)
3
that th1s compound
together with the ruthenium compound suggested a model route2 ' 11 for biological
dinttroten
fixation.it
was hoped to study why thethen in
2+ of impurities in samples of [Ru(NH
3) · prepared utdno bydrazine hydrate • the original reperted60 methcn:i • The
impurities contain b:ydrc:uctne, probably as the complex ion
N2H4H2
o
<o
0c .. ,
10 mins.
(Ru(NH
3)
Cool in Air.
Itt"
Mixture (Ru(NH
3)5(N2)J Cl2
Hydrazine reduced
"'---llHJ.---
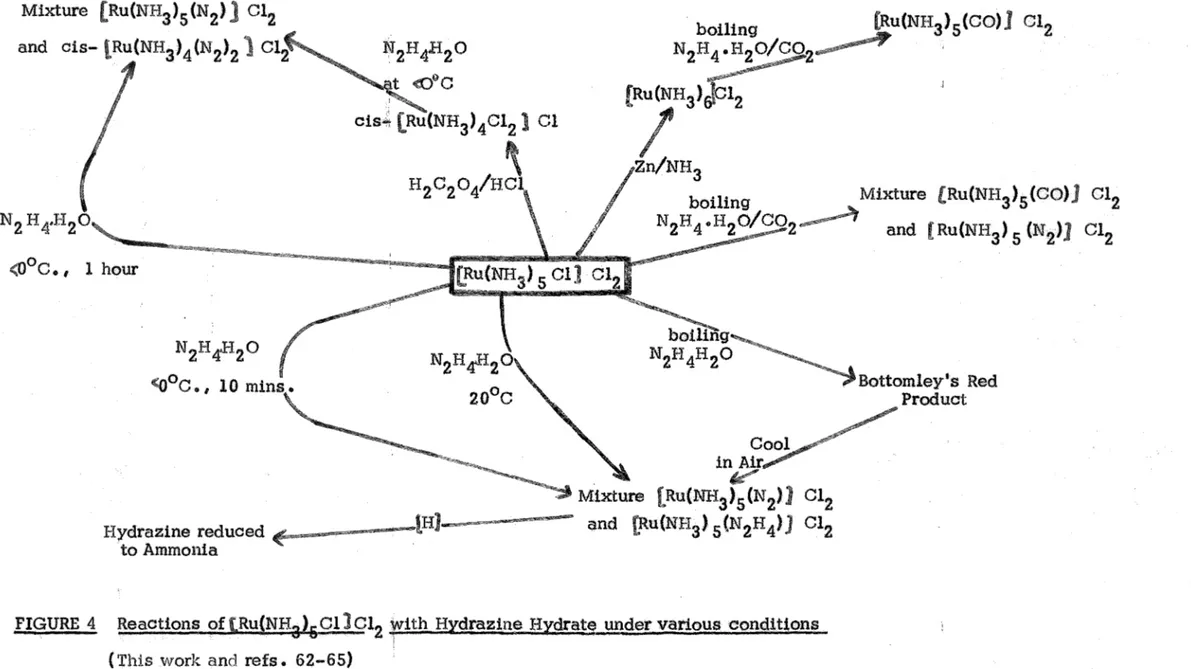
and (Ru(NH3)5(N2H4)J Cl2·to Ammonia
FIGURE 4 Reactions of tRu(NHa )
6 Cl
l
012 7"-'ith H:ydrazine Hydrate under various conditions(This work and refs. 62-65)
Mixture (Ru(NH
3)5(GO)j Cl2
and [Ru(NH
3) 5 (N2
)J
Cl2by a lingle ..,..,,.,..,,.. 1> ThiS 111'1'1'11.1t'1'1:'UI'~ iS ~ ... ~.,...; $:tld
the
oa ...
N2 bond length l84;o2 , the N N bond length is:U2 pm and the average
os ....
(NH3) bond lfllngth is 214 pm.OaNN bond unole 17tl'' the environment the osmtum is
•
(dJ The solid
solu"ttons
[Ru(NH3) 5(N2)] 012 in
(~u(NH3)50l] Olz ~nd in
LRu
)
s(CO)J
have been inveltigated.These
mixture•form solid solution•
in whioh the dtnitrovengroup
probably no
longet aulllorcLenu:t withthe
:::~~mmn1ru :::~~ ligands a1 oac1urspure [Ru(NH3) 8(N2
)J
•
of this work it decided to investigate complexes of penta•valent rhenium containing the species Re ...
o.
The short rhenium-oxygen bond confers a strong tetragonal dist,or.tion to octahedral tbenium (V) complexes and this gives tise to spin pairing • The complex s'
2ReOC15 has been reported as patamagnettc:-? 0
20
complex is in fact spin paired with a small positive molar
susceptibility due to temperature independent paramagnetism.
