metal-organic papers
Acta Cryst.(2005). E61, m1075–m1076 doi:10.1107/S1600536805013954 Zou and Xu [Zn(C
10H14N4)3](NO3)2
m1075
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
Tris(4,4
000,5,5
000-tetramethyl-2,2
000-biimidazole)-zinc(II) dinitrate
Ru-Qiang Zoua,band Qiang Xua,b*
a
National Institute of Advanced Industrial Science and Technology (AIST), Ikeda, Osaka 563-8577, Japan, andbGraduate School of
Science and Technology, Kobe University, Nada Ku, Kobe, Hyogo 657-8501, Japan
Correspondence e-mail: q.xu@aist.go.jp
Key indicators Single-crystal X-ray study
T= 293 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.043
wRfactor = 0.111
Data-to-parameter ratio = 16.1
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
A new six-coordinate monomeric ZnII complex, [Zn(L)3
]-(NO3)2, where L is 4,40,5,50-tetramethyl-2,20-biimidazole
(C10H14N4), has been synthesized and characterized by
X-ray diffraction techniques. The ZnII atom, on a twofold axis, has a ZnN6 distorted octahedral coordination
environ-ment. 4,40,5,50-Tetramethyl-2,20-biimidazole is bonded as a
chelating ligand through its unprotonated N atoms. These discrete monomeric units possesses excellent hydrogen-bonding sites which further self-assemble into a three-dimensional supramolecular architecture through intermole-cular N—H O and C—H O hydrogen bonds.
Comment
The unique strength, directionality and complementarity of non-covalent interactions, such as hydrogen bonding and coordination bonding, play an important role in the creation of a variety of molecular architectures (Tadokoroet al., 1999; Lehn, 1995). The building blocks possessing such non-covalent interaction sites can produce one-, two- and three-dimensional molecular arrangements with long-range order (Subramanian & Zaworotko, 1994; Ermer, 1988). We present here a new three-dimensional hydrogen-bonding network with discrete monomeric ZnIIbuilding blocks, [Zn(L)3](NO3)2, (I), whereL
is 4,40,5,50-tetramethyl-2,20-biimidazole.
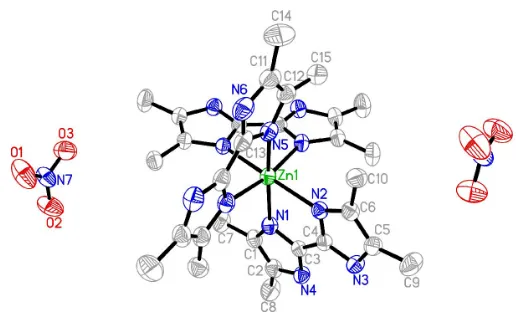
X-ray structure analysis reveals that (I) is a monomeric ZnII complex (Fig. 1). The coordination geometry around the ZnII
atom, which lies on a twofold rotation axis, is octahedral, with Zn—N distances in the range 2.149 (3)–2.208 (2) A˚ . The 4,40,5,50-tetramethyl-2,20-biimidazole ligand is bonded as a
chelating ligand through its unprotonated N atoms. A striking structural feature of (I) is the formation of a three-dimen-sional supramolecular architecture through intermolecular N—H O and C—H O hydrogen-bonding interactions (Table 1).
Experimental
Complex (I) was prepared by reacting an acetonitrile solution (15 ml) of zinc(II) nitrate with 4,40,5,50-tetramethyl-2,20-biimidazole in a 1:3
ratio. Red block-shaped crystals were recovered by filtration and dried in air. The yield wasca78% (based on Zn). IR (KBr, cm1): 3232 (m), 2980 (m), 1696 (s), 1517 (s), 1456 (m), 1200 (m), 841 (m), 751 (w), 624 (w) cm1.
Crystal data
[Zn(C10H14N4)3](NO3)2
Mr= 760.15 Hexagonal,P64
a= 13.900 (2) A˚
c= 16.809 (3) A˚
V= 2812.7 (8) A˚3
Z= 3
Dx= 1.346 Mg m 3
MoKradiation Cell parameters from 6512
reflections
= 2.9–24.1
= 0.72 mm1
T= 293 (2) K Block, red
0.200.200.15 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1998)
Tmin= 0.868,Tmax= 0.898 17035 measured reflections
4093 independent reflections 3093 reflections withI> 2(I)
Rint= 0.116
max= 28.3
h=12!18
k=18!15
l=17!21
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.043
wR(F2) = 0.111
S= 0.96 4093 reflections 254 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0565P)2] whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.68 e A˚ 3
min=0.31 e A˚ 3
Absolute structure: Flack (1983), 1683 Friedel pairs
[image:2.610.309.567.67.227.2]Flack parameter =0.027 (13)
Table 1
Hydrogen-bonding geometry (A˚ ,).
