metal-organic papers
Acta Cryst.(2005). E61, m1221–m1222 doi:10.1107/S1600536805016156 Fuet al. [FeCl
2(C12H8N2)2]
m1221
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
cis
-Dichlorobis(1,10-phenanthroline)iron(II)
Xu-Cheng Fu,a,bMing-Tian Lia and Cheng-Gang Wanga*
a
Department of Chemistry, Central China Normal University, Wuhan, Hubei 430079, People’s Republic of China, andbChemistry and
Biology Department, West Anhui University, Liuan, Anhui 237000, People’s Republic of China
Correspondence e-mail: wangcg23@yahoo.com.cn
Key indicators
Single-crystal X-ray study
T= 292 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.067
wRfactor = 0.149
Data-to-parameter ratio = 17.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
In the title compound,cis-[FeCl2(C12H8N2)2], the Fe atom has
a distorted octahedral coordination composed of four N atoms from two phenanthroline groups and two Cl atoms. The crystal packing is stabilized by weak – stacking of neighboring phenanthroline groups.
Comment
The title compound, cis-[Fe(phen)2Cl2], (I) (phen is
1,10-phenanthroline), was previously reported by Baker & Bobo-nich (1963), and its magnetic behavior has been studied (Ko¨niget al., 1967). However, its crystal structure has not yet been reported.cis-[Fe(phen)2Cl2], was unexpectedly obtained
while attempting to prepare [Fe(terephth)(phen)(H2O)]
(terephth is terephthalate). The FeII atom has a distorted
octahedral coordination composed of a pair of phen groups and two Cl atoms. The Cl1—Fe1—Cl2 angle is 100.09 (4). The
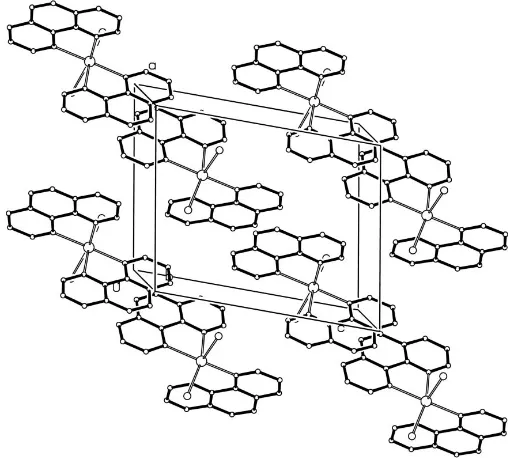
crystal packing of (I) is stabilized by extended–stacking of the conjugated phen ring systems, characterized by interplanar distances in the range 3.404 (6)–3.608 (6) A˚ . (see Fig.2).
Experimental
The title compound was prepared from a mixture of FeCl3, tereph-thalic acid, 1,10-phenanthroline (monohydrated), NaOH and EtOH with a molar ratio of 1:2:1:2:206. The mixture was stirred for 2 h, sealed in a 15 ml Teflon-lined stainless steel bomb, kept at 413 K for 96 h, and then cooled slowly to ambient temperature. The resulting black–red crystals of (I) were filtered and washed with acetone.
Crystal data
[FeCl2(C12H8N2)2] Mr= 487.16 Monoclinic,P21=n a= 10.1699 (18) A˚
b= 16.883 (3) A˚
c= 12.490 (2) A˚
= 100.126 (3)
V= 2111.1 (6) A˚3 Z= 4
Dx= 1.533 Mg m
3 MoKradiation Cell parameters from 3005
reflections
= 2.7–25.1 = 0.99 mm1 T= 292 (2) K Block, black–red 0.400.200.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 2000)
Tmin= 0.693,Tmax= 0.908 13352 measured reflections
4760 independent reflections 3251 reflections withI> 2(I)
Rint= 0.060
max= 27.5
h=10!13
k=21!21
l=15!14
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.067
wR(F2) = 0.149 S= 1.08 4760 reflections 280 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.059P)2 + 0.7354P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 0.66 e A˚
3
min=0.69 e A˚
3
Table 1
Selected geometric parameters (A˚ ,).
