metal-organic papers
Acta Cryst.(2007). E63, m227–m229 doi:10.1107/S160053680605272X Shiet al. (C
39H36P2)[Mo(C2O4)O(O2)2]H2O
m227
Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
Propane-1,3-diylbis(triphenylphosphonium)
(oxalato)oxodiperoxomolybdate(VI) monohydrate
Xian-Ying Shi, Jun-Fa Wei,* Xi-Ru Zhang and Jing Cao
School of Chemistry and Materials Science, Shaanxi Normal University, Xi’an 710062, People’s Republic of China
Correspondence e-mail: weijf@snnu.edu.cn
Key indicators
Single-crystal X-ray study
T= 296 K
Mean(C–C) = 0.013 A˚
Rfactor = 0.057
wRfactor = 0.151
Data-to-parameter ratio = 13.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 24 October 2006 Accepted 6 December 2006
#2007 International Union of Crystallography All rights reserved
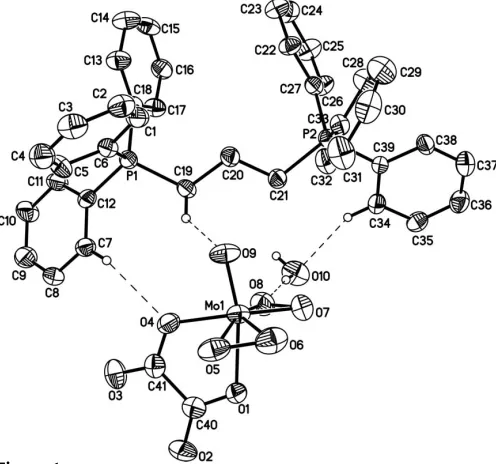
In the structure of the title compound, (C39H36P2)[Mo(C2O4 )-O(O2)2]H2O, the Mo atom in the complex anion shows a distorted pentagonal–bipyramidal geometry and the six phenyl rings in the cation form a double propeller linked by a propane group. The cations, anions and water molecules are connectedviahydrogen bonds and electrostatic interactions to form a three-dimensional extended network.
Comment
As an important class of reactive intermediates in catalytic oxidation reactions, peroxomolybdate complexes have attracted considerable attention owing to their excellent catalytic ability to activate H2O2in selective organic oxidation (Buhlet al., 2004; Du & Espenson, 2005; Xiet al., 2001; Weiet al., 2002; Shi & Wei, 2005). In this paper, we report the structure of a new bis-quaternary phosphonium perox-omolybdate complex, [Ph3P(CH2)3PPh3][MoO(O2)2(C2O4)] -H2O, (I).
Complex (I) consists of a peroxomolybdate anion, a 1,3-bis(triphenylphosphonium)propane (btppp) cation and a water molecule (Fig. 1). The six phenyl rings in the cation form a double propeller linked by a propane group. In the anion, the Mo atom is coordinated by an oxalate ligand, two peroxo groups and an oxo group. The coordinated atoms adopt a distorted pentagonal–bipyramidal geometry, in which two peroxo groups and atom O4 from the oxalate ligand form a pentagonal equatorial plane. Oxo atom O9 and the other atom O1 from the oxalate ligand occupy the axial positions. The bond lengths and angles in the anion are similar to those in the reported peroxomolybdate complex (Djordjevic & Covert, 1985). As a result of coordination of the peroxo groups to Mo, the O5—O6 and O7—O8 bond lengths are shortened from 1.48 A˚ (Shi et al., 1994) to 1.456 (10) A˚ and 1.451 (9) A˚. Compared with the peroxo ligands, weak coordination occurs between the Mo atom and oxalate, as evidenced by the bond distances of Mo—O1 = 2.213 (5) A˚ and Mo—O4 = 2.043 (6) A˚ , whereas the longest Mo—peroxo bond distance is 1.945 (7) A˚ .
complicated so that each anion is joined to four cations and
vice versa. The solvent water molecule is linked to anions and cations via three hydrogen bonds. Atom O10 of the water molecule acts as an acceptor to form a C34—H34 O10 hydrogen bond, and it donates H10Dand H10Eto atoms O2ii and O8i of the peroxo groups from two neighboring anions (Table 1). It is noteworthy that the peroxomolybdate anions form a zigzag chainviahydrogen-bonding interactions invol-ving the water molecules (Fig. 2). The cations are located between the anionic zigzag chains.
Experimental
K2[MoO(O2)2(C2O4)] (0.171 g, 0.5 mmol) was dissolved in water
After stirring for 15 min, a solution of [Ph3P(CH2)3PPh3]Br22H2O
(0.381 g, 0.5 mmol) in methanol (20 ml) was added to the mixture with stirring. The resulting mixture was allowed to stand at 278 K for several days. Yellow crystals of (I), suitable for X-ray diffraction, were obtained. Analysis, calculated for C41H38MoO10P2: C 58.03, H
4.51, O2 2
7.54, Mo 11.30%; found: C 58.31, H 4.27, O2 2
7.14, Mo 10.97%. IR(cm1):(O—O) 855,(MoO2)sym690,(MoO2)asym580,
(M O) 937.
