Penile hypoplasia and rudimentary prepuce in a dog
(78,XY; SRY-positive): a case report
D. Rozanska
1, I. Szczerbal
2, M. Stachowiak
2, P. Debiak
1, A. Smiech
1,
P. Rozanski
1, M. Orzelski
1, B. Zylinska
1, M. Switonski
2, B. Slaska
11University of Life Sciences in Lublin, Lublin, Poland 2Poznan University of Life Sciences, Poznan, Poland
ABSTRACT: This is the first report in the literature concerning penile hypoplasia and rudimentary prepuce in a 78,XY; SRY-positive male that received a successful surgical treatment. A six-month-old male mixed-breed dog with body weight of 3.5 kg and with symptoms of prolonged stranguria and abnormalities of the external genitalia were presented by the owner at the University Surgical Clinic. Clinical, biochemical, radiological, pathological, and genetic examinations of the dog were carried out. Penile hypoplasia and rudimentary prepuce were diagnosed. Early diagnosis of penile hypoplasia and rudimentary prepuce in small animals requires a high level of vigilance and is based on clinical and ultrasonographical findings. The radiography revealed a fan-shaped widening of the caudal part and shortening of the os penis. A hyperechogenic os penis with a wide posterior part and a slightly curved, smooth anterior end was imaged. No normal prepuce structures were observed. The endocrinological examina-tion showed a substantially decreased testosterone level. Fast surgical intervenexamina-tion is preferable and confirms the diagnosis. In the presented case report enlargement of the preputial orifice was applied in order to prevent urinary retention and recurrent urinary tract infections. Orchiectomy was also performed. After the surgery, immediate clinical improvement was noted. The testicular atrophy diagnosed in the histological analysis explains the low level of the hormone. The cytogenetic analysis revealed a normal male set of chromosomes – 78,XY. The molecular analysis showed presence of the SRY gene as well as the ZFY gene, which reside on the Y chromosome and the ZFX, which is X-linked. The successful amplification of the SRY, ZFX, and ZFY genes confirmed the presence of both X and Y chromosomes. Sequencing and comparison with the reference sequence of the canine SRY gene indicated a normal sequence in the examined dog. Given the absence of polymorphisms and mutations in the coding sequence of the SRY gene, it can be assumed that it is not associated with the observed phenotype. The studied case was classified as testicular XY (SRY-positive) disorder of sex development (DSD), the aetiology of which remains unknown.
Keywords: dog; disorder of sex development; penile hypoplasia; rudimentary prepuce; SRY; ZFX/ZFY
Determination of sex in mammals consists of a number of genetically and hormonally controlled phases. Disturbances in successive developmental stages result in congenital disorders collectively referred to as disorders of sex development (DSD; Meyers-Wallen 2012). The causes of DSD include sex chromosome abnormalities, mutations of genes involved in sex determination, and environmen-tal factors that disturb the process (Poth et al. 2010; Meyers-Wallen 2012).Among chromosomal DSD the most frequent in dogs are X monosomy (77,X), as well as trisomies (79,XXX and 79,XXY).
Case Report Veterinarni Medicina, 61, 2016 (5): 279–287
doi: 10.17221/8884-VETMED
monogenic DSD with a defined molecular back-ground is the persistent Müllerian duct syndrome – PMDS (Wu et al. 2009). However, it should be noted that, besides the aforementioned disorders of sex development, there are important defects with a complex background such as cryptorchidism and hypospadias diagnosed in male individuals with a normal set of sex chromosomes (78,XY; Meyers-Wallen 2012; Switonski et al. 2012).
Present knowledge regarding the genetic back-ground of canine DSD is obscure and thus a de-tailed description of a larger number of such cases is important. The aim of this study was to perform radiological, histological, cytological, and molecu-lar analyses, as well as a successful surgical treat-ment of the first reported case of a DSD male dog with a hypoplastic penis and a rudimentary prepuce and preputial orifice.