The reason for the high magnetism reported earlier is due to the
first
no~)Gd. that ..
u•••v would enable
to understandthe abilitY
nitrogenase
this knowt.d;e may
the of cunttt<>Oelrh
molecular dimtrooen.. Eventually
Qther eoonomlo !ndustrtal method$ for
This imminent when Allen and Sal1toff
... Ql ... ..,Ui [Ru(NH
3) 5(N2)] ,2·+ could
to yi$ld mole of Nthl!l.inium.
time, CoH(N
2
)(P~) 3tr(Na)CUP~) 2 , csec,omiDOJ•ed. wtth evolution dinittogen on reduot1on36 ..
the
the The
0 .. 0 ({.} c: ro ;.... £,-; ~
0 ..
4000
FIGURE 5
.
'
•• < ~
Infrared
;lio~ .. ·~·--" ...
2000
..
.
i ' · ... ,..
.
'.
' ;..
~~ ' ..
' ''.
' . '.
' I.
1200
1600 l
Wavenumber (em- )
(NH3) 5 ) ] ZnC14 i) . . .
(i}, product is
t
800
.H2
oxidation
Pudfioatton
Reaction• cf [ Ru(NH3)
s
(N2)J
Cla were initially our!ed out on products at room temperature by treating(Ru(NH
3) 501
1
013
with ·bydtalid.fte hydratE'+When this
is evolved but a "'u''lii!UJLt.;U1..Jull! I,;I,It:;l,!iUI.i.t.dl~11!:!
of
with [ rtu(NH3)
61
(HgCl3) $ , m-eoared t14
by the addition of mercuricto (itu(NH
3) 6] • However, I!SP&ottum
2015 om ""'1• The
an
attemptto
~ ... ..,..,li ofanions
a-Table 4
Property
)Leff•
Infrared ~ (NN)
Ss(NH)
fr(NH)
+
Analysis
Cl; H: N/Ratio
Properties of the Two Products formed from
the Mercuric Chloride Oxidation of
[Ru(NH3)
5 (N2)) 012 (Hydrazine Prep.)
Product 1
0.6 B.M.
2115 cm-1
1302;1285 cm-1
775 cm-1
4
.o:
16.5:7.o
Product 2
2.3 B.M.
1340cm-1
800 cm-1
4.8:20.0:6.0
Conductivity/Cone* 2.22 x 10•3
(in H2o) Moles/1
1.93 X 10-3
Moles/1
+
*
Molar* Cond. 240No effect
440
Reduced to (Ru(NH
3)6 ] Cl2
The analytical figures given in the experimental section at the end of this chapter.
Based on the molecular formulation [Ru(NH3)
5(N2Jznc14 for
Most of
the
remainin~ruthenium
could be recoveredfrom
the filtrate;from
wbiob theZnCl~""
aalt the dtnitto;en product h$d been removed, by addition ofmethanol
(product 2). The properties ofthe
dinitrogen prOduct and the methanol precipitated product outlined in Table 4*
The latter product, product ~ ~ bas
ProPerties
wh1<:.1h areconsistent wtth a
oompound
ofoompc:ur;ltion
l
i\u(N.H3)6) Ol.Zn014 ~ A product with identioal K•taY powder photo, infrared t~peotrum
and
analysis toproouot
a ,
is obtained when thecation
[ rtu(NH
3) 6 ] $+ !s precipitated from an
aqueou•
solution by useof I!l'lino chloride and methanol.
Produot
1,
the dinitrogen complex
precipitated as theZnOl~
salt, was mOta difficult to explain~ Thex ...
ray pQ'W'der photo of this product is identlaal to the POWder photo of (Ru(NH3) 6J
Zn014, hence the low ma~netic moment of this compound is consi~Jtent witha.
monomeric ruthenium<tl)
complex~ However~ some ptQpetties of this new complex (Produot 1)in the air at
room
temperature and the paleyellow
so11d darkens to a blf.tok powder after •few
days if The product obtained by theKUt1'4H,.)
B
}
J ,
(Ru(NH3)5 (N2)
c1
2 • and possibly1\U\,N,H'!!it)
J
a
of
(Ru(14NH3)(15N1SN)
J
2+ •' ooraftnned this.tesult
80 • They found norGcovered from the d.ec:::onl:PC,S1t1on
,·a number
entiohment of the
(Ru(14 )
5
t
15w15:N) ] (IF 4)3 with hot o.i:meentlratE~a
sulphutio or alkali borohydrtde ,,; also found £~Jolut1ons ofIUttilOaJedlY pure (Ru(NH3)
5
(N2J
,
usinghydrcu:ine
hydrate, a positive than 6%
hydrazine was found to be present• Allen•s6
0
original aompound a high hydrogenanalysis
Whiob his preparations26
tdx moles of ammonia" The additional of anunonia comes
from the hydrazine imput!ty and the co-ordinated c:Unitrogen.
in th1$ chapter (see beloW') it was found th-.~ approximately S. 7 moles of ammonia obtained from the decomposition, of th~ hydtazine pteperatton of
~ ' '
( Ru(NH
3) 5 (N2)] 012 , by strong alkaU in the presence of
Devardi!l 's
alloy.