D—H A D—H H A D A D—H A
N3—H3A O1i
0.86 2.01 2.852 (4) 167 N4—H4A O3i
0.86 2.01 2.818 (5) 156 N6—H6A O2ii
0.86 2.03 2.849 (5) 157 N6—H6A O3ii
0.86 2.60 3.276 (5) 136 C9—H9A O3iii
0.96 2.47 3.403 (7) 163 C9—H9B O1iv
0.96 2.51 3.431 (7) 162 C10—H10C O2v
0.96 2.46 3.235 (6) 138
Symmetry codes: (i) xy;x;z1
3; (ii) y;1xþy; 1
3þz; (iii) 1x;1y;z; (iv) 1xþy;2x;z1
3; (v)x1;y;z.
All H atoms were placed in calculated positions and allowed to ride on their parent atoms [C—H = 0.93 A˚ andUiso(H) = 1.2Ueq(C)].
Data collection:SMART(Bruker, 1998); cell refinement:SAINT
(Bruker, 1998); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1997a); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997a); molecular graphics:
SHELXTL(Sheldrick, 1997b); software used to prepare material for publication:SHELXTL.
The authors thank AIST and Kobe University for financial support.
References
Bruker (1998).SMARTandSAINT.Bruker AXS Inc., Madison, Wisconsin, USA.
Ermer, O. (1988).J. Am. Chem. Soc.110, 3747–3754. Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Lehn, J.-M. (1995).Supramolecular Chemistry. Weinheim: VCH. Sheldrick, G. M. (1998).SADABS.University of Go¨ttingen, Germany. Sheldrick, G. M. (1997a). SHELXS97 and SHELXL97. University of
Go¨ttingen, Germany.
Sheldrick, G. M. (1997b).SHELXTL.Version 5.1. Bruker AXS Inc., Madison, Wisconsin, USA.
Subramanian, S. & Zaworotko, M. J. (1994).Coord. Chem. Rev.137, 357–401. Tadokoro, M., Isobe, K., Uekusa, H., Ohashi, Y., Toyoda, J., Tashiro, K. &
Nakasuji, K. (1999).Angew. Chem. Int. Ed.38, 95–98.
Figure 1
[image:2.610.44.293.470.553.2]supporting information
sup-1
Acta Cryst. (2005). E61, m1075–m1076
supporting information
Acta Cryst. (2005). E61, m1075–m1076 [https://doi.org/10.1107/S1600536805013954]
Tris(4,4
′
,5,5
′
-tetramethyl-2,2
′
-biimidazole)zinc(II) dinitrate
Ru-Qiang Zou and Qiang Xu
Tris(4,4′,5,5′-tetramethyl-2,2′-biimidazole)zinc(II) dinitrate
Crystal data
[Zn(C10H14N4)3](NO3)2
Mr = 760.15
Hexagonal, P64
a = 13.900 (2) Å
c = 16.809 (3) Å
V = 2812.7 (8) Å3
Z = 3
F(000) = 1194
Dx = 1.346 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 6512 reflections
θ = 2.9–24.1°
µ = 0.72 mm−1
T = 293 K Block, red
0.20 × 0.20 × 0.15 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
Detector resolution: 8.366 pixels mm-1
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 1998)