Case description
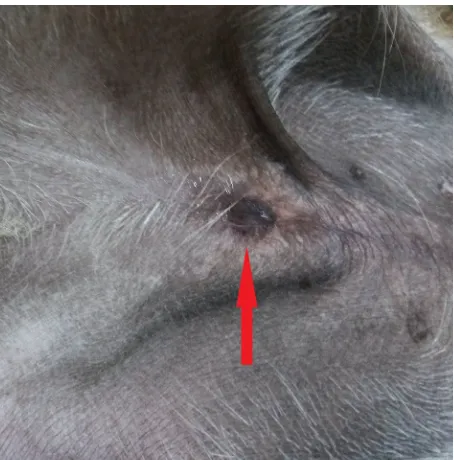
[image:2.595.306.533.94.324.2]A six-month-old male mixed-breed dog with body weight of 3.5 kg and with symptoms of dif-ficult urination was presented by the owner at the Department and Clinic of Animal Surgery, Faculty of Veterinary Medicine. The dog had been kept by the owner since birth. The dysfunction, which was manifested by painful and prolonged urination, was noted after one month of life. From then until the time of the appointment at our clinic, the owner helped the animal in removing urine retained in the preputial cavity by manual massage. The clini-cal examination revealed a rudimentary prepuce. The preputial orifice was located distally relative to the nipples of the preputial region. It had a form of a 2-mm slit extending in the skin of the rudi-mentary prepuce over the external urethral meatus (Figure 1). In the preputial cavity, large amounts of retained urine were observed as a fluctuating bulge in the preputial region, which was clearly visible in the standing position. Palpation revealed penile and testicular atrophy; both testicles were located in the scrotum. Measurements of rectal temperature as well as the pulse and respiratory rates showed physiological values. Thoracic aus-cultation revealed no abnormalities. Additional ex-aminations were performed, including biochemical blood tests, chest and abdominal radiography and abdominal ultrasound. The dog was qualified for reconstruction of the preputial orifice and
castra-tion. Pathomorphological examination of the testes as well as cytogenetic analysis, molecular analysis of the SRY gene, and identification of the ZFX and
ZFY genes were performed.
Biochemical and endocrinological blood tests were carried out in a specialised veterinary labo-ratory. Apart from an elevated level of blood urea nitrogen (62 mg/dl; reference range: 8–28 mg/dl), biochemical parameters were within physiologi-cal ranges. Serum testosterone concentration was decreased (0.17 ng/ml, range for intact male dog from 1 to 10 ng/ml), while the level of oestradiol in the patient was normal.
Abdominal radiographical examinations were performed in two projections: right lateral and ventro-dorsal. Ultrasonography of the abdomen was performed using a microconvex 6.5 MHz probe and the region of the scrotum and penis were ex-amined with a 12 MHz linear probe.
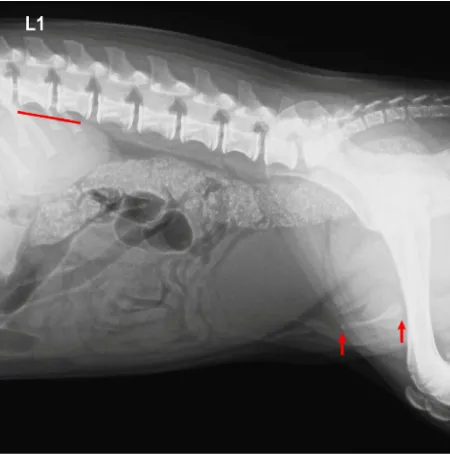
The radiological and ultrasonographical exami-nations showed a normal image of the abdominal organs. The radiography revealed a fan-shaped widening of the caudal part and shortening of the os penis. For objective estimation of the size of penile bone (baculum), retrospective measure-ments of the length of the bone in twelve previ-ously examined six-month-old different-breed dogs with similar body weights and normally developed genital organs were performed. The length of the
baculus was compared with the length of the first lumbar vertebrae. X-ray examinations performed in the analysed case revealed shortening of the penile bone, as its length relative to the lumbar spine reached ¾ of the L2 vertebra length. In all the dogs from the compared group, the baculus was longer in relation to the lumbar spine and was more than half the length of the L3 vertebra (Figure 2). Ultrasonography of the scrotum showed no changes in the echogenicity and morphology of both descended testes. Longitudinal ventro-dorsal B-mode ultrasonograms of the penile region
re-vealed structures typical of this organ and located entirely under the skin (Figure 3). A hyperechogen-ic os penis with a wide posterior part and a slightly curved, smooth anterior end was imaged. No nor-mal prepuce structures were shown. The urethral meatus was located on the penis in the typical site approximately 0.5 cm under the skin. Close to the urethral meatus, a 1-cm long liquid (urine) cistern was found in the subcutaneous tissues.