ruthenium complexes suchl
Ru(NH3) f~NaJJ
'
(a•ide preparation), ( Ru(N:a3) 5ot
1
012 and(Ru(NH
3)
al
012
giveonly
the molei:i ammonia predictedsupports the that their oom~X'sit1on. This
otdinated hydtazine 1$ the SOUirCe the additional •
l;),..u, .. o this published et al. confirmed
tha' co-ordinated d.tnitrogen.in {Ru(NH3)
s
(N2)] 012 oanno1:
with alkaline borohydride • However, these workers on to say that even ( Ru( ) 6
J
moles of ammonia, when hydride, in
Attempts
potential$ of
without
conditions
gives ~eater than the predicted
is
with alkalinebore•
by them.
reduotion
{ Ru(NH ) (N
J
J
z+,
but3 5 2
that oo..,.otdinated dinitrogen "stable" oornpleXE~s m:UlllC:)t
(
]
Lever
28
relatively
(a) ,Rf!&qtii!l
w1tb
merow1C' oblgride .-. Tht• work
been described :ln the .,...,,v .. ;ru;.,. secltlc~n... Both
oontam1lna1:•ea ( Ru(NM
3) 5
(Nz)1
012
I"A!ll>l"'''''with
.,,. ... .,..,,.. OOCUt$ wrul!t'tiiU;il!!
73 ,7~
and hydtaa:ine
ln solution
evolution with
formation of a yellOW'solution.
purplefrom
both"'"'""'~,.,,.,1;:>
to theV
(NN) yellow solutionruthenium (UI) ~Au,u~·~~"""" while the u.: . . n •. U.H, from the purple solution
did
net contain
oo-"o~a.J.natEiaammonia.
the formatton
(o) Reaction with ( Go(N
eimU.arly
to HgGlz.-.olution. The
hydtazine impurity[l\u(N'H
8
)
5
(Nia
4)1
l
Ru(NH3) 61
i+,
//
4 are both
...
~,
... .,...,, ... to
rutheruum (ttl) ; but [ Ru(NH3)5 (N2)
1
3
+
(d)• d ruthenium
tn
[l\u(NH3) 5 (N2)J
c1
2ami
(Ru(NH3) 61
to
ruthenium (U.t) with
dltfowuted tn in 5" The
hexaammtne
[Ru(NH
3)6
J
012 tepgrtedto react
dt~erent1y1
3,'1<4
linoe
ord.btated ammoma 1$ al•o lost, and the
produat ( Ru(NH3) S (HzO)ClaJ
01.ED
• The two rutbentum (t!) ClOrrtPle:xes[au( ) 5 (N2)] o12, L4""'''4".l;!..l!!l) 6
J
o1213
, '
4 wuhnitrous
give
nttto•Yll
Ru(~p)CNH
3) 51 ·
(g) ... sodium
. 30
(autN:a3
J
6
J
cn
3
give
• The
~~P~ trtu(NHa) s(Nz)J r tAu(NH3) s (N2H4)
J
, .
deocmn:Jes••d
.underln
theaDaJenc'e
.of
'l"'hMr~tmof ant.mpn!a1 wmare1m the complex[Ru(NH3) 6
Jo1
2rea,ots in hot
wflltl!r-whUe
thllil
~IIU!J.f!~ny CO!lrJPI.i'X l& ""'""""''""'""•~,. ....with mUd oxidising agerntl,
4itUtf00'4\Jl'l OOll!i:PlEtlC
have
13,
Mbenium as
metal,
ruthenium (UI)
ammtnes I!(Ru(NH$)
S
(N2)J
012oondittonl ,
hut
and (
co(NHa)6
1 ,
ru-c:neru\Jtm
(Ill)
..
l
au(NH3) 6
J
oxid1aecs'3 •
14
[
au(NH3) 6 whUe (Ru(NH3) 5 (N2)
J
:t+
o1v~:ls
pent~uamm!ne suoh ( ltu( )
5 Cl
1 ..
v"""''t.Uo.,i>Q to similar pteduota as obtaine<I the <lm&vogen oompl~.
1
32
(Found N, 34.6~, 012
a
15N71tu reqUires N, 34.2"). other $altsaueh
theZnOl~'""
aalt we prepared .bydissolving
tht• impute complex in Wi\ter, then $dd1Uon of the apptopt1ate anton precipitatesrequt.t.d
lalt.
These salts were QOt analysed asthey
wereknown
at
tht•to
be impure.. Put1f1oation[1\uCN ) (N2}] 01
2(bydta~Sine preparation) with HtC12 • ~e product obtained Rnt'i'Va
(0 .1 g;m) was treated with saturat~ aqueoua metrourio oblotlde solution
(1.1
mls). After the prea1p1tat.a mercury oompounds hadflltetf~Jd off, ammonium chloride and
21tno
chloride w•re added to preciplta'e the purec:Unitrogen
complex[Ru(NH
3
)s_lNa~no1
4
•
Thill~ repreotp1tated ftom water NH 4 01 and.
Ltli."-"'*"'. (Yield 0.02 gms). (Pound
01,
*4~- (NUa
only),
16.1%# Cl4Hu;NJN' (total) ,
-·--... Cl, 33
.6"1,
3.6~;N
ft:ota1),
.~~~N(NH* only)
ts ..
s~l.~l!PM•UPD
Qt
hexapmm&ne . rutl1en1gm.