Tmin = 0.868, Tmax = 0.898
17035 measured reflections 4093 independent reflections 3093 reflections with I > 2σ(I)
Rint = 0.116
θmax = 28.3°, θmin = 1.7°
h = −12→18
k = −18→15
l = −17→21
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.043
wR(F2) = 0.111
S = 0.96 4093 reflections 254 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0565P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.68 e Å−3
Δρmin = −0.31 e Å−3
Absolute structure: Flack (1983), 1683 Friedel pairs
Absolute structure parameter: −0.027 (13)
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Zn1 0.5000 0.5000 0.17471 (3) 0.05577 (14)
C1 0.6948 (2) 0.6142 (3) 0.0302 (2) 0.0631 (7)
C2 0.7235 (3) 0.7016 (3) −0.0192 (2) 0.0690 (8)
C3 0.5999 (2) 0.6903 (2) 0.06630 (18) 0.0547 (6)
C4 0.5190 (2) 0.7054 (2) 0.11051 (18) 0.0537 (6)
C5 0.4118 (3) 0.7647 (3) 0.1567 (2) 0.0660 (8)
C6 0.3870 (2) 0.6676 (3) 0.19359 (18) 0.0602 (7)
C7 0.7377 (4) 0.5361 (4) 0.0327 (3) 0.0853 (11)
H7A 0.6786 0.4624 0.0208 0.124 (18)*
H7B 0.7663 0.5371 0.0849 0.101 (15)*
H7C 0.7959 0.5580 −0.0058 0.110 (14)*
C8 0.7982 (4) 0.7433 (5) −0.0887 (3) 0.1006 (14)
H8A 0.8315 0.6981 −0.0971 0.19 (3)*
H8B 0.8553 0.8187 −0.0792 0.090 (13)*
H8C 0.7564 0.7404 −0.1350 0.18 (3)*
C9 0.3652 (4) 0.8406 (4) 0.1662 (3) 0.0975 (14)
H9A 0.2878 0.7978 0.1795 0.097 (12)*
H9B 0.3737 0.8797 0.1173 0.088 (12)*
H9C 0.4043 0.8931 0.2079 0.32 (6)*
C10 0.3023 (3) 0.6062 (3) 0.2551 (3) 0.0779 (10)
H10A 0.2674 0.6482 0.2702 0.092 (12)*
H10B 0.3370 0.5952 0.3008 0.13 (2)*
H10C 0.2473 0.5355 0.2343 0.118 (17)*
C11 0.2728 (3) 0.3279 (3) 0.3719 (3) 0.0809 (10)
C12 0.2826 (3) 0.3402 (3) 0.2912 (2) 0.0713 (9)
C13 0.4431 (3) 0.4572 (3) 0.3424 (2) 0.0676 (8)
C14 0.1718 (5) 0.2489 (5) 0.4200 (3) 0.1225 (18)
H14A 0.1899 0.2590 0.4756 0.184*
H14B 0.1489 0.1738 0.4051 0.184*
H14C 0.1125 0.2637 0.4097 0.184*
C15 0.1956 (3) 0.2814 (4) 0.2278 (3) 0.0860 (11)
H15A 0.1288 0.2240 0.2518 0.14 (2)*
H15B 0.2224 0.2491 0.1896 0.096 (13)*
H15C 0.1803 0.3337 0.2017 0.096 (14)*
N1 0.6162 (2) 0.6072 (2) 0.08389 (16) 0.0578 (6)
N2 0.45547 (18) 0.63124 (19) 0.16345 (16) 0.0554 (5)
N3 0.4953 (2) 0.7866 (2) 0.10390 (16) 0.0618 (6)
H3A 0.5266 0.8426 0.0723 0.060 (9)*
N4 0.6626 (2) 0.7494 (2) 0.00532 (17) 0.0624 (6)
supporting information
sup-3
Acta Cryst. (2005). E61, m1075–m1076
