metal-organic papers
m568
Jian-Yi Wuet al. [Co(C4H4O4S)(C12H8N2)(H2O)] doi:10.1107/S1600536805005076 Acta Cryst.(2005). E61, m568–m570
Acta Crystallographica Section E Structure Reports Online
ISSN 1600-5368
Aqua(1,10-phenanthroline)(thiodiglycolato)cobalt(II)
Jian-Yi Wu,aLin-Ming Xie,a Hong-Yin He,aXia Zhouaand Long-Guan Zhub*
aDepartment of Chemical Engineering, Jiaxing
College, Jiaxing 314001, People’s Republic of China, andbDepartment of Chemistry, Zhejiang
University, Hangzhou 310007, People’s Republic of China
Correspondence e-mail: chezlg@zju.edu.cn
Key indicators
Single-crystal X-ray study
T= 295 K
Mean(C–C) = 0.004 A˚
Rfactor = 0.038
wRfactor = 0.091
Data-to-parameter ratio = 15.9
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
In the title cobalt compound, [Co(C4H4O4S)(C12H8N2
)-(H2O)], the distorted octahedron around the Co atom is
formed by the O atom from a water molecule, two N atoms from the heterocycle, two carboxyl O atoms and an S atom from the dianion. Hydrogen bonds extend the structure into a two-dimensional network.
Comment
The S atom of thiodiglycolic acid may be coordinated to metal atoms. However, only a few metal–thiodiglycolate complexes have been reported (Bonomo et al., 1982; Baggioet al., 1996, 1999; Kopelet al., 2003; Grirraneet al., 2003).
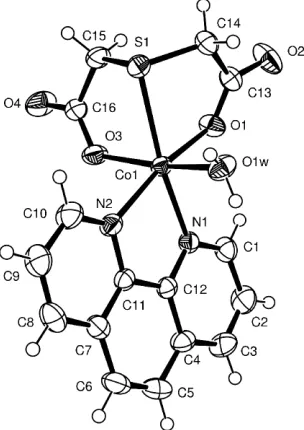
In the title compound, (I), the coordination polyhedron around the Co atom can be described as a distorted octa-hedron consisting of two N-atom donors from a 1,10-phen-anthroline, one O atom from the water molecule and three donors from the thiodiglycolate ligand (Fig. 1 and Table 1). The flexible dicarboxylate dianion is converted to a rigid ligand when the S-atom donor coordinates to the CoIIatom, giving rise to the formation of two five-membered chelate rings. Both rings display a twist conformation. Each carboxyl group is coordinated in monodentate fashion to the cobalt centre. Both uncoordinated carboxyl O atoms form hydrogen bonds with water molecules, resulting in a two-dimensional hydrogen-bonding network (Fig. 2 and Table 2).
Experimental
A mixture of cobalt(II) acetate tetrahydrate (0.0747 g, 0.30 mmol), thiodiglycolic acid (0.0452 g, 0.30 mmol), 1,10-phenanthroline (0.0595 g, 0.30 mmol) and water (10 ml) was heated at 393 K for 24 h in a 20 ml Teflon-lined stainless steel autoclave. After cooling, blue block-shaped crystals of (I) were obtained.
Crystal data
[Co(C4H4O4S)(C12H8N2)(H2O)]
Mr= 405.28
Monoclinic, P21=n a= 8.0127 (9) A˚
b= 22.524 (3) A˚
c= 9.733 (1) A˚
= 113.420 (1)
V= 1611.9 (3) A˚3
Z= 4
Dx= 1.670 Mg m
3
MoKradiation Cell parameters from 3581
reflections
= 2.8–24.7 = 1.23 mm1
T= 295 (2) K Block, blue
0.140.140.09 mm
Data collection
Bruker SMART APEX area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 2002)
Tmin= 0.784,Tmax= 0.898
18 142 measured reflections
3688 independent reflections 2997 reflections withI> 2(I)
Rint= 0.050 max= 27.5
h=10!10
k=29!29
l=12!12
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.038
wR(F2) = 0.091
S= 1.02 3688 reflections 232 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo2) + (0.0432P)2
+ 0.4148P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.38 e A˚ 3
min=0.27 e A˚ 3
Table 1
Selected geometric parameters (A˚ ,).