Fe1—N4 2.179 (3)
Fe1—N1 2.179 (3)
Fe1—N3 2.246 (3)
Fe1—N2 2.276 (3)
Fe1—Cl1 2.3604 (12)
Fe1—Cl2 2.4696 (11)
N4—Fe1—N1 156.32 (12)
N4—Fe1—N3 74.47 (11)
N1—Fe1—N3 87.65 (11)
N4—Fe1—N2 87.49 (11)
N1—Fe1—N2 73.94 (11)
N3—Fe1—N2 79.90 (11)
N4—Fe1—Cl1 100.19 (9)
N1—Fe1—Cl1 96.32 (9)
N3—Fe1—Cl1 93.85 (8)
N2—Fe1—Cl1 168.52 (8)
N4—Fe1—Cl2 93.00 (8)
N1—Fe1—Cl2 100.72 (9)
N3—Fe1—Cl2 162.77 (8)
N2—Fe1—Cl2 87.91 (8)
Cl1—Fe1—Cl2 100.09 (4)
H atoms were placed in calculated positions and refined using a riding model, withUiso(H) = 1.2Ueq(C) and C–H distances of 0.93 A˚ . Data collection:SMART(Bruker, 2000); cell refinement:SAINT (Bruker, 2000); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: SHELXTL (Bruker, 1997); software used to prepare material for publication:SHELXTL.
The authors thank the Education Office of Anhui Province, People’s Republic of China, for research grant No. 200161.
References
Baker, W. A. & Bobonich, H. M. (1963).Inorg. Chem.2, 1071–1073. Bruker (1997). SHELXTL. Version 5.10. Bruker AXS Inc., Madison,
Wisconsin, USA.
Bruker (2000).SMART,SAINTandSADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
Ko¨nig, E., Charkravarty, A. S. & Madeja, K. (1967).Theor. Chim. Acta,9, 171– 173.
[image:2.610.45.293.72.241.2]Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Figure 2
A view of the–stacking. H atoms have been omitted. Figure 1
[image:2.610.42.297.305.534.2]supporting information
sup-1 Acta Cryst. (2005). E61, m1221–m1222
supporting information
Acta Cryst. (2005). E61, m1221–m1222 [https://doi.org/10.1107/S1600536805016156]
cis
-Dichlorobis(1,10-phenanthroline)iron(II)
Xu-Cheng Fu, Ming-Tian Li and Cheng-Gang Wang
Dichlorobis(1,10-phenanthroline)iron(II)
Crystal data [FeCl2(C12H8N2)2] Mr = 487.16
Monoclinic, P21/n
Hall symbol: -P2yn a = 10.1699 (18) Å b = 16.883 (3) Å c = 12.490 (2) Å β = 100.126 (3)° V = 2111.1 (6) Å3 Z = 4
F(000) = 992 Dx = 1.533 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 3005 reflections θ = 2.7–25.1°
µ = 0.99 mm−1 T = 292 K Block, black–red 0.40 × 0.20 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 2000) Tmin = 0.693, Tmax = 0.908