Crystal data
(C39H36P2)[Mo(C2O4)O(O2)2]H2O
Mr= 848.59
Monoclinic,P21
a= 10.9385 (8) A˚ b= 15.6425 (12) A˚ c= 11.8100 (9) A˚
= 109.040 (1)
V= 1910.2 (2) A˚3
Z= 2
Dx= 1.475 Mg m3
MoKradiation
= 0.49 mm1
T= 296 (2) K Block, yellow 0.340.280.15 mm
Data collection
Bruker SMART APEXII CCD diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 1999) Tmin= 0.852,Tmax= 0.930
9549 measured reflections 6593 independent reflections 5391 reflections withI> 2(I) Rint= 0.031
max= 25.1
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.057
wR(F2) = 0.151 S= 1.05 6593 reflections 494 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo2) + (0.0606P)2
+ 3.2002P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.53 e A˚3
min=0.76 e A˚3
Extinction correction:SHELXL97 Extinction coefficient: 0.0132 (12) Absolute structure: Flack (1983),
[image:2.610.47.295.73.305.2]1458 Friedel pairs Flack parameter: 0.02 (5)
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O10—H10D O8i
0.86 (8) 2.06 (8) 2.916 (10) 174 (9) O10—H10E O2ii
0.86 (8) 2.32 (4) 3.131 (11) 158 (9)
C7—H7 O4i 0.93 2.60 3.484 (9) 159
C19—H19A O9i
0.97 2.36 3.159 (9) 140
C16—H16 O8ii
0.93 2.59 3.511 (11) 170
C19—H19B O2ii
0.97 2.31 3.243 (9) 161
C26—H26 O3ii 0.93 2.55 3.257 (11) 133
C34—H34 O10 0.93 2.34 3.224 (11) 158
C36—H36 O6iii
0.93 2.56 3.408 (11) 152
C1—H1 O5iv 0.93 2.55 3.306 (11) 139
C22—H22 O5iv
0.93 2.42 3.280 (10) 153
C23—H23 O9iv
0.93 2.55 3.225 (10) 130
Symmetry codes: (i)x;y1;z; (ii)xþ1;y1
2;zþ1; (iii)xþ1;y 1 2;z; (iv) xþ2;y1
2;zþ1.
H atoms bound to C atoms were positioned geometrically and refined as riding, with C—H = 0.93 A˚ (CH) and 0.97 A˚ (CH2) and Uiso(H) = 1.2Ueq(C). H atoms of the water molecule were located in a difference Fourier map and refined withUiso(H) = 0.10 A˚2.
Data collection:SMART(Bruker, 1999); cell refinement: SAINT-Plus(Bruker, 1999); data reduction:SAINT-Plus; program(s) used to solve structure:SHELXS97(Sheldrick, 1997a); program(s) used to
Figure 1
[image:2.610.47.295.353.515.2]The molecular structure of (I). Displacement ellipsoids are drawn at the 30% probability level. H atoms are omitted except those involved in hydrogen bonds (dashed lines).
Figure 2
[image:2.610.314.565.502.624.2]SHELXTL(Sheldrick, 1997b); software used to prepare material for publication:SHELXTL.
The authors are grateful to theNational Natural Science Foundation (grant No. 20572066), the Natural Science Foun-dation of Shaanxi Province (grant No. 2006B20) and the Graduate Innovation Foundation of Shaanxi Normal Univer-sity for providing financial support for this research.
References
Bruker (1999). SMART(Version 5.624),SAINT-Plus(Version 6.02A) and SADABS(Version 2.0). Bruker AXS Inc., Madison, Wisconsin, USA.
Buhl, M., Schurhammer, R. & Imhof, P. (2004).J. Am. Chem. Soc.126, 3310– 3320.
Djordjevic, C. & Covert, K. J. (1985).Inorg. Chim. Acta,101, L37–L39. Du, G. & Espenson, J. H. (2005).Inorg. Chem.44, 2465–2471. Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Sheldrick, G. M. (1997a). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Sheldrick, G. M. (1997b). SHELXTL. Version 5.10. Bruker AXS Inc., Madison, Wisconsin, USA.
Shi, X. B., Li, C. G., Wu, S. H., Chen, J. & Xie, G. Y. (1994).Chin. Sci. Bull.39, 1572.
Shi, X. Y. & Wei, J. F. (2005).J. Mol. Catal. A Chem.229, 13–17.
Wei, J. F., Shi, X. Y., He, D. P. & Zhang, M. (2002).Chin. Sci. Bull.47, 2060– 2063.