Co1—O1 2.033 (2)
Co1—O3 2.038 (2)
Co1—O1W 2.084 (2)
Co1—N1 2.114 (2)
Co1—N2 2.149 (2)
Co1—S1 2.5191 (7)
O1—Co1—O3 94.78 (8)
O1—Co1—O1W 92.07 (8)
O3—Co1—O1W 166.26 (8)
O1—Co1—N1 91.64 (7)
O3—Co1—N1 100.60 (7)
O1W—Co1—N1 91.08 (7)
O1—Co1—N2 169.55 (7)
O3—Co1—N2 87.07 (7)
O1W—Co1—N2 88.34 (8)
N1—Co1—N2 77.91 (7)
O1—Co1—S1 80.51 (5)
O3—Co1—S1 81.51 (5)
O1W—Co1—S1 87.90 (5)
N1—Co1—S1 172.03 (5)
[image:2.610.95.247.76.291.2]N2—Co1—S1 109.95 (5)
Table 2
Hydrogen-bonding geometry (A˚ ,).
D—H A D—H H A D A D—H A
O1W—H1W1 O2i
0.84 (1) 1.93 (1) 2.764 (3) 170 (3)
O1W—H1W2 O4ii
0.85 (3) 1.82 (3) 2.662 (3) 179 (3)
Symmetry codes: (i)x1 2;
3 2y;z
1
2; (ii)x1;y;z.
The H atoms bonded to C atoms were positioned geometrically and included in the refinement using the riding-model approximation [C—H = 0.93 A˚ for CH and C—H = 0.97 A˚ for CH2, andUiso(H) = 1.2Ueq(C)]. The water H atoms were located in a difference Fourier map and refined with distance restraints of O—H = 0.85 (1) A˚ and with fixed isotropic displacement parameters ofUiso(H) = 0.05 A˚
2 . Data collection:SMART(Bruker, 2002); cell refinement:SAINT
(Bruker, 2002); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3 for Windows(Farrugia, 1997); software used to prepare material for publication:WinGX(Farrugia, 1999).
We thank Professor Seik Weng Ng for his kind help in the single-crystal structure analysis and reference information and the National Natural Science Foundation of China (grant No. 50073019).
References
Baggio, R., Perec, M. & Garland, M. T. (1996).Acta Cryst.C52, 2457–2460. Baggio, R., Garland, M. T., Manzur, J. Pena, O., Perec, M., Spodine, E. & Vega,
A. (1999).Inorg. Chim. Acta,286, 74–79.
Bonomo, R. P., Rizzarelli, E., Bresciani-Pahor, N. & Nardin, G. (1982).J. Chem. Soc. Dalton Trans.pp. 681–685.
Bruker (2002).SMART,SAINTandSADABS.Bruker AXS Inc., Madison, Wisconsin, USA.
metal-organic papers
Acta Cryst.(2005). E61, m568–m570 Jian-Yi Wuet al. [Co(C
4H4O4S)(C12H8N2)(H2O)]
m569
Figure 1
ORTEP-3view (Farrugia, 1997) of the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Figure 2
[image:2.610.44.298.353.506.2]Farrugia, L. J. (1997).J. Appl. Cryst.30, 565. Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Grirrane, A., Pastor, A., Galindo, A., Ienco, A., Mealli, C. & Rosa, P. (2003).
Chem. Commun.pp. 512–513.
Kopel, P., Travnicek, Z., Marek, J., Korabik, M. & Mrozinski, J. (2003).