13352 measured reflections 4760 independent reflections 3251 reflections with I > 2σ(I) Rint = 0.060
θmax = 27.5°, θmin = 2.1° h = −10→13
k = −21→21 l = −15→14
Refinement Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.067 wR(F2) = 0.149 S = 1.08 4760 reflections 280 parameters 3 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.059P)2 + 0.7354P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.66 e Å−3
Δρmin = −0.69 e Å−3
Special details
Experimental. Dichlorobis(1,10-phenanthroline)iron(II)
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Fe1 0.09953 (5) 0.23100 (3) 0.77849 (4) 0.02622 (17)
Cl1 0.25988 (12) 0.13361 (6) 0.84460 (9) 0.0499 (3)
Cl2 −0.09344 (10) 0.14563 (6) 0.70783 (8) 0.0363 (3)
N1 0.0526 (3) 0.26409 (18) 0.9362 (2) 0.0314 (7)
N2 −0.0404 (3) 0.33691 (18) 0.7473 (2) 0.0312 (7)
N3 0.2491 (3) 0.33013 (17) 0.7987 (2) 0.0290 (7)
N4 0.1475 (3) 0.25080 (17) 0.6173 (2) 0.0277 (7)
C1 0.0983 (4) 0.2276 (2) 1.0291 (3) 0.0379 (10)
H1 0.1510 0.1827 1.0278 0.045*
C2 0.0707 (5) 0.2538 (3) 1.1291 (3) 0.0526 (12)
H2 0.1052 0.2267 1.1927 0.063*
C3 −0.0062 (5) 0.3187 (3) 1.1329 (3) 0.0520 (12)
H3 −0.0266 0.3358 1.1989 0.062*
C4 −0.0555 (4) 0.3602 (3) 1.0365 (3) 0.0424 (11)
C5 −0.1361 (5) 0.4293 (3) 1.0320 (4) 0.0570 (13)
H5 −0.1587 0.4491 1.0958 0.068*
C6 −0.1801 (5) 0.4666 (3) 0.9377 (4) 0.0568 (13)
H6 −0.2311 0.5123 0.9374 0.068*
C7 −0.1499 (4) 0.4370 (2) 0.8377 (4) 0.0455 (11)
C8 −0.1958 (5) 0.4737 (3) 0.7367 (4) 0.0568 (13)
H8 −0.2465 0.5198 0.7325 0.068*
C9 −0.1639 (5) 0.4399 (3) 0.6449 (4) 0.0595 (14)
H9 −0.1937 0.4627 0.5772 0.071*
C10 −0.0878 (4) 0.3720 (3) 0.6531 (3) 0.0424 (10)
H10 −0.0685 0.3495 0.5896 0.051*
C11 −0.0722 (4) 0.3692 (2) 0.8381 (3) 0.0328 (9)
C12 −0.0228 (4) 0.3297 (2) 0.9400 (3) 0.0327 (9)
C13 0.2995 (4) 0.3693 (2) 0.8890 (3) 0.0348 (9)
H13 0.2786 0.3519 0.9546 0.042*
C14 0.3817 (4) 0.4349 (2) 0.8901 (4) 0.0435 (11)
H14 0.4145 0.4605 0.9552 0.052*
C15 0.4141 (4) 0.4617 (2) 0.7949 (4) 0.0430 (11)
H15 0.4676 0.5063 0.7942 0.052*