'BI!
~o~lortde,,teuaon4Qtgatno
.. , . ' '(Dl . ....
'M~
''t
Methanol was"'""'Y.'""'
[Ru(NH3) 3
(N2)
J
-~.. --..
atvan aomTe., 4.4"J N, 18.9~; Cl~a
11
N6
RuZn .. ,... ... ~ .... N,18.8"h
MeJhP$1
{e)·
The -w•••.r ...U'll~n.ea with NH4Cl, and ZnC12• The product was preotp!tated
Preearljlttm.
gg
:Pep,tatmt.llinedintttgflcan
Qrth~a&urn.JWt.eir•~~l~~o~ino ~}q
•..~
Ru{NH3li,
~~
3
)]
.zncl
4~
34
First
rowcomplexes
11 "
ln contrast,
ruthetdum and a nurnbet
othet
•econdand
third
tow
tranettton metal comp"'xf!J.uJ react to
etve
e.
number
produots whichAm•r>llilSt the ""'""L·""·""'"" .. J.O.I>UU!.4.,.. • • mDOI'tll:tt!! and a yellow
aomrx>und whlch wtth
red aon!lPO'una has mote recently been
of 4umeJ
[fb.t01
5
(H~O )}
3~
or
'
the
IW'I!!Inall'attt\'1'1'l'tttl"l"\ttAn
aom:Ptex
£
Ru
) s (Ns)
1
byAllen
the
thatcompound has
~BubsE~tquentlyDI'C4!lUO'l8 af8 OD'9;,BlJl~Ml QCHltlil!UilU
""'""'-'"'""'"'Ill}~ )
J
I ( AWU'll&1f6# ~]
a .bisdinitrogen
36
. 2+
complex ion ( Ru(NH3) 4 (N2)2
J
and the new complex[ Os(NH
3)5 (CO)
J
c12 has been obtained. The latter compoundwas also prepared pure by a different route. Evidence for a species
Fe(dtc)2 (CO)n has also been obtained.
Results and Discussion (a) Compounds •
The work will now be discussed in tE;1rms of the compounds
prepared.
( Ru(NH
3)5 (CO)
j
Cl2•The tervalent complex (Ru(NH
3)5cl] 012 gives a deep red
solution when it is dissolved in hot (80°C) hydrazine hydrate, and a
vigorous gas evolution occurs • The ruthenium containing complexes
can be precipitated with either alcohol or ammonium chloride, after
cooling. The product may be either a deep red or yellow, the colour
depends on the time taken to cool the solution in contact with air.
The red product is obtained after rapid cooling, while the yellow
product is obtained if precipitation is delayed until the solution has
stood for about one hour. The infrared spectra of both these products
are identical and the same as the infrared spectrum of mixtures of
[Ru(NH3)5 (N2)] Cl2
I
[Ru(NH3)5(N2H4)j
c12 (see Chapter 2)
except for the addition of a sharp absorption at 193 0 em -l • The
component, giving rise to the 1930 cm- 1 absorption, could not be
separated from the
l
Ru(NH3)5(N2)
J
z+
known to be present ..A similar reaction occurs when divalent ruthenium (as
LRu(NH
3)6
J
Cl2 is heated in hydrazine hydrate, but relatively more(j)
u
c:
ro
+-'
+'
...
8
~
o .
4000
FIGURE 6
2000
Infrared Spectrum
1200 800
Wavenumber (em -1)
[Ru(NH
Table 6 Comparison of the Properties of (Rhli(NH
3) 5) Br2
Properties from Propertieu Reported
Present 'Work ,by Wilkinson
Infrared S;eeotrum (om -l)
~(NH) 3285,3190 (sh) 3276, 3186
~(Rh•H) 2013 2015
Os(NH) 1610 1609
.5a(NH) 1275 1278
Pr(NH3) 813 820
~(Rh-NH
3
) 480,448 483,4528(NH
3
·Rh~NH3
) and 265,157 (broad),Low
freq. Lattice Modes 87
I.R.
Other, probably S (Rh-H) 1185, 1170 (sh) 1171
Ultraviolet, v;t,sible Spectrum (nm)
205
233 (sh)
305 (w ,sh) 3 07 (reflectance)
350 (w ,sh)
Reactions Dilute acid - New Product, as CC, [RhH (NH3) 4
Halide ion
in neutral
solution.
~(Rh-H) at (H2
o)]
S04
2138cm-1•
Mbced rhodi urn (III)
amine halide
products together
with .rhnrHum m@btL
formed,~ (Rh-H)
at 2146cm-l
Rhodium metal
and mixed
ammine halide
of the
by repeated r<eact!ons, on one rutheniun1 sample, . u.stn9 fresh
•ample•
hYdrill~ine hydrate • · MalY~is of a •ample obtained from
{Ru,Nl3;~)
el
c1
2 showed that it contained 3 ·1" carbon, it appeared that the ~>roduct Wll$. <;=~mpQsed prtnoiPallY of [ lu(Nii3) 5(NalJ
Claand
(Ru(NH3) S (CO)J..c1
2 • The . .,rocluot oan be obtained in the .... of
organic
solve~tsand
carbonoontaininr;
compoundS~ t pt ·for the traces of carbon dioxide dissolv$d in hYdtazine hydtate.