[image:3.595.307.536.96.324.2]The dog was pre-medicated intramuscularly with xylazine hydrochloride (Sedazin, Biowet, Pulawy, Poland) at a dose of 2 mg/kg, atropine sulphate
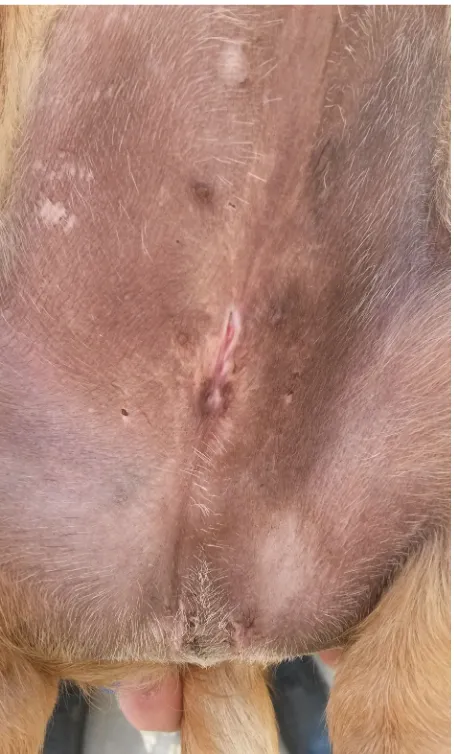
[image:3.595.61.290.573.728.2]Figure 5. Penis visible in the reconstructed preputial ori-fice
[image:3.595.306.535.581.728.2]Figure 2. Radiographic image of the abdomen. A wid-ened os penis (marked with red arrows) in the caudal part. The length of the os penis measured relative to the lumbar spine reaches ¾ of the L2 vertebra length (red line). This implies shortening of the os penis
Figure 3. Longitudinal section of the apex of the penis
(pars longa glandis) using B-mode ultrasound. 0 =
sub-cutaneous tissue; 1 = area of urine; 2 = external urethral orifice; 3 = erectile tissue; 4 = fibro-cartilaginous portion of the os penis; 5 = os penis
Case Report Veterinarni Medicina, 61, 2016 (5): 279–287
doi: 10.17221/8884-VETMED
(Atropinum Sulfuricum, Polfa, Warsaw, Poland) at a dose of 0.05 mg/kg, and butorphanol(Butomidor, Boehringer, Richter Pharma AG, Austria) at a dose of 0.2 mg/kg. General anaesthesia was achieved by intravenous administration of a mixture of keta-mine(Vetaketam 10%, Vet Agro, Lublin, Poland) and midazolam (Midanium, WZF Polfa, Warsaw, Poland)at doses of 5 mg/kg and 0.3 mg/kg, respec-tively. A 1-mm diameter catheter was inserted into the rudimentary preputial orifice and the preputial cavity was thoroughly rinsed with an antiseptic
[image:4.595.66.290.95.268.2]so-lution. The hair on the dog’s abdomen was clipped and the skin was aseptically prepared. The dog was placed in dorsal recumbency with tied legs. Next, a grooved guide was inserted into the rudimen-tary preputial orifice and a 12-mm incision of the outer preputial lamina was made along the long axis of the body in the cranial direction. The length of the incision was correlated with the perimeter of the penis and was sufficiently large to prevent paraphimosis (Figures 4, 5). After incision of the tissues, the inner preputial lamina was found and sewn to the skin with an absorbable polyglycolic acid surgical suture (5-0 Dexon, Vetoquinol Biowet, Gorzow Wlkp., Poland) using a Chassaignac and Halsted absorbable intradermal suture (Figure 6). Afterwards, the dog was castrated (Figure 7), and a catheter was inserted into the bladder to check urethral patency.