C16 0.3657 (4) 0.4209 (2) 0.6979 (3) 0.0337 (9)
C17 0.3974 (4) 0.4423 (2) 0.5947 (4) 0.0441 (11)
H17 0.4512 0.4863 0.5900 0.053*
C18 0.3512 (4) 0.4005 (2) 0.5044 (3) 0.0434 (11)
H18 0.3760 0.4148 0.4388 0.052*
C19 0.2646 (4) 0.3342 (2) 0.5080 (3) 0.0323 (9)
supporting information
sup-3 Acta Cryst. (2005). E61, m1221–m1222
H20 0.2311 0.3022 0.3480 0.052*
C21 0.1303 (4) 0.2275 (3) 0.4273 (3) 0.0454 (11)
H21 0.0950 0.1969 0.3672 0.054*
C22 0.0997 (4) 0.2095 (2) 0.5296 (3) 0.0359 (10)
H22 0.0437 0.1668 0.5358 0.043*
C23 0.2298 (3) 0.3123 (2) 0.6074 (3) 0.0268 (8)
C24 0.2834 (4) 0.3556 (2) 0.7049 (3) 0.0282 (8)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Fe1 0.0297 (3) 0.0306 (3) 0.0190 (3) −0.0019 (2) 0.0058 (2) 0.0001 (2)
Cl1 0.0520 (7) 0.0503 (6) 0.0463 (7) 0.0141 (5) 0.0055 (5) 0.0075 (5)
Cl2 0.0352 (5) 0.0378 (5) 0.0381 (6) −0.0060 (4) 0.0126 (4) −0.0042 (4)
N1 0.0324 (18) 0.0357 (17) 0.0255 (16) −0.0026 (15) 0.0037 (14) 0.0003 (14)
N2 0.0266 (17) 0.0372 (17) 0.0285 (17) −0.0002 (14) 0.0011 (14) −0.0050 (14)
N3 0.0319 (17) 0.0327 (17) 0.0211 (15) −0.0016 (14) 0.0015 (14) −0.0035 (13)
N4 0.0286 (17) 0.0344 (16) 0.0206 (15) −0.0054 (14) 0.0055 (13) −0.0017 (12)
C1 0.037 (2) 0.053 (3) 0.0221 (19) 0.000 (2) 0.0020 (17) 0.0041 (18)
C2 0.060 (3) 0.076 (3) 0.022 (2) −0.009 (3) 0.008 (2) −0.001 (2)
C3 0.048 (3) 0.081 (4) 0.029 (2) −0.013 (3) 0.013 (2) −0.020 (2)
C4 0.042 (3) 0.054 (3) 0.034 (2) −0.004 (2) 0.017 (2) −0.013 (2)
C5 0.056 (3) 0.071 (3) 0.048 (3) 0.005 (3) 0.020 (2) −0.027 (3)
C6 0.056 (3) 0.052 (3) 0.065 (3) 0.014 (2) 0.017 (3) −0.014 (3)
C7 0.043 (3) 0.045 (2) 0.048 (3) 0.008 (2) 0.010 (2) −0.004 (2)
C8 0.060 (3) 0.048 (3) 0.060 (3) 0.024 (2) 0.006 (3) 0.005 (2)
C9 0.067 (3) 0.062 (3) 0.045 (3) 0.023 (3) −0.002 (3) 0.007 (2)
C10 0.045 (3) 0.052 (3) 0.028 (2) 0.014 (2) 0.0017 (19) 0.0002 (19)
C11 0.032 (2) 0.034 (2) 0.033 (2) 0.0004 (18) 0.0071 (17) −0.0057 (16)
C12 0.034 (2) 0.039 (2) 0.0254 (19) −0.0067 (18) 0.0067 (17) −0.0074 (17)
C13 0.030 (2) 0.044 (2) 0.029 (2) −0.0003 (19) −0.0002 (17) −0.0056 (17)