N5 0.3904 (2) 0.4200 (2) 0.27343 (17) 0.0642 (6)
N6 0.3722 (3) 0.4018 (3) 0.4034 (2) 0.0811 (9)
H6A 0.3879 0.4121 0.4533 0.16 (3)*
N7 0.9754 (2) 0.3546 (3) 0.2781 (2) 0.0815 (9)
O1 0.9869 (3) 0.3607 (4) 0.3503 (2) 0.1347 (15)
O2 1.0435 (2) 0.4214 (3) 0.2309 (2) 0.1163 (11)
O3 0.8911 (3) 0.2796 (2) 0.2513 (2) 0.1055 (9)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Zn1 0.0570 (3) 0.0602 (3) 0.0561 (2) 0.0338 (2) 0.000 0.000
C1 0.0560 (15) 0.0766 (18) 0.0615 (17) 0.0366 (15) 0.0040 (14) −0.0008 (15)
C2 0.0590 (16) 0.086 (2) 0.0663 (19) 0.0395 (16) 0.0103 (15) 0.0047 (17)
C3 0.0489 (14) 0.0591 (15) 0.0550 (15) 0.0262 (12) −0.0049 (13) −0.0024 (13)
C4 0.0545 (14) 0.0557 (14) 0.0536 (15) 0.0297 (12) −0.0026 (13) −0.0034 (13)
C5 0.0655 (17) 0.0639 (17) 0.074 (2) 0.0368 (14) 0.0057 (16) −0.0038 (16)
C6 0.0574 (15) 0.0658 (16) 0.0626 (18) 0.0348 (13) 0.0053 (13) −0.0056 (15)
C7 0.086 (2) 0.106 (3) 0.088 (3) 0.067 (2) 0.021 (2) 0.009 (2)
C8 0.101 (3) 0.125 (4) 0.098 (3) 0.074 (3) 0.035 (3) 0.029 (3)
C9 0.114 (3) 0.092 (3) 0.115 (4) 0.073 (2) 0.043 (3) 0.023 (3)
C10 0.0674 (19) 0.071 (2) 0.098 (3) 0.0369 (18) 0.025 (2) 0.0087 (19)
C11 0.082 (2) 0.080 (2) 0.089 (3) 0.047 (2) 0.024 (2) 0.020 (2)
C12 0.076 (2) 0.071 (2) 0.080 (2) 0.0462 (18) 0.0135 (18) 0.0096 (18)
C13 0.090 (2) 0.0718 (17) 0.0630 (18) 0.0565 (16) 0.0125 (18) 0.0107 (17)
C14 0.129 (4) 0.117 (4) 0.100 (4) 0.046 (3) 0.037 (3) 0.034 (3)
C15 0.079 (2) 0.082 (2) 0.098 (3) 0.041 (2) 0.004 (2) 0.009 (2)
N1 0.0575 (13) 0.0668 (14) 0.0560 (13) 0.0363 (11) 0.0043 (11) −0.0020 (11)
N2 0.0530 (11) 0.0583 (12) 0.0586 (14) 0.0307 (10) −0.0009 (11) −0.0061 (11)
N3 0.0693 (15) 0.0602 (14) 0.0623 (15) 0.0373 (12) 0.0063 (12) 0.0047 (12)
N4 0.0618 (13) 0.0696 (14) 0.0608 (14) 0.0365 (12) 0.0081 (13) 0.0129 (14)
N5 0.0745 (16) 0.0661 (14) 0.0636 (16) 0.0439 (14) 0.0054 (13) 0.0063 (13)
N6 0.103 (2) 0.094 (2) 0.0638 (19) 0.062 (2) 0.0129 (17) 0.0149 (16)
N7 0.0563 (16) 0.099 (2) 0.075 (2) 0.0273 (16) −0.0004 (16) −0.0075 (18)
O1 0.0894 (19) 0.212 (4) 0.068 (2) 0.049 (2) −0.0029 (16) −0.022 (2)
O2 0.0803 (17) 0.119 (2) 0.099 (2) 0.0118 (17) 0.0190 (18) −0.0052 (19)
O3 0.100 (2) 0.0918 (19) 0.087 (2) 0.0202 (17) −0.0072 (17) 0.0084 (16)
Geometric parameters (Å, º)
Zn1—N5i 2.149 (3) C9—H9A 0.9600
Zn1—N5 2.149 (3) C9—H9B 0.9600
Zn1—N1i 2.180 (3) C9—H9C 0.9600
Zn1—N1 2.180 (3) C10—H10A 0.9600