[image:4.595.303.534.99.224.2]Figure 7. Postoperative status
Figure 6. Posthioplasty: sewing the outer preputial lamina to the skin with a Chassaignac and Halsted intra-dermal suture
Figure 9. Metaphase spread (78,XY) from the examined dog. The arrows indicate sex chromosomes
[image:4.595.64.291.472.698.2] [image:4.595.304.528.498.724.2]Cimicoxib (Cimalgex, Vetoquinol, Lure, France) at a dose of 2 mg/kg, q 24 h for four days was pre-scribed perorally as an analgesic and amoxicillin with clavulanic acid (Synulox, Pfizer, Warsaw, Poland) at a dose of 8.75 mg/kg, q 24 h for seven days was administered intramuscularly as a protec-tive agent. The movement of the animal was limited and the sutures were protected from destruction using an Elizabethan collar. Hygienic treatment of the wound was applied three times a day using 0.5% povidone iodine (Betadine, Egis Pharmaceuticals, Budapest, Hungary) compresses until removal of the sutures, which was done after 10 days. The sur-gery yielded a distinct improvement in patient’s urination comfort. After the surgery, no urine re-tention in the preputial cavity was observed.
The pathomorphological examination of the tes-tes was carried out after the samples were fixed in 10% buffered formaldehyde, pH = 7.2, and passed through a series of increasing alcohol concentra-tions, acetone, and xylene into paraffin blocks in a tissue processor. Tissue sections with 4-μm-thickness were cut in a sled microtome and placed on slides. For histopathological analysis, the slides were stained with haematoxylin and eosin (HE) and evaluated under a light microscope. The histopatho-logical examinations of the testes showed a normal microscopic structure of the organ in the pre-sexual maturity stage of development. Histological
[image:5.595.63.291.93.322.2]analy-sis showed a predominance of elongated Sertoli cells resting on the basilar membrane; they had oval nuclei and cytoplasmic projections protruding into the tubule lumen. Among them were round seminal cells exhibiting features of spermatogonia with a spherical nucleus. The loose interlobular connective tissue comprised spherical interstitial cells with a vesicular cytoplasm (Figure 8). In the microscopic structure of the testis with a normal spermatogenesis process there are seminal cells in different developmental stages, from stem forms to mature germ cells. The histological image reveals spermatogonia, over which there are large spheri-cal meiotispheri-cally dividing first-order spermatocytes. Closer to the tubule lumen, smaller second-order spermatocytes can be seen. The smallest spherical or oval spermatids transforming into sperm are lo-cated most superficially. In the analysed case, sper-matogonia lying between the sustentacular (Sertoli) cells were the only germ cells found in the wall of convoluted tubules, which may indicate impair-ment of the spermatogenesis process caused by a low testosterone level. The macroscopic image of the epididymis showed bands of connective tissue surrounding the seminiferous tubules and efferent ducts. The wall of the tubules was formed of cylin-drical and cubic cells located on the basilar mem-brane, and the structure of the epididymal ducts comprised a double-layered epithelium composed
[image:5.595.305.537.98.325.2]Figure 11. Molecular detection (PCR-RFLP) of the ZFX/ ZFY genes in the DSD case and the controls
Case Report Veterinarni Medicina, 61, 2016 (5): 279–287
doi: 10.17221/8884-VETMED
of columnar and basal cells. Small lymphatic and blood vessels were present between these struc-tures in the connective tissue.