C14 0.042 (2) 0.044 (2) 0.043 (3) −0.005 (2) 0.003 (2) −0.018 (2)
C15 0.038 (2) 0.032 (2) 0.059 (3) −0.0125 (19) 0.007 (2) −0.005 (2)
C16 0.034 (2) 0.0258 (19) 0.042 (2) −0.0011 (17) 0.0069 (18) 0.0046 (16)
C17 0.045 (3) 0.041 (2) 0.048 (3) −0.010 (2) 0.013 (2) 0.014 (2)
C18 0.042 (2) 0.051 (3) 0.039 (2) −0.004 (2) 0.011 (2) 0.017 (2)
C19 0.031 (2) 0.040 (2) 0.028 (2) 0.0058 (18) 0.0105 (17) 0.0109 (17)
C20 0.043 (3) 0.066 (3) 0.023 (2) −0.002 (2) 0.0101 (19) 0.0062 (19)
C21 0.048 (3) 0.068 (3) 0.021 (2) −0.014 (2) 0.0082 (19) −0.009 (2)
C22 0.037 (2) 0.048 (2) 0.025 (2) −0.0145 (19) 0.0108 (18) −0.0082 (17)
C23 0.0226 (19) 0.034 (2) 0.0235 (18) 0.0045 (16) 0.0026 (15) 0.0051 (15)
C24 0.0255 (19) 0.0295 (19) 0.029 (2) 0.0012 (16) 0.0033 (16) 0.0013 (15)
Geometric parameters (Å, º)
Fe1—N4 2.179 (3) C7—C8 1.410 (6)
Fe1—N1 2.179 (3) C8—C9 1.370 (7)
Fe1—N2 2.276 (3) C9—C10 1.378 (6)
Fe1—Cl1 2.3604 (12) C9—H9 0.9300
Fe1—Cl2 2.4696 (11) C10—H10 0.9300
N1—C1 1.324 (5) C11—C12 1.446 (5)
N1—C12 1.352 (5) C13—C14 1.386 (5)
N2—C10 1.330 (5) C13—H13 0.9300
N2—C11 1.348 (5) C14—C15 1.366 (6)
N3—C13 1.330 (4) C14—H14 0.9300
N3—C24 1.350 (5) C15—C16 1.405 (6)
N4—C22 1.318 (4) C15—H15 0.9300
N4—C23 1.352 (4) C16—C24 1.397 (5)
C1—C2 1.400 (6) C16—C17 1.429 (5)
C1—H1 0.9300 C17—C18 1.344 (6)
C2—C3 1.352 (7) C17—H17 0.9300
C2—H2 0.9300 C18—C19 1.430 (5)
C3—C4 1.407 (6) C18—H18 0.9300
C3—H3 0.9300 C19—C23 1.399 (5)
C4—C12 1.404 (5) C19—C20 1.404 (6)
C4—C5 1.421 (6) C20—C21 1.354 (6)
C5—C6 1.341 (7) C20—H20 0.9300
C5—H5 0.9300 C21—C22 1.400 (5)
C6—C7 1.429 (6) C21—H21 0.9300
C6—H6 0.9300 C22—H22 0.9300
C7—C11 1.389 (5) C23—C24 1.443 (5)
N4—Fe1—N1 156.32 (12) C7—C8—H8 120.8
N4—Fe1—N3 74.47 (11) C8—C9—C10 119.8 (4)
N1—Fe1—N3 87.65 (11) C8—C9—H9 120.1
N4—Fe1—N2 87.49 (11) C10—C9—H9 120.1
N1—Fe1—N2 73.94 (11) N2—C10—C9 123.2 (4)
N3—Fe1—N2 79.90 (11) N2—C10—H10 118.4
N4—Fe1—Cl1 100.19 (9) C9—C10—H10 118.4
N1—Fe1—Cl1 96.32 (9) N2—C11—C7 123.4 (4)
N3—Fe1—Cl1 93.85 (8) N2—C11—C12 117.1 (3)
N2—Fe1—Cl1 168.52 (8) C7—C11—C12 119.6 (4)
N4—Fe1—Cl2 93.00 (8) N1—C12—C4 123.5 (4)