. .
P~te [Ru(NH3) 5 (CO)]
c1
2 was then found to be readilyob~ained if additional carbon dioxide was dissolved in the bydrazine
. . . , . . ' ' . . . '
hydrate, u•ed in the reaction. Vndet.these conditions no deep solution is formed~ . This carbonyl product, the infraril:ld spEH,trum of . which ts shown tn Figure 6, has been prepared Ptt!Wiously by the more
lab~ous
67,GS
method of reaoting carbon monoxide withl
Ru(NH3) 5 (H2o)J
2+ ... The properties desotibedfor
this oomplei7 ,sa,851ate identical to those of the carbonyl ptepared duting thia present
,
l}~hH(NHals}
,Bt3 Rhodium trichloride and (R11(NH3) ] c12teact With :~lno in ammonia to gtve the hydrtdo-oomplex ion
l
Rh(NH3)sH ]
•
The pure CQm:Plti!'¢ can be Obtained by precipitationof this bromide salt have been measured (Table 6). Wilkinaon et l;ll. 66 have since reported the isolaUon of this compound and their re~Sults are similar
0 0
Vi
:z: """
.::>:::
,__
""'"
0 '
4000
I
~
0 0 0
~ 0 0 0 Cl> N
""'
• 2000
. . ( -1)
Vlavenumber em
FIGURE 7 Spectrum of [Ir(NH
3) 4 (CO) Cl]
ert shows formed diss
' ~
800 400
The complex RhC1
3 .nHzO reacts with hydrazine hydrate ot
hYdtazinium carbonate 9iVe a product with no absorptions near 2000 om""'lin its infrared
spectrum~~
The ptoduots appeared complex andthe infrared.
speotta sugc:;ested that o~ordtnated hydrazine was present-. Further inve&~Jtigation did not seem warranted,l,
Ir(NHI) 4 (CO), C1 ] 01~ ~ An ilu;oluble formof trcla
and the complex ir(NH3) 3 Cia:, react with hydrazine hydrate with zinc dust in oonoenttated ammonia give similar products all of whioh have an infrated absorption 2078 om ...
1..
The product obttd.ned from the zino in ammonia reaotlon was the only one th<at oould be purifiedfurther and was the only one to be
more
fully investigated \oAfter reaotion with z1no tn ammonia, irtdium product
in water and quickly removing an insoluble of mainly zinc
hydto~ide t The ptod.uot is obtained by freeze drying the filtrate~
lt
is necessary to wotk rapidly as the carbonyl decomposes significantly within five m!nutes tn watet, but it isThe product, wh1oh never obtained eompletely pure because of 1ti instability in and insolubility in othet solvents is postu;.. lated as a carbon monoxide derivative on following evldenoea (l) !hAlt complex an intense infrared absorption at 2078 om"" 1 ,
39
I.!Oid produced a gas of mass 28 together with a
•li'•,.,a
of hydroqen. The iridium product remaining in solution is ~={ ir(NH3) 4
c1
2
l
Cl whiob was identified by analysis and X•rav powder photography,.(3) Aoidio oerto "'"'""""9".""'"'~ and thermal
decomposition
both gtve aoat~ of maas 2$ whioh was identified a mixture of dinittooen
oarbon monoxide. Some
d!nittogen
aan be obtained from[ tr(w
3) 801] 012 ,
by similardecompositions. Analysts
of asample of the complex shQWed carbon to be present, and the
amount (2%) $U9frJests appronimately 60% purity, based on the formulation [ lr(NH
3) 4 (OO)Cl] 012•
(4) The compound is
isomorphous with [Ru(NH
3) 501
J
o12• This
suggestat a di positive cation with two chloride anions" (Faint :Unes corresponding to mt
4
Cl
present).
fS) The compound is diamagnetic.
'.l:m1o
6=
-113 .. Diamagnetic corrections for (lr(NH3)4 (CO).Ol ] 012 are
•.138 x Uf"'6 oga,. {tnalus!on of eontrJ.hutione the probable impurities,
evic:tanoe il oonsl~S,ent with the propcuual that the new proc:tuct (Jr(NH3) 4 (00)01] Cl2 h~,t$ bf:)EU\ fotmedtt
]
1919
l&(c)
a
ceo)
2051
40
at 2120 om·1 , (Figure 7)., Dilute acid catalyses this reaetion whereas strong ammonia solution or reverses the reaction.