Polyhedron,22, 411–418.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
metal-organic papers
m570
Jian-Yi Wuet al. [Co(Csupporting information
sup-1
Acta Cryst. (2005). E61, m568–m570
supporting information
Acta Cryst. (2005). E61, m568–m570 [https://doi.org/10.1107/S1600536805005076]
Aqua(1,10-phenanthroline)(thiodiglycolato)cobalt(II)
Jian-Yi Wu, Lin-Ming Xie, Hong-Yin He, Xia Zhou and Long-Guan Zhu
Aqua(1,10-phenanthroline)(thiodiglycolato)cobalt(II)
Crystal data
[Co(C4H4O4S)(C12H8N2)(H2O)]
Mr = 405.28 Monoclinic, P21/n Hall symbol: -P 2yn a = 8.0127 (9) Å b = 22.524 (3) Å c = 9.733 (1) Å β = 113.420 (1)° V = 1611.9 (3) Å3
Z = 4
F(000) = 828 Dx = 1.670 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 3581 reflections θ = 2.8–24.7°
µ = 1.23 mm−1
T = 295 K Block, blue
0.14 × 0.14 × 0.09 mm
Data collection
Bruker APEX area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 2002) Tmin = 0.784, Tmax = 0.898
18142 measured reflections 3688 independent reflections 2997 reflections with I > 2σ(I) Rint = 0.050
θmax = 27.5°, θmin = 1.8°
h = −10→10 k = −29→29 l = −12→12
Refinement
Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.038
wR(F2) = 0.091
S = 1.02 3688 reflections 232 parameters 2 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0432P)2 + 0.4148P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001 Δρmax = 0.38 e Å−3 Δρmin = −0.27 e Å−3
Special details
supporting information
sup-2
Acta Cryst. (2005). E61, m568–m570
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Co1 0.44105 (4) 0.657445 (13) 0.73224 (3) 0.0260 (1)
S1 0.49229 (8) 0.71389 (3) 0.52895 (7) 0.0307 (2)
O1 0.5329 (2) 0.73547 (7) 0.84119 (18) 0.0353 (4)
O2 0.5421 (3) 0.83347 (8) 0.8371 (2) 0.0545 (6)
O3 0.6934 (2) 0.62578 (8) 0.7685 (2) 0.0397 (4)
O4 0.9588 (3) 0.63361 (10) 0.7525 (3) 0.0566 (6)
O1W 0.1734 (2) 0.68777 (9) 0.6415 (2) 0.0387 (4)
H1W1 0.123 (4) 0.6839 (13) 0.548 (1) 0.050*
H1W2 0.104 (3) 0.6705 (11) 0.676 (3) 0.050*
N1 0.4044 (3) 0.62143 (8) 0.9190 (2) 0.0276 (4)
N2 0.3401 (3) 0.57003 (9) 0.6543 (2) 0.0316 (4)
C1 0.4337 (4) 0.64808 (11) 1.0481 (3) 0.0363 (6)
H1 0.4873 0.6855 1.0660 0.044*
C2 0.3871 (4) 0.62209 (13) 1.1578 (3) 0.0474 (7)
H2 0.4075 0.6423 1.2464 0.057*
C3 0.3116 (4) 0.56696 (13) 1.1348 (3) 0.0462 (7)
H3 0.2801 0.5494 1.2076 0.055*
C4 0.2813 (3) 0.53677 (11) 1.0015 (3) 0.0347 (6)
C5 0.2045 (4) 0.47843 (12) 0.9680 (3) 0.0448 (7)
H5 0.1744 0.4584 1.0385 0.054*
C6 0.1753 (4) 0.45217 (12) 0.8360 (3) 0.0433 (7)