Further diagnostics involved cytogenetic analy-sis. Chromosome preparations were obtained from skin fibroblasts cultured in vitro using standard methods. Conventional staining with the Giemsa solution was applied to assess the normal diploid number and morphology of the chromosomes. The sex chromosomes of the dog were identified easily given their bi-armed morphology (X – large and sub-metacentric, Y – small and metacentric). The cytogenetic analysis revealed a normal male set of chromosomes (78,XY; Figure 9). The next step of the investigations was the molecular analy-sis. The presence of X-linked (ZFX) and Y-linked (SRY and ZFY) genes was detected with the use of PCR. The primer sequences and length of the
amplicons were, respectively, for the SRY gene fragment - F: 5'CTTTCCAACTTCCCTCCGTA; R: 5' GGACGTTTCGTTAGCCAGAG, 813 bp (according to Switonski et al. 2012); for the ZFX and ZFY gene fragments – F: 5' ATAATCACATG- GAGAGCCACCAGCT; R: 5' GCACTTCTTTG- GTATCTGAGAAAGT, 448 bp. The RFLP (restric-tion fragments length polymorphism) technique was applied to distinguish between the ZFX and
ZFY genes. The PCR product (448 bp) was digested using the HaeIII enzyme. The fragments obtained were 403 and 45 bp for the ZFX and 448 bp for the
ZFY gene (according to Senese et al. 1999). The
SRY gene coding sequence (663 bp) important for normal male sex development was selected for se-quence analysis and compared to the canine SRY
gene reference sequence (AF107021). Amplicons were sequenced using the same primers as above.
The molecular analysis showed the presence of the SRY gene (Figure 10) as well as the ZFY gene, which reside on the Y chromosome and ZFX, which is X-linked (Figure 11). Sequencing and comparison with the reference sequence of the canine SRY gene indicated a normal sequence in the examined dog.
DISCUSSION AND CONCLUSIONS
The case described concerns a six-month-old male dog in which we diagnosed DSD of the penis, prepuce, and preputial orifice. To the authors’ best knowledge, this is the first report of this type of developmental disorder diagnosed in a male dog.
Penile hypoplasia is usually asymptomatic and diagnosed incidentally during routine examination. Currently, there are no literature data concerning radiographic imaging of the size and structure of the os penis in dogs. The comparative measure-ments performed in this study indicated that the length of this organ in the examined patient was shorter than the total length of the first two lumbar vertebrae. In all the dogs from the compared group, the os penis was longer than the total length of the first two lumbar vertebrae.
[image:6.595.64.291.94.471.2]Investigations presented in the literature have main-ly focused on urethral abnormalities (Adelsberger and Smeak 2007; Vnuk et al. 2014).The authors of the few papers available presenting the results of penile bone radiography describe problems related to post-traumatic, inflammatory, or neoplastic lesions of
this organ (Bleier et al. 2003; Erne and McNicholas 2009). Reports in the literature data hardly ever in-clude ultrasonographic examinations of the canine penis, although the technical ease and great diag-nostic potential of this examination in evaluation of penile structure and pathological processes affecting the organ has been demonstrated (Payan-Carreira and Bessa 2008; Goericke-Pesch et al. 2013).Hence, we recommend ultrasonography to be an adequate diagnostic tool for evaluation of penile soft tissue structures in the case of physiological abnormalities. Analysis of the reported case proves that there is a need for radiological and ultrasound examinations of the penis and penile bone in dogs in order to de-termine the normal value range. Knowledge of this range would contribute to diagnosis of anatomical defects of these organs.
The most common defects of the prepuce com-prise phimosis, paraphimosis, and a short prepuce. In terms of function, the defect of the structure presented in the study resembles phimosis, which involves prepuce tightness and its inability to be retracted (England 1998).The presented case was related to hypoplasia of the prepuce skinfold and partial atrophy of the preputial orifice. Phimosis can have a congenital, traumatic, or post-inflam-matory background (Gobello et al. 2003; Feldman and Nelson 2004).In the case of the examined dog, acquired causes were excluded given the fact that the animal had been kept since birth by the same owner, who did not report any traumatic event. Therefore, a congenital factor at the developmental level was assumed to be the most probable cause. Genital malformations constitute a high propor-tion of congenital defects reported in animals and humans. More than 85% of genital anomalies in populations of domestic animals and humans are not associated with chromosomal aberrations or mutations of genes known to be responsible for sex differentiation (Basrur and Basrur 2004).The high prevalence of defects rather seems to be a result of the emergence of new mutations, genetic-envi-ronmental interactions, or epigenetic regulation.