N1—Fe1—Cl2 100.72 (9) N1—C12—C11 117.3 (3)
N3—Fe1—Cl2 162.77 (8) C4—C12—C11 119.2 (4)
N2—Fe1—Cl2 87.91 (8) N3—C13—C14 123.2 (4)
Cl1—Fe1—Cl2 100.09 (4) N3—C13—H13 118.4
C1—N1—C12 117.7 (3) C14—C13—H13 118.4
C1—N1—Fe1 125.0 (3) C15—C14—C13 119.6 (4)
C12—N1—Fe1 117.2 (2) C15—C14—H14 120.2
C10—N2—C11 117.5 (3) C13—C14—H14 120.2
C10—N2—Fe1 128.3 (3) C14—C15—C16 119.1 (4)
C11—N2—Fe1 114.2 (2) C14—C15—H15 120.5
C13—N3—C24 117.2 (3) C16—C15—H15 120.5
supporting information
sup-5 Acta Cryst. (2005). E61, m1221–m1222
C24—N3—Fe1 114.4 (2) C24—C16—C17 119.3 (4)
C22—N4—C23 118.2 (3) C15—C16—C17 123.5 (4)
C22—N4—Fe1 125.3 (3) C18—C17—C16 121.5 (4)
C23—N4—Fe1 116.4 (2) C18—C17—H17 119.2
N1—C1—C2 122.7 (4) C16—C17—H17 119.2
N1—C1—H1 118.7 C17—C18—C19 120.7 (4)
C2—C1—H1 118.7 C17—C18—H18 119.6
C3—C2—C1 119.7 (4) C19—C18—H18 119.6
C3—C2—H2 120.1 C23—C19—C20 117.7 (4)
C1—C2—H2 120.1 C23—C19—C18 119.3 (4)
C2—C3—C4 119.7 (4) C20—C19—C18 123.1 (4)
C2—C3—H3 120.2 C21—C20—C19 118.8 (4)
C4—C3—H3 120.2 C21—C20—H20 120.6
C12—C4—C3 116.7 (4) C19—C20—H20 120.6
C12—C4—C5 119.3 (4) C20—C21—C22 120.1 (4)
C3—C4—C5 124.0 (4) C20—C21—H21 119.9
C6—C5—C4 121.4 (4) C22—C21—H21 119.9
C6—C5—H5 119.3 N4—C22—C21 122.3 (4)
C4—C5—H5 119.3 N4—C22—H22 118.8
C5—C6—C7 120.9 (4) C21—C22—H22 118.8
C5—C6—H6 119.5 N4—C23—C19 122.8 (3)
C7—C6—H6 119.5 N4—C23—C24 117.3 (3)
C11—C7—C8 117.7 (4) C19—C23—C24 119.9 (3)
C11—C7—C6 119.6 (4) N3—C24—C16 123.7 (3)
C8—C7—C6 122.7 (4) N3—C24—C23 117.0 (3)
C9—C8—C7 118.4 (4) C16—C24—C23 119.3 (3)
C9—C8—H8 120.8
N4—Fe1—N1—C1 −140.7 (3) C10—N2—C11—C7 −0.7 (6)
N3—Fe1—N1—C1 −100.2 (3) Fe1—N2—C11—C7 176.1 (3)
N2—Fe1—N1—C1 179.6 (3) C10—N2—C11—C12 178.7 (4)
Cl1—Fe1—N1—C1 −6.6 (3) Fe1—N2—C11—C12 −4.5 (4)
Cl2—Fe1—N1—C1 95.0 (3) C8—C7—C11—N2 −0.7 (7)
N4—Fe1—N1—C12 35.3 (4) C6—C7—C11—N2 179.2 (4)
N3—Fe1—N1—C12 75.8 (3) C8—C7—C11—C12 179.9 (4)
N2—Fe1—N1—C12 −4.4 (3) C6—C7—C11—C12 −0.2 (6)
Cl1—Fe1—N1—C12 169.4 (3) C1—N1—C12—C4 −0.6 (6)
Cl2—Fe1—N1—C12 −89.1 (3) Fe1—N1—C12—C4 −176.8 (3)
N4—Fe1—N2—C10 16.0 (3) C1—N1—C12—C11 179.9 (3)
N1—Fe1—N2—C10 −178.9 (4) Fe1—N1—C12—C11 3.7 (4)