H6 0.1244 0.4144 0.8164 0.052*
C7 0.2214 (3) 0.48165 (10) 0.7249 (3) 0.0332 (5)
C8 0.1943 (4) 0.45607 (12) 0.5857 (3) 0.0438 (7)
H8 0.1450 0.4182 0.5615 0.053*
C9 0.2411 (4) 0.48749 (12) 0.4870 (3) 0.0473 (7)
H9 0.2260 0.4710 0.3951 0.057*
C10 0.3122 (4) 0.54466 (12) 0.5248 (3) 0.0433 (7)
H10 0.3413 0.5659 0.4553 0.052*
C11 0.2966 (3) 0.53850 (10) 0.7548 (2) 0.0271 (5)
C12 0.3293 (3) 0.56654 (10) 0.8958 (2) 0.0271 (5)
C13 0.5170 (3) 0.78437 (10) 0.7742 (3) 0.0315 (5)
C14 0.4632 (4) 0.78457 (10) 0.6054 (3) 0.0339 (6)
H14A 0.5353 0.8142 0.5816 0.041*
H14B 0.3366 0.7963 0.5568 0.041*
C15 0.7362 (3) 0.70339 (12) 0.6152 (3) 0.0387 (6)
H15A 0.7808 0.6990 0.5366 0.046*
H15B 0.7909 0.7390 0.6712 0.046*
supporting information
sup-3
Acta Cryst. (2005). E61, m568–m570 Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Co1 0.02576 (18) 0.02661 (18) 0.02795 (18) −0.00360 (12) 0.01311 (14) −0.00112 (13)
S1 0.0311 (3) 0.0337 (3) 0.0279 (3) −0.0011 (2) 0.0121 (3) −0.0002 (2)
O1 0.0444 (10) 0.0290 (9) 0.0273 (9) −0.0062 (8) 0.0088 (8) −0.0015 (7)
O2 0.0804 (16) 0.0294 (10) 0.0393 (11) 0.0079 (10) 0.0085 (11) −0.0037 (8)
O3 0.0327 (10) 0.0391 (10) 0.0543 (12) 0.0058 (8) 0.0247 (9) 0.0179 (9)
O4 0.0317 (11) 0.0696 (14) 0.0757 (15) 0.0152 (10) 0.0292 (11) 0.0302 (12)
O1W 0.0279 (9) 0.0537 (12) 0.0348 (10) −0.0003 (8) 0.0129 (8) 0.0060 (9)
N1 0.0279 (10) 0.0274 (10) 0.0283 (10) 0.0002 (8) 0.0119 (9) −0.0016 (8)
N2 0.0363 (11) 0.0325 (11) 0.0309 (11) −0.0055 (9) 0.0186 (9) −0.0045 (9)
C1 0.0430 (15) 0.0345 (13) 0.0305 (13) 0.0010 (11) 0.0136 (12) −0.0050 (11)
C2 0.063 (2) 0.0507 (17) 0.0313 (15) 0.0040 (14) 0.0218 (14) −0.0041 (12)
C3 0.0597 (18) 0.0545 (18) 0.0316 (14) 0.0030 (14) 0.0259 (14) 0.0098 (13)
C4 0.0361 (13) 0.0385 (14) 0.0315 (13) 0.0015 (11) 0.0156 (11) 0.0080 (11)
C5 0.0541 (17) 0.0371 (15) 0.0455 (16) −0.0028 (13) 0.0223 (14) 0.0140 (13)
C6 0.0472 (16) 0.0284 (13) 0.0551 (18) −0.0058 (11) 0.0213 (14) 0.0062 (12)
C7 0.0319 (13) 0.0280 (12) 0.0385 (14) 0.0003 (10) 0.0125 (11) 0.0002 (10)
C8 0.0463 (16) 0.0307 (14) 0.0496 (17) −0.0054 (12) 0.0138 (14) −0.0113 (12)
C9 0.0575 (19) 0.0450 (16) 0.0395 (16) −0.0077 (14) 0.0194 (14) −0.0173 (13)
C10 0.0549 (17) 0.0459 (16) 0.0349 (14) −0.0084 (13) 0.0240 (13) −0.0078 (12)
C11 0.0244 (11) 0.0298 (12) 0.0283 (12) 0.0019 (9) 0.0119 (10) 0.0008 (10)
C12 0.0233 (11) 0.0297 (12) 0.0282 (12) 0.0033 (9) 0.0102 (10) 0.0026 (9)