a sample of product with both the 2120 om ... 1 and 2078 om'""1
absorptions in the infrared spectrum is dissolved in
o.sso
ammonia, thenfre•~e
dried, the resulting productM.a
only the 2018 om-1o.sso
(i) The addition of ammonia reverses the !n~tial reaction, this
would
requite that the initial reaction involves replacement of' '
some ot all the co-ordinated ammine groups with solvent water. (id The addition of ammonia ' the component of the mixture having a 2078 om·1 absorption in the infrared specttumJ the other component; with the 2120 om ... l absorption, still decomposes • but with no repleniehment through reaction of the "2078 om-
1
component~~
A having only the 2120 om""l absorption in its infrared' spectrum could not isolated, hence the two explanations (1, il) Dl"ti'WII'A could not be """""' ... """' to distinguish whtoh aorreat"
No evidenae obtained completely confirms the
dinitrogen but
on
..,o,~"'"" of·oomparisons betweenknown d1nttrogen it that iridium
e.rnrn1ne, dinttrogen
COltnUIO\U'lds unstable isolate"Monooarbonyl such as ruthenium (Ii)
supporting evidence to the proposal that the compound prepared during this work is an iridium (II!) carbonyl. Presumably the iridium must first be reduced f probably to Ir(l), before any stable dinitrogen
complexes are likely to be isolated"
The compound It(NH
3) 5, proposed by Watt88 to be the product of the reaction of [Ir(NH
3) 5
c1]
012 with sodium in liquid ammonia, was prepared to see if it was a dinitrogen complex but the infraredspectrum of this product showed no absorptions near 2000 cm""' 11
ruling out a mononuclear dinitrogen complex. No investigations to check for a dimer were made •
The complex {Ru(NH
3) 5
c1]
012 reacts with cold ( < 0°0) hydrazine hydrate to form a brown solution. No further visible change occurs in this solution after standing at < 0°0for one to two hours •
The ruthenium containing species in this solution can be
isolated as a brown solid by precipitation with sodium bromide. The infrared spectrum of the solid, after precipitation shows absorptions characteristic of hydrazine together with a absorption at 2105 om"'" 1 , attributable to the ~ (NN) stretch in monodinitrogen, ruthenium (It)
If the [Ru(NH3)
sCl]
012I
hydrazine hydrate solution is leftat "0°C for longer than one hour before the brown ruthenium containing
l1.4YENUHBLR
2200 2100 2000
After one hour
(no change a ftar OM week)
After one year
FIGURE 8
two ~ (NN)
44
identical infrared speotrum tba't obtained ptevtously exoept the· 2230 clm*1 and 2130 om""1 both relatively more :c.n'tl"lul
mre~'!t.er
proportion
new
pr()duot( Ru(NH3) 4 012
J
of~is;,.( Ru( )
4CN2)2
J
Bt2,of
rreeu11rulr>l'Y this reaonc>n
co ... ·ora.ln~lea hYdlral!!t1ne in this
obtained by
and
,
All
the1e PNPM''El'C11()Ill QOllll.lll\lragmuoh
the
43
instability of the complex in solution" Hence the existence of this
bisdinitrogen cornplex can only be proposed on the basis of its
infrared spectrum, preparative method and comparison with
ci,!_ ,... (Ru(en)
2 (N2) 2} Ph4B)2
44.. Possibly the most interesting
thing about the two ruthenium bisdinitr:ogen complexes
cis -(Ru(NH
3)4 (N2)2
J
Br2 and cis .. [ Ru{en)2(N2)2]CBPh4)2 44 is that they differ significantly in their reported stabilities in the solidstate.. Co-ordinated ammonia or ethylenediamine could be expected
to behave as very similar ligands , in complexes but the stability of
dinitrogen as a ligand has previously been noted93 to be very
sensitive to minor changes in the metal complex to which it is .bonded,
Impurities can also have larg-e effeots on the stability of dinitrogen
complexes and on the frequency of the
\>
(NN) infrared absorption(re£ .. 26 and ChapterS}.. An additional reason for the observed
stability of the bisdinitrogen complex cis -(Ru(NH
3)4(N2}2 JBr2
relative to .2!!,. -
L
Ru(en)2 (N2)2
J
(BPh4)2 could be that hydrazineimpurities in samples of the former complex, continually decompose,
to produce more of the bisdinitrogen complex. :Outing this present
work an attempt was made to prepare
.9!!.. ....
(Ru(en)2(N2)2 ] Br2
using the low temperature hydrazine hydrate reaction on
s!L.. ...
[ Ru(en)2 Cl
2} Cl but without success • This observation suggests
additional factors may be involved other than the hydrazine
impurities •
( Os (NH3}5 (CO)
J
ClzTraces of the carbonyl
l
Os(NH3)5 (CO) ] c12 can be prepared
;)(GO)
Sa(NH)
~(NH)
fr(Nma>
1898,1848 (sh) 1633
1316 ,13 OO(sh), 1298 808
~ (Os-CO), 8(0sCO) 604
Ultraviolet-visible ·spectrum No absorptions at energies below
21 Olllllm but has an absorption
above this energy (!.e. similar to the ruthenium complex).
Stabilit,X ....
'
.