There is extensive data available on the ab-normalities of the reproductive system in dogs (England 1998; Wierzbowski 1999; Switonski et al. 2000; Switonski et al. 2003; Switonski et al. 2004; Ndikuwera 2005; Christensen 2012).The most common penile defects include persistent frenu-lum, hypospadias, penile bone deformation, and penile hypoplasia (England 1998; Gobello et al.
2003; Feldman and Nelson 2004).Penile hypoplasia is usually one of the symptoms of testicular DSD. An abnormal karyotype was detected in some dogs with penile hypoplasia; therefore, cytogenetic analysis should be performed in similar cases (Gobello et al. 2003; Gurel et al. 2014).In the presented case, the chromosomal and gonadal sex coincided, and no female traits were detected. The successful amplifi-cation of the SRY, ZFX, and ZFY genes confirmed the presence of the X and Y chromosomes. Given the absence of polymorphisms and mutations in the coding sequence of the SRY gene, it can be assumed that this gene is not associated with the observed phenotype, as the sequence is present in dogs with normally developed external genital organs. In ac-cordance with the current nomenclature proposed by Poth et al. and Meyers-Wallen, the reported case can be classified as a testicular XY (SRY-positive) DSD (Poth et al. 2010; Meyers-Wallen 2012). It should be mentioned that phenotypic variation of sex development in XY dogs is quite wide and in-cludes cryptorchidism, hypospadias and ambiguous external genitalia. In the latter group an enlarged clitoris (Meyers-Wallen et al. 1999; Wernham and Jerram 2006; Alam et al. 2007; Nowacka-Woszuk et al. 2007; Dianovsky et al. 2013) was usually reported. In addition, a rare case manifested by lack of scrotum and penis, but with the presence of a vulva was also described (Bigliardi et al. 2011). Penile hypoplasia may also develop as a secondary disorder due to early neutering (Switonski et al. 2004).It should be noted, however, that the examined dog was young (six months) and had not been castrated, while the blood testosterone level was 0.17 ng/ml. In healthy adult dogs, the level ranges between 1 and 10 ng/ml; the wide range results from pulsed hormone release in canine males. In neutered dogs, the level of tes-tosterone declines below 0.5 ng/ml (Kaneko et al. 2008).Small- and miniature-breed dogs reach sexual maturity at the age of five to six months, therefore the testosterone level in the examined patient should be regarded as decreased (Johnston et al. 2001; Gobello 2014). The testicular inactivity diagnosed in the histological analysis explains the low level of the hormone.
Case Report Veterinarni Medicina, 61, 2016 (5): 279–287
doi: 10.17221/8884-VETMED
without obvious discomfort for the patient as re-ported by the owner. The aim of surgery was cor-rection of anatomical abnormalities that pose a risk of recurrent urinary tract infections, kidney failure, and irritation of the penis and urethra (Adelsberger and Smeak 2007; Guimaraes et al. 2013; Vnuk et al. 2014). The causes of underdevelopment of the prepuce or penis are not fully known. Therefore, assuming that developmental disorders can be po-tentially heritable (Gobello et al. 2003; Vnuk et al. 2014),it is important that individuals affected by the problem should be eliminated from reproduc-tion if fertility is suspected (Meyers-Wallen et al. 1999; Switonski et al. 2012).
The presented case of penile hypoplasia com-bined with rudimentary prepuce and accompanied by difficult urination was effectively treated with surgery. Since no familial information was avail-able, it was impossible to establish whether the disorder had a hereditary background or was an idiopathic defect.
RefeReNCeS
Adelsberger ME, Smeak DD (2007): Repair of extensive perineal hypospadias in a Boston terrier using tubularized incised plate urethroplasty. Canadian Veterinary Journal 50, 937–942.
Alam MR, Cho YG, Cho SJ, Lee JI, Lee HB, Tae HJ, Kim IS, Kim NS (2007): Male pseudohermaphroditism in dogs: three case reports. Veterinarni Medicina 52, 74–78. Basrur PK, Basrur VR (2004): Genes in genital
malforma-tions and male reproductive health. Animal Reproduction 1, 64–85.