C13 0.0277 (12) 0.0291 (13) 0.0309 (13) 0.0023 (10) 0.0044 (10) −0.0001 (10)
C14 0.0359 (14) 0.0292 (13) 0.0331 (13) 0.0048 (10) 0.0101 (11) 0.0033 (10)
C15 0.0305 (13) 0.0399 (14) 0.0536 (17) 0.0034 (11) 0.0251 (13) 0.0115 (12)
C16 0.0321 (13) 0.0320 (13) 0.0364 (14) −0.0001 (10) 0.0164 (11) 0.0020 (11)
Geometric parameters (Å, º)
Co1—O1 2.033 (2) C3—C4 1.398 (4)
Co1—O3 2.038 (2) C3—H3 0.9300
Co1—O1W 2.084 (2) C4—C12 1.404 (3)
Co1—N1 2.114 (2) C4—C5 1.433 (4)
Co1—N2 2.149 (2) C5—C6 1.348 (4)
Co1—S1 2.5191 (7) C5—H5 0.9300
S1—C15 1.810 (3) C6—C7 1.437 (4)
S1—C14 1.811 (2) C6—H6 0.9300
O1—C13 1.260 (3) C7—C11 1.396 (3)
O2—C13 1.241 (3) C7—C8 1.407 (3)
O3—C16 1.257 (3) C8—C9 1.361 (4)
O4—C16 1.234 (3) C8—H8 0.9300
O1W—H1W1 0.84 (1) C9—C10 1.397 (4)
O1W—H1W2 0.85 (3) C9—H9 0.9300
N1—C1 1.327 (3) C10—H10 0.9300
N1—C12 1.354 (3) C11—C12 1.437 (3)
supporting information
sup-4
Acta Cryst. (2005). E61, m568–m570
N2—C11 1.361 (3) C14—H14A 0.9700
C1—C2 1.393 (4) C14—H14B 0.9700
C1—H1 0.9300 C15—C16 1.528 (3)
C2—C3 1.360 (4) C15—H15A 0.9700
C2—H2 0.9300 C15—H15B 0.9700
O1—Co1—O3 94.78 (8) C6—C5—H5 119.5
O1—Co1—O1W 92.07 (8) C4—C5—H5 119.5
O3—Co1—O1W 166.26 (8) C5—C6—C7 121.0 (2)
O1—Co1—N1 91.64 (7) C5—C6—H6 119.5
O3—Co1—N1 100.60 (7) C7—C6—H6 119.5
O1W—Co1—N1 91.08 (7) C11—C7—C8 117.7 (2)
O1—Co1—N2 169.55 (7) C11—C7—C6 119.1 (2)
O3—Co1—N2 87.07 (7) C8—C7—C6 123.2 (2)
O1W—Co1—N2 88.34 (8) C9—C8—C7 119.1 (2)
N1—Co1—N2 77.91 (7) C9—C8—H8 120.4
O1—Co1—S1 80.51 (5) C7—C8—H8 120.4
O3—Co1—S1 81.51 (5) C8—C9—C10 119.4 (3)
O1W—Co1—S1 87.90 (5) C8—C9—H9 120.3
N1—Co1—S1 172.03 (5) C10—C9—H9 120.3
N2—Co1—S1 109.95 (5) N2—C10—C9 123.2 (3)
C15—S1—C14 102.5 (1) N2—C10—H10 118.4
C15—S1—Co1 93.02 (8) C9—C10—H10 118.4
C14—S1—Co1 91.88 (8) N2—C11—C7 122.8 (2)
C13—O1—Co1 123.0 (2) N2—C11—C12 117.1 (2)
C16—O3—Co1 124.2 (2) C7—C11—C12 120.1 (2)
Co1—O1W—H1W1 113 (2) N1—C12—C4 123.1 (2)
Co1—O1W—H1W2 114 (2) N1—C12—C11 117.4 (2)
H1W1—O1W—H1W2 106 (3) C4—C12—C11 119.4 (2)
C1—N1—C12 118.1 (2) O2—C13—O1 124.1 (2)
C1—N1—Co1 127.4 (2) O2—C13—C14 116.7 (2)
C12—N1—Co1 114.3 (2) O1—C13—C14 119.2 (2)
C10—N2—C11 117.8 (2) C13—C14—S1 114.4 (2)
C10—N2—Co1 129.1 (2) C13—C14—H14A 108.7
C11—N2—Co1 113.1 (2) S1—C14—H14A 108.7
N1—C1—C2 122.4 (2) C13—C14—H14B 108.7
N1—C1—H1 118.8 S1—C14—H14B 108.7
C2—C1—H1 118.8 H14A—C14—H14B 107.6
C3—C2—C1 119.7 (2) C16—C15—S1 115.4 (2)
C3—C2—H2 120.2 C16—C15—H15A 108.4
C1—C2—H2 120.2 S1—C15—H15A 108.4
C2—C3—C4 119.9 (2) C16—C15—H15B 108.4