Stable in hot concentrated hydrochloric acidf
N
2H4 .H20
Boil with
co
2Os(III) or Os(IV) salt as cC
N2H4 .H2
o
Boil with
co
212 oYJ.dation
in HCl
FIGURE 9 Cycle. for the Pl.\rification of
When a mbd:ure of hydtazine hydrate and hydrazinium carbonate is heated with either [ Os(NH
3) 5 C'l] 012 or (NH4) 2 (OsC10) ~ the product has a weak infrared absorption at 1887 em -l, corresponding to the frequency of the
J
(CO) ab(lotption in pure ( Os(NH3) 5
(co)]
012,(see below) together with a strong absorption at
.
2008 om ... 1 correspond.-ing to thedtnitrogen oomple'c [os(NH3) 5 (N2
)J
c12• The yield ofcarbonyl could not be increased by any changes of conditions (e.g. by using Zn dust in ammonia.
The carbonyl is stable to concentrated hydtochlorio acid and iodine O!}ddation, hence it may be partially purified by the reaction cycle shown in Figure 9. Mechanical losses prevent this method being used for small scale preparations of the pure carbonylt but on a larger scale it would probably be feasible to separate the carbonyl after only one or two cycles •
However, a much better method to prepare the pute osmium carbonyl complex is to use a method analogous to that reported67 108 previously to prepare ( Ru{NH
3)5 (CO)
J
012• The complexlOs
(NH3) 5 01
J
012 was reduced with Zinc amalgam, then carbon monoxide was bubbled through for sixty hours .. The properties of this osmium carbonyl are shown in Table 6 and Figure l 0.as well as the absorption at 1885 om- 1 due to the
~(CO)
absorptionof the carbonyl component •. Reactions carried out in air with relatively
low
partial pressures of carbon monoxide (about 40 mm) c;J.f:ve products which have little detectable carbonyl but have strongv
(N:til
absorptions in their infrared spectra. Even at higher carbon monoXide ooncentrat ... ions, a little dinitrogen product was detected. This qualitative. evidence suggests that the reduced species; obtained fromlOs (NH
3) 5 Ol ] 012 ~ reacts with dinitrogen at a comparable rate to
its
reaction with carbon monoxide, especially as the latter gas is approximately 1.5 times as soluble in water (at 0°0 3.5 om3• per 100 mls compared with 2.33 cm3 per 100 mls for dinitrogen)12• Previously ithas
been ob~Served for ruthenium (II), that the rateconstants for addition of
co
97 ot N2 48 to tRu(NH3) 5 (H2o)
J
2+'
aresimilar.
In this case it appears 48 that the rate detetmining step is the
initial dissociation of the [M(NH
3) 5 (H
2
b}J
2+ into a five co-ordinatespecies which reacts rapidly with dinitrogen 1 carbon monoXide or
other substrates •
Fe(dtc)
2 (CO) n - The complex trisdiethyldithiocarbamate
iron (III) , Fe(dto) 3 , reacts with hydrazine hydrate solution to give
a product with an infrared absorption at 2070 cm- 1 • Originally it was
hoped that this new complex would be a dinitrogen complex, which
~··· (]) () c fd 4-' +-' -rl E U) c fd I-. £:--; ~ o ,
.woo
FIGURE 11
/-~~/
,..,.., .. .;~·~
..
~
tl t • • ., "' •' Ita.. 111o ~ " f l • 1
2000
r~ . .~·
:. / '!. .. ,.,... ! '•
..
~ ·"\ .... _ : .... : ~: \ :_./ \ ; ..,
:· '•' 1200 (cm- 1)Infrared Spectra of samples of Fe (dtc)
2 CO(n)
Product evaporation reaction mixture.
~~~~
·--- Product after reaction of above with HgC1
2 s c
,
-800
.,_
~.
proposed to exist in nitrogenase. The complex Fe(dto)3 readily loses at least one dithiooarbamato ligand to give a variety of products, such as the halides Fe(dto)
2X (X-Cl, Br, 1,)90 and the nitrosyl Fe(dto)2 (No)91 , while the carbonyl cis Fe(dtc)
2 (C:o)2 92
has been prepared although not by oarbon monoxide displacement of dithiocarbamate,.
Solutions of Fe(dtc)
3 or Fe(dtc)20l both react similarly in ethanol with hydtazine hydrate to give a product infrared absorption at 2010 cm- 1 • In both products Fe(dtc)
3 and hydrazine hydrate can be detected (irifra ted spectra) and use of t'e(dto)
2cl appeared to offer no advantage. The Fe (dtc)
3 can be removed by dissolving the product in water while the excess hydrazine hydrate is removed by oxidation with mercuric chloride solution. The yield of product, the infrared spectrum of which is shown in Figure ll,
is extremely low.. The two products in this figure are considered to contain an identical complex with an infrared absorption at
2010 cm- 1 .. The differences between the spectra are thought to be
due to variations in the impurities present. (Product(l) is known to have hydrazine hydrate present.
The evidence below suggests that the product with the 2070 cm-1 infrared absorption contains co-ordinated carbon monoxide rather
than the hoped for dinitrogen •
TABLE 9
Property
Infrared
)ceo)
Stability Heat
Colour
Comparison of Properties of cis ... f'e(dtc)
2 (Co)2 92
and Hydrazine PrepE!.red Fe(dto) ·'CO) .. Pro<\uct
New Product
207Scm""' 1
Both compounds have numerous other absorptions due to the dithiocarbamate ligands.