Zn1—N2 2.208 (2) C10—H10B 0.9600
Zn1—N2i 2.208 (2) C10—H10C 0.9600
C1—C2 1.356 (5) C11—N6 1.351 (6)
C1—C7 1.477 (5) C11—C14 1.512 (6)
C2—N4 1.375 (4) C12—N5 1.380 (5)
C2—C8 1.476 (6) C12—C15 1.509 (6)
C3—N1 1.318 (4) C13—N5 1.331 (5)
C3—N4 1.330 (4) C13—N6 1.363 (5)
C3—C4 1.448 (4) C13—C13i 1.426 (7)
C4—N2 1.313 (4) C14—H14A 0.9600
C4—N3 1.329 (4) C14—H14B 0.9600
C5—C6 1.364 (5) C14—H14C 0.9600
C5—N3 1.370 (4) C15—H15A 0.9600
C5—C9 1.497 (5) C15—H15B 0.9600
C6—N2 1.378 (4) C15—H15C 0.9600
C6—C10 1.476 (5) N3—H3A 0.8600
C7—H7A 0.9600 N4—H4A 0.8600
C7—H7B 0.9600 N6—H6A 0.8600
C7—H7C 0.9600 N7—O3 1.202 (4)
C8—H8A 0.9600 N7—O1 1.221 (5)
C8—H8B 0.9600 N7—O2 1.228 (4)
C8—H8C 0.9600
N5i—Zn1—N5 78.88 (16) H9B—C9—H9C 109.5
N5i—Zn1—N1i 170.31 (10) C6—C10—H10A 109.5
N5—Zn1—N1i 95.51 (10) C6—C10—H10B 109.5
N5i—Zn1—N1 95.51 (10) H10A—C10—H10B 109.5
N5—Zn1—N1 170.31 (10) C6—C10—H10C 109.5
N1i—Zn1—N1 91.09 (14) H10A—C10—H10C 109.5
N5i—Zn1—N2 93.33 (9) H10B—C10—H10C 109.5
N5—Zn1—N2 94.26 (9) N6—C11—C12 107.1 (4)
N1i—Zn1—N2 94.97 (9) N6—C11—C14 124.6 (4)
N1—Zn1—N2 78.07 (9) C12—C11—C14 128.3 (5)
N5i—Zn1—N2i 94.26 (9) C11—C12—N5 108.5 (4)
N5—Zn1—N2i 93.33 (9) C11—C12—C15 129.1 (4)
N1i—Zn1—N2i 78.07 (9) N5—C12—C15 122.4 (3)
N1—Zn1—N2i 94.97 (9) N5—C13—N6 109.5 (3)
N2—Zn1—N2i 170.17 (14) N5—C13—C13i 119.3 (2)
C2—C1—N1 109.3 (3) N6—C13—C13i 131.2 (2)
C2—C1—C7 129.1 (3) C11—C14—H14A 109.5
N1—C1—C7 121.6 (3) C11—C14—H14B 109.5
C1—C2—N4 105.9 (3) H14A—C14—H14B 109.5
C1—C2—C8 132.1 (3) C11—C14—H14C 109.5
N4—C2—C8 122.0 (3) H14A—C14—H14C 109.5
N1—C3—N4 111.6 (3) H14B—C14—H14C 109.5
N1—C3—C4 119.9 (3) C12—C15—H15A 109.5
N4—C3—C4 128.5 (3) C12—C15—H15B 109.5
N2—C4—N3 111.6 (3) H15A—C15—H15B 109.5
N2—C4—C3 120.0 (2) C12—C15—H15C 109.5
N3—C4—C3 128.3 (3) H15A—C15—H15C 109.5
supporting information
sup-5
Acta Cryst. (2005). E61, m1075–m1076
C6—C5—C9 131.7 (3) C3—N1—C1 105.5 (3)
N3—C5—C9 122.0 (3) C3—N1—Zn1 111.30 (19)
C5—C6—N2 108.5 (3) C1—N1—Zn1 142.6 (2)
C5—C6—C10 128.6 (3) C4—N2—C6 106.1 (2)
N2—C6—C10 122.9 (3) C4—N2—Zn1 110.51 (18)
C1—C7—H7A 109.5 C6—N2—Zn1 143.3 (2)
C1—C7—H7B 109.5 C4—N3—C5 107.4 (3)
H7A—C7—H7B 109.5 C4—N3—H3A 126.3
C1—C7—H7C 109.5 C5—N3—H3A 126.3
H7A—C7—H7C 109.5 C3—N4—C2 107.7 (3)
H7B—C7—H7C 109.5 C3—N4—H4A 126.1
C2—C8—H8A 109.5 C2—N4—H4A 126.1
C2—C8—H8B 109.5 C13—N5—C12 106.8 (3)