C2—C3—H3 120.0 S1—C15—H15B 108.4
C4—C3—H3 120.0 H15A—C15—H15B 107.5
C3—C4—C12 116.8 (2) O4—C16—O3 124.0 (2)
C3—C4—C5 123.9 (2) O4—C16—C15 116.5 (2)
C12—C4—C5 119.3 (2) O3—C16—C15 119.5 (2)
supporting information
sup-5
Acta Cryst. (2005). E61, m568–m570
O1—Co1—S1—C15 77.1 (1) C12—C4—C5—C6 −1.0 (4)
O3—Co1—S1—C15 −19.2 (1) C4—C5—C6—C7 0.5 (4)
O1W—Co1—S1—C15 169.5 (1) C5—C6—C7—C11 −0.4 (4)
N2—Co1—S1—C15 −103.0 (1) C5—C6—C7—C8 179.7 (3)
O1—Co1—S1—C14 −25.6 (1) C11—C7—C8—C9 −0.3 (4)
O3—Co1—S1—C14 −121.9 (1) C6—C7—C8—C9 179.6 (3)
O1W—Co1—S1—C14 66.9 (1) C7—C8—C9—C10 −1.0 (4)
N2—Co1—S1—C14 154.4 (1) C11—N2—C10—C9 −0.1 (4)
O3—Co1—O1—C13 106.6 (2) Co1—N2—C10—C9 −178.4 (2)
O1W—Co1—O1—C13 −61.4 (2) C8—C9—C10—N2 1.3 (5)
N1—Co1—O1—C13 −152.6 (2) C10—N2—C11—C7 −1.3 (4)
N2—Co1—O1—C13 −153.5 (4) Co1—N2—C11—C7 177.2 (2)
S1—Co1—O1—C13 26.1 (2) C10—N2—C11—C12 179.5 (2)
O1—Co1—O3—C16 −61.9 (2) Co1—N2—C11—C12 −2.0 (2)
O1W—Co1—O3—C16 57.7 (4) C8—C7—C11—N2 1.5 (4)
N1—Co1—O3—C16 −154.5 (2) C6—C7—C11—N2 −178.4 (2)
N2—Co1—O3—C16 128.4 (2) C8—C7—C11—C12 −179.3 (2)
S1—Co1—O3—C16 17.7 (2) C6—C7—C11—C12 0.8 (3)
O1—Co1—N1—C1 1.6 (2) C1—N1—C12—C4 0.0 (3)
O3—Co1—N1—C1 96.8 (2) Co1—N1—C12—C4 −175.1 (2)
O1W—Co1—N1—C1 −90.5 (2) C1—N1—C12—C11 179.4 (2)
N2—Co1—N1—C1 −178.5 (2) Co1—N1—C12—C11 4.2 (3)
O1—Co1—N1—C12 176.2 (2) C3—C4—C12—N1 1.1 (4)
O3—Co1—N1—C12 −88.6 (2) C5—C4—C12—N1 −179.2 (2)
O1W—Co1—N1—C12 84.1 (2) C3—C4—C12—C11 −178.2 (2)
N2—Co1—N1—C12 −3.96 (18) C5—C4—C12—C11 1.4 (4)
O1—Co1—N2—C10 −177.6 (4) N2—C11—C12—N1 −1.5 (3)
O3—Co1—N2—C10 −77.0 (2) C7—C11—C12—N1 179.3 (2)
O1W—Co1—N2—C10 90.0 (2) N2—C11—C12—C4 177.9 (2)
N1—Co1—N2—C10 −178.5 (2) C7—C11—C12—C4 −1.4 (3)
S1—Co1—N2—C10 2.9 (2) Co1—O1—C13—O2 166.8 (2)
O1—Co1—N2—C11 4.1 (5) Co1—O1—C13—C14 −13.0 (3)
O3—Co1—N2—C11 104.7 (2) O2—C13—C14—S1 163.0 (2)
O1W—Co1—N2—C11 −88.3 (2) O1—C13—C14—S1 −17.2 (3)
N1—Co1—N2—C11 3.2 (2) C15—S1—C14—C13 −64.6 (2)
S1—Co1—N2—C11 −175.5 (1) Co1—S1—C14—C13 28.9 (2)
C12—N1—C1—C2 −1.2 (4) C14—S1—C15—C16 116.0 (2)
Co1—N1—C1—C2 173.2 (2) Co1—S1—C15—C16 23.4 (2)
N1—C1—C2—C3 1.2 (4) Co1—O3—C16—O4 173.8 (2)
C1—C2—C3—C4 0.0 (4) Co1—O3—C16—C15 −6.2 (3)
C2—C3—C4—C12 −1.1 (4) S1—C15—C16—O4 163.5 (2)
C2—C3—C4—C5 179.3 (3) S1—C15—C16—O3 −16.5 (3)
supporting information
sup-6
Acta Cryst. (2005). E61, m568–m570 Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O1W—H1W1···O2i 0.84 (1) 1.93 (1) 2.764 (3) 170 (3)
O1W—H1W2···O4ii 0.85 (3) 1.82 (3) 2.662 (3) 179 (3)