Bigliardi E, Parma P, Peressotti P, De Lorenzi L, Wohlsein P, Passeri B, Jottini S, Cantoni AM (2011): Clinical, genetic, and pathological features of male pseudohermaphroditism in dog. Reproductive Biology and Endocrinology 9, 12. Bleier T, Lewitschek HP, Reinacher M (2003): Canine
os-teosarcoma of the penile bone. Journal of Veterinary Medicine Series A 50, 397–398.
Christensen BW (2012): Disorders of sexual development in dogs and cats. The Veterinary Clinics of North Amer-ica: Small Animal Practice 42, 515–526.
Dianovsky J, Holeckova B, Hajurka J, Sivikova K, Cigankova V (2013): Disorder of sexual development in a Yorkshire terrier (78, XY; SRY-positive). Journal of Applied Genet-ics 54, 193–199.
England GCW (1998): Abnormalities of the Reproductive Tract of the Dog. In: England GCW (ed.): Allen’s Fertility
and Obstetrics in the Dog. 1st ed. SIMA WLW, Warszawa.
115–134.
Erne JB, McNicholas WT (2009): What is your diagnosis? Urethral obstruction and fracture of os penis. Journal of the American Veterinary Medical Association 234, 201–202. Feldman EC, Nelson RW (2004): Disorders of the penis and
prepuce. In: Feldman EC, Nelson RW (eds.): Canine and Feline Endocrinology and Reproduction. 3rd ed. Elsevier
Science, St. Louis. 953–961.
Gobello C (2014): Prepubertal and pubertal canine repro-ductive studies: conflicting aspects. Reproduction in Domestic Animals 49, 70–73.
Gobello C, De Luca JC, Corrada Y, Garcia M, Peral Garcia P (2003): Penile hypoplasia in a rottweiler: a case report. Analecta Veterinaria 23, 38–41.
Goericke-Pesch S, Holscher C, Failing K, Wehrend A (2013): Functional anatomy and ultrasound examination of the canine penis. Theriogenology 80, 24–33.
Guimaraes LD, Bourguignon E, Santos LC, Duarte TS, An-drade EC, Borges APB (2013): Canine perineal hypospadias. Arquivo Brasileiro De Medicina Veterinaria E Zootecnia 65, 1647–1650.
Gurel A, Yildirim F, Sennazli G, Ozer K, Karabagli M, Deviren A, Cirakoglu A (2014): Hermaphroditism in two dogs – pathological and cytogenetic studies: a case report. Veterinarni Medicina 59, 51–55.
Johnston SD, Root Kustritz MV, Olson PN (2001): The ca-nine estrous cycle. In: Kersey R (ed.): Caca-nine and Feline Theriogenology. Saunders, Philadelphia. 16–31. Kaneko JJ, Harvey JW, Bruss ML (2008): Clinical
biochem-istry of domestic animals. In: Kaneko JJ, Harvey JW, Bruss ML (eds): Clinical Reproductive Endocrinology, Aca-demic Press. 654 pp.
Marcinkowska-Swojak M, Szczerbal I, Pausch H, Nowacka-Woszuk J, Flisikowski K, Dzimira S, Nizanski W, Payan-Carreira R, Fries R, Kozlowski P, Switonski M (2015): Copy number variation in the region harboring SOX9 gene in dogs with testicular/ovotesticular disorder of sex development (78,XX; SRY-negative). Reproductive Sci-ences 5, 14696.
Meyers-Wallen VN (2012): Gonadal and sex differentiation abnormalities of dogs and cats. Sexual Development 6, 46–60.
Meyers-Wallen VN, Schlafer D, Barr I, Lovell-Badge R, Keyzner A. (1999): Sry-negative XX sex reversal in pure-bred dogs. Molecular Reproduction and Development 53, 266–273.
Ndikuwera J (2005): A case of hypospadias in a dog. Irish Veterinary Journal 58, 504–506.
lack of the SRY and SOX9 gene mutations in a male pseu-dohermaphrodite dog. Animal Reproduction Science 98, 371–376.