H8A—C8—H8B 109.5 C13—N5—Zn1 111.2 (2)
C2—C8—H8C 109.5 C12—N5—Zn1 142.0 (3)
H8A—C8—H8C 109.5 C11—N6—C13 108.1 (4)
H8B—C8—H8C 109.5 C11—N6—H6A 125.9
C5—C9—H9A 109.5 C13—N6—H6A 125.9
C5—C9—H9B 109.5 O3—N7—O1 118.0 (4)
H9A—C9—H9B 109.5 O3—N7—O2 117.5 (4)
C5—C9—H9C 109.5 O1—N7—O2 124.4 (4)
H9A—C9—H9C 109.5
N1—C1—C2—N4 0.7 (4) C5—C6—N2—Zn1 177.1 (3)
C7—C1—C2—N4 −177.6 (4) C10—C6—N2—Zn1 −3.3 (5)
N1—C1—C2—C8 −176.6 (4) N5i—Zn1—N2—C4 94.5 (2)
C7—C1—C2—C8 5.1 (7) N5—Zn1—N2—C4 173.5 (2)
N1—C3—C4—N2 −5.6 (4) N1i—Zn1—N2—C4 −90.6 (2)
N4—C3—C4—N2 172.3 (3) N1—Zn1—N2—C4 −0.48 (19)
N1—C3—C4—N3 176.5 (3) N5i—Zn1—N2—C6 −82.4 (3)
N4—C3—C4—N3 −5.6 (5) N5—Zn1—N2—C6 −3.3 (3)
N3—C5—C6—N2 0.3 (3) N1i—Zn1—N2—C6 92.6 (3)
C9—C5—C6—N2 −179.4 (4) N1—Zn1—N2—C6 −177.3 (3)
N3—C5—C6—C10 −179.3 (3) N2—C4—N3—C5 0.8 (3)
C9—C5—C6—C10 1.1 (7) C3—C4—N3—C5 178.8 (3)
N6—C11—C12—N5 2.1 (4) C6—C5—N3—C4 −0.6 (3)
C14—C11—C12—N5 −178.6 (4) C9—C5—N3—C4 179.0 (4)
N6—C11—C12—C15 −177.6 (3) N1—C3—N4—C2 0.4 (3)
C14—C11—C12—C15 1.8 (7) C4—C3—N4—C2 −177.6 (3)
N4—C3—N1—C1 0.0 (3) C1—C2—N4—C3 −0.7 (4)
C4—C3—N1—C1 178.3 (3) C8—C2—N4—C3 177.0 (4)
N4—C3—N1—Zn1 −173.5 (2) N6—C13—N5—C12 1.0 (3)
C4—C3—N1—Zn1 4.7 (3) C13i—C13—N5—C12 −178.9 (3)
C2—C1—N1—C3 −0.5 (4) N6—C13—N5—Zn1 −177.79 (18)
C7—C1—N1—C3 178.0 (3) C13i—C13—N5—Zn1 2.4 (4)
C2—C1—N1—Zn1 169.6 (3) C11—C12—N5—C13 −1.9 (4)
C7—C1—N1—Zn1 −11.9 (6) C15—C12—N5—C13 177.8 (3)
N1i—Zn1—N1—C3 92.6 (2) C15—C12—N5—Zn1 −4.1 (5)
N2—Zn1—N1—C3 −2.3 (2) N5i—Zn1—N5—C13 −0.8 (3)
N2i—Zn1—N1—C3 170.7 (2) N1i—Zn1—N5—C13 171.2 (2)
N5i—Zn1—N1—C1 95.8 (4) N2—Zn1—N5—C13 −93.4 (2)
N1i—Zn1—N1—C1 −77.1 (3) N2i—Zn1—N5—C13 92.9 (2)
N2—Zn1—N1—C1 −172.0 (4) N5i—Zn1—N5—C12 −178.9 (4)
N2i—Zn1—N1—C1 1.0 (4) N1i—Zn1—N5—C12 −6.9 (3)
N3—C4—N2—C6 −0.6 (3) N2—Zn1—N5—C12 88.5 (3)
C3—C4—N2—C6 −178.8 (3) N2i—Zn1—N5—C12 −85.2 (3)
N3—C4—N2—Zn1 −178.7 (2) C12—C11—N6—C13 −1.4 (4)
C3—C4—N2—Zn1 3.1 (3) C14—C11—N6—C13 179.2 (4)
C5—C6—N2—C4 0.2 (3) N5—C13—N6—C11 0.3 (3)
C10—C6—N2—C4 179.8 (3) C13i—C13—N6—C11 −179.9 (4)
Symmetry code: (i) −x+1, −y+1, z.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
N3—H3A···O1ii 0.86 2.01 2.852 (4) 167
N4—H4A···O3ii 0.86 2.01 2.818 (5) 156
N6—H6A···O2iii 0.86 2.03 2.849 (5) 157
N6—H6A···O3iii 0.86 2.60 3.276 (5) 136
C9—H9A···O3i 0.96 2.47 3.403 (7) 163
C9—H9B···O1iv 0.96 2.51 3.431 (7) 162
C10—H10C···O2v 0.96 2.46 3.235 (6) 138