N3—Fe1—N2—C10 90.7 (3) C3—C4—C12—N1 −0.4 (6)
Cl1—Fe1—N2—C10 148.4 (4) C5—C4—C12—N1 −179.6 (4)
Cl2—Fe1—N2—C10 −77.1 (3) C3—C4—C12—C11 179.1 (4)
N4—Fe1—N2—C11 −160.4 (3) C5—C4—C12—C11 −0.1 (6)
N1—Fe1—N2—C11 4.7 (3) N2—C11—C12—N1 0.7 (5)
N3—Fe1—N2—C11 −85.8 (3) C7—C11—C12—N1 −179.8 (4)
Cl1—Fe1—N2—C11 −28.1 (6) N2—C11—C12—C4 −178.8 (4)
N4—Fe1—N3—C13 −179.9 (3) C24—N3—C13—C14 1.4 (5)
N1—Fe1—N3—C13 15.8 (3) Fe1—N3—C13—C14 −173.4 (3)
N2—Fe1—N3—C13 89.9 (3) N3—C13—C14—C15 −0.1 (6)
Cl1—Fe1—N3—C13 −80.4 (3) C13—C14—C15—C16 −1.4 (6)
Cl2—Fe1—N3—C13 135.5 (3) C14—C15—C16—C24 1.5 (6)
N4—Fe1—N3—C24 5.1 (2) C14—C15—C16—C17 −177.8 (4)
N1—Fe1—N3—C24 −159.2 (3) C24—C16—C17—C18 −0.9 (6)
N2—Fe1—N3—C24 −85.1 (3) C15—C16—C17—C18 178.4 (4)
Cl1—Fe1—N3—C24 104.6 (2) C16—C17—C18—C19 2.2 (6)
Cl2—Fe1—N3—C24 −39.4 (5) C17—C18—C19—C23 −1.1 (6)
N1—Fe1—N4—C22 −140.0 (3) C17—C18—C19—C20 178.6 (4)
N3—Fe1—N4—C22 177.7 (3) C23—C19—C20—C21 −0.5 (6)
N2—Fe1—N4—C22 −102.1 (3) C18—C19—C20—C21 179.8 (4)
Cl1—Fe1—N4—C22 86.5 (3) C19—C20—C21—C22 0.8 (7)
Cl2—Fe1—N4—C22 −14.3 (3) C23—N4—C22—C21 −0.6 (6)
N1—Fe1—N4—C23 37.8 (4) Fe1—N4—C22—C21 177.2 (3)
N3—Fe1—N4—C23 −4.5 (2) C20—C21—C22—N4 −0.2 (7)
N2—Fe1—N4—C23 75.7 (3) C22—N4—C23—C19 0.8 (5)
Cl1—Fe1—N4—C23 −95.7 (2) Fe1—N4—C23—C19 −177.1 (3)
Cl2—Fe1—N4—C23 163.5 (2) C22—N4—C23—C24 −178.6 (3)
C12—N1—C1—C2 0.6 (6) Fe1—N4—C23—C24 3.4 (4)
Fe1—N1—C1—C2 176.5 (3) C20—C19—C23—N4 −0.3 (6)
N1—C1—C2—C3 0.4 (7) C18—C19—C23—N4 179.4 (3)
C1—C2—C3—C4 −1.4 (7) C20—C19—C23—C24 179.1 (3)
C2—C3—C4—C12 1.3 (6) C18—C19—C23—C24 −1.2 (5)
C2—C3—C4—C5 −179.4 (4) C13—N3—C24—C16 −1.3 (5)
C12—C4—C5—C6 −0.9 (7) Fe1—N3—C24—C16 174.3 (3)
C3—C4—C5—C6 179.9 (5) C13—N3—C24—C23 179.3 (3)
C4—C5—C6—C7 1.4 (8) Fe1—N3—C24—C23 −5.2 (4)
C5—C6—C7—C11 −0.9 (7) C15—C16—C24—N3 −0.2 (6)
C5—C6—C7—C8 179.1 (5) C17—C16—C24—N3 179.2 (4)
C11—C7—C8—C9 1.3 (7) C15—C16—C24—C23 179.3 (3)
C6—C7—C8—C9 −178.6 (5) C17—C16—C24—C23 −1.4 (5)
C7—C8—C9—C10 −0.6 (8) N4—C23—C24—N3 1.3 (5)
C11—N2—C10—C9 1.6 (6) C19—C23—C24—N3 −178.1 (3)
Fe1—N2—C10—C9 −174.7 (3) N4—C23—C24—C16 −178.2 (3)