Payan-Carreira R, Bessa AC (2008): Application of B-mode ultrasonography in the assessment of the dog penis. Animal Reproduction Science 106, 174–180.
Poth T, Breuer W, Walter B, Hecht W, Hermanns W (2010): Disorders of sex development in the dog – Adoption of a new nomenclature and reclassification of reported cases. Animal Reproduction Science 121, 197–207. Rossi E, Radi O, De Lorenzi L, Vetro A, Groppetti D,
Bigli-ardi E, Luvoni GC, Rota A, Camerino G, ZuffBigli-ardi O, Parma P (2014): Sox9 duplications are a relevant cause of Sry-negative XX sex reversal dogs. PLoS One 97, 101244.
Salamon S, Nowacka-Woszuk J, Szczerbal I, Dzimira S, Ni-zanski W, Ochota M, Switonski M (2014): A lack of as-sociation between polymorphisms of three positional candidate genes (CLASP2, UBP1, and FBXL2) and canine disorder of sexual development (78,XX; SRY-Negative). Sexual Development 8, 160–165.
Senese C, Penedo MC, Shiue YL, Bowlin AT, Millon LV (1999): A HaeIII PCR-RFLP in the ZFY/ZFX genes of horses. Animal Genetics 30, 390–391.
Switonski M, Godynicki S, Jackowiak H, Pienkowska A, Turczuk-Bierla I, Szymas J, Golinski P, Bereszynski A (2000): X trisomy in an infertile bitch: cytogenetic, ana-tomic, and histologic studies. Journal of Heredity 91, 149–150.
Switonski M, Szczerbal I, Grewling J, Antosik P, Nizanski W, Yang F (2003): Two cases of infertile bitches with 78,XX/77,X mosaic karyotype: a need for cytogenetic evaluation of dogs with reproductive disorders. Journal of Heredity 94, 65–68.
Switonski M, Nowacka J, Skorczyk A, Chmurzynska A, Ni-zanski W (2004): Hereditary sex-reversal syndrome
(78,XX; SRY-negative) in German Shepherd puppies. Medycyna Weterynaryjna 60, 705–707.
Switonski M, Payan-Carreira R, Bartz M, Nowacka-Woszuk J, Szczerbal I, Colaco B, Pires MA, Ochota M, Nizanski W (2012): Hypospadias in a male (78, XY; SRY-positive) dog and sex reversal female (78, XX; SRY-negative) dogs: clinical, histological and genetic studies. Sexual Develop-ment 6, 128–134.
Szczerbal I, Nowacka-Woszuk J, Nizanski W, Salamon S, Ochota M, Dzimira S, Atamaniuk W, Switonski M (2014): A case of leucocyte chimerism (78, XX/78, XY) in a dog with a disorder of sexual development. Reproduction in Domestic Animals 49, 31–34.
Vnuk D, Brkljaca Bottegaro N, Slunjski L, Skrlin B, Musulin A, Stejskal M (2014): Prepubic urethrostomy opening within a prepuce in a dog: a case report. Veterinarni Me-dicina 59, 107–111.
Wernham BG, Jerram RM (2006): Male pseudohermaph-roditism in a Labrador Retriever, and a review of mam-malian sexual differentiation. New Zealand Veterinary Journal 54, 248–252.
Wierzbowski S (1999): Congenital defects of genital organs. In: Wierzbowski S (ed.): Andrology. 1st ed. Platan, Krakow.
347–355.
Wu X, Wan S, Pujar S, Haskins ME, Schlafer DH, Lee MM, Meyers-Wallen VN (2009): A single base pair mutation encoding a premature stop codon in the MIS type II re-ceptor is responsible for canine persistent Mullerian duct syndrome. Journal of Andrology 30, 46–56.
Received: 2015–06–11 Accepted after corrections: 2016–03–09
Corresponding Author:
Brygida Slaska, University of Life Sciences in Lublin, Department of Biological Bases of Animal Production, Akademicka 13 Street, 20-950 Lublin, Poland