CO lost in cases after short periods of
warming.
gas composed of a mixture of carbon monoxide and dinttrogen. The infrared spectra of the sample decomposed above showed some hydrazine present, hence the evolved dinittogen. The carbon
monoxide suggests the presence of a carbonyl complex in the mixture.
(b) The compound cis•Fe(dto)2 (Oo)2 ts k.nown92 and this
compound has properties which are similar but not identical to those
of the product above. A comparison of the properties of these two compounds is shown in Table 9 •
The most probable composition for this new compound appears
t6
be one of the following two:(i) trans Fe(dtc)
2 (00)2
absorption.
(ii) Fe(dtc)
2 (CO)
Fe(dtc)
2 (NO).
- one ~ (00) infrared
- analogous to the ni trosy 1
The lack of thermal stability of this new compound, together with the reported observation92 that cis - (Fe(dto)2 (00)2 can only be prepared pUre at temperature lesS than 0°0 1 makes
it
UnlikelyHydrazine Hydrate
hydrazine containing products
J
and
1
Tbe
aotu~l .DI'Gidu~ot• 0D1:mz1eaare summarited in
( Ru(NH3) 4 (N2) g
J
l
4J
sl
IS (CC))]
not been
were • ( [RhH(bUi
3) 5
J
present worlt that
l
"'"""\"'U•it<'Jj/ ~V\ll!IU&V.Ul
dinitroten
[ Os(J{rH
3
)
4 (N2) rea.u.Qtaon of Os (ll!)and .. ~~\itV,Ul~ n,,.. ... H,.,.. {fl •l'!nlllnOl!l:l.f!llil
the 'I"W'o~:~tntl>,~•tt
(l) Reduotion
from. the
.;: .. ,:a%"""''".,."'~, probably the oat~nOJnv 1 omn.p,~;exe's from
pre:&Enlce Of using
49
(it) Co-ordination of the dissolved carb~n dioxide to suitable
metal ions ; then reduction of this group to give co-ordinated carbon monoxide.
The preparation of pure (Ru(NH3) 5 (00)] c12 using hydrazine hydrate/ carbon dioxide , but does not prove, that free carbon monoxide is not involved.. Ruthenium metal complexes; cause vigorous catalytic decomposition of hot hydrazine hydrate
solution to dinttrogen and dihydrogen
~
The proposed42 ,g 7 mechanism of carbon monoXide and dinitrogen addition to ruthenium (II) wouldSU~iHJest that this dinitrogen $hould compete with any free carbon
monoxide for oo ... ordina.Uon to the ruthenium iont but no dinittogen product is obtained. Also the iron complex, Fe(dtc)2 (CO) n
cannot be prepared using carbon monoxi.de gas and t'e(dto}3 solution. ln this reaction; an 1.ron carbonyl complex (probably Fe(C0)4, from infrared spectra 41), is formed and :l.t certainly is not the same product as obtained using hydra.zine hydrate. These observations make it unlikely that any free carbon monoxide is involved in the reactions.
The preparation of the dinitrogen complexes from the reaction
of ruthenium osmium complexes with reducing metals in ammonia is interesting as this reaction involves the o:ltidat1on of ammonia nitrogen in the of strong reducing agents, Chatt and
50
the reduction of RuCl3 with zinc in concentrated ammoni.a 1 but the
reaction with osmium, (as. Los(NH3) 5 01
J
C12), under ·similar
conditions, to form the bisdinittogen complex cis [ ps (NH
3) 4 (N2)2] 012 does not seem to able to be exp~atined . by any hydride ·intermediate.
in neutral ~nd slightly acidic oondttions, with ~oth the +3 and +2 oxidation states
(Osmium (ttl) as [Os(Nii3)5Cl] 2
+
is qult.e stable, even to strong acid while osmium (II) as [ Os(:NH3) 5 X) n+ (X probably t:l H20) is stable enough for it to take part in a relatively slow reaction w!th carbon monoxide or dinitrogen). It seems unreasonable to suppose that the LOs(NH3) 5
1
group will be less stable in concentrated ammonia, but it is still pos$ible undet these conditions to prepare a bisdinitrogen complex in a reaction of less than ten minutes~ It seems that the oo .... otttinated ammonia, nitrogen atoms must supply at least one of the nitrogen atoms in the resulting oo•ordinated dinitrogen groups.The initial step of the reaction
must
involve some change to the oo ... ordinated ammonia so as to enableit
to dimerise with anotherammonia to form a NN bonded group which can be degraded into co ... ordinated dlnltrogen~ /A possible reaction route could involve inter-mediates, similar to the nitrene { Ru(NH
3) 5 (NH)
J
a+,
40 said toform during the acid degradation of ( Ru(NH
3) 5 (N3)
1
to give (Ru(NH3) 5 (N2))a+
and [ Ru2 (NH3) lO (N2)