Xi, Z. W., Zhou, N., Sun, Y. & Li, K. L. (2001).Science,292, 1139–1140.
metal-organic papers
Acta Cryst.(2007). E63, m227–m229 Shiet al. (C
supporting information
Acta Cryst. (2007). E63, m227–m229 [https://doi.org/10.1107/S160053680605272X]
Propane-1,3-diylbis(triphenylphosphonium)
(oxalato)oxodiperoxomolybdate(VI) monohydrate
Xian-Ying Shi, Jun-Fa Wei, Xi-Ru Zhang and Jing Cao
Propane-1,3-diylbis(triphenylphosphonium) (oxalato)oxodiperoxomolybdate(VI) monohydrate
Crystal data
(C39H36P2)[Mo(C2O4)O(O2)2]·H2O Mr = 848.59
Monoclinic, P21
Hall symbol: P 2yb
a = 10.9385 (8) Å
b = 15.6425 (12) Å
c = 11.8100 (9) Å
β = 109.040 (1)°
V = 1910.2 (2) Å3
Z = 2
F(000) = 872.0
Dx = 1.475 Mg m−3
Mo Kα radiation, λ = 0.71073 Å
Cell parameters from 6593 reflections
θ = 1.8–25.1°
µ = 0.49 mm−1
T = 296 K
Plate, yellow
0.34 × 0.28 × 0.15 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 1999)
Tmin = 0.852, Tmax = 0.930
9549 measured reflections 6593 independent reflections 5391 reflections with I > 2σ(I)
Rint = 0.031
θmax = 25.1°, θmin = 1.8°
h = −12→13
k = −17→18
l = −14→8
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.057 wR(F2) = 0.151
S = 1.05
6593 reflections 494 parameters 3 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0606P)2 + 3.2002P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 0.53 e Å−3
Δρmin = −0.76 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.0132 (12)
Absolute structure: Flack (1983), 1458 Friedel pairs
supporting information
sup-2
Acta Cryst. (2007). E63, m227–m229
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Mo1 0.74619 (6) 1.04409 (4) 0.39693 (6) 0.0512 (2)
P1 0.85369 (18) 0.27691 (12) 0.76147 (16) 0.0443 (4)
P2 0.72354 (17) 0.39688 (12) 0.29927 (16) 0.0446 (4)
O1 0.6088 (5) 0.9357 (4) 0.3607 (5) 0.0568 (14)
O2 0.5347 (6) 0.8341 (4) 0.4553 (6) 0.0798 (19)
O3 0.6385 (6) 0.9300 (5) 0.6625 (6) 0.0842 (19)
O4 0.7246 (5) 1.0160 (3) 0.5583 (5) 0.0614 (15)
O5 0.8669 (6) 0.9494 (4) 0.4208 (7) 0.077 (2)
O6 0.8289 (7) 0.9866 (5) 0.3013 (7) 0.090 (2)
O7 0.6356 (7) 1.0981 (4) 0.2580 (6) 0.086 (2)
O8 0.5851 (6) 1.1081 (4) 0.3567 (6) 0.0742 (17)
O9 0.8485 (6) 1.1221 (4) 0.4591 (6) 0.0691 (17)
O10 0.3728 (8) 0.2304 (5) 0.3049 (8) 0.092 (2)
C1 1.0914 (8) 0.2784 (6) 0.7270 (8) 0.056 (2)
H1 1.0563 0.3205 0.6701 0.067*
C2 1.2174 (8) 0.2521 (6) 0.7480 (8) 0.064 (2)
H2 1.2664 0.2762 0.7051 0.077*
C3 1.2701 (9) 0.1904 (6) 0.8325 (9) 0.071 (2)
H3 1.3542 0.1715 0.8454 0.085*
C4 1.1988 (9) 0.1565 (6) 0.8978 (9) 0.072 (2)
H4 1.2359 0.1161 0.9568 0.087*
C5 1.0715 (8) 0.1819 (5) 0.8768 (8) 0.059 (2)
H5 1.0237 0.1583 0.9212 0.071*
C6 1.0162 (7) 0.2426 (5) 0.7897 (6) 0.0487 (17)
C7 0.7510 (7) 0.1298 (5) 0.8188 (7) 0.0525 (19)
H7 0.7651 0.1062 0.7519 0.063*
C8 0.6984 (8) 0.0806 (6) 0.8884 (7) 0.064 (2)
H8 0.6802 0.0231 0.8704 0.077*
C9 0.6731 (9) 0.1171 (7) 0.9848 (8) 0.076 (3)
H9 0.6349 0.0842 1.0297 0.092*
C10 0.7028 (10) 0.1997 (6) 1.0151 (8) 0.074 (3)
H10 0.6864 0.2228 1.0812 0.089*
C11 0.7570 (8) 0.2494 (5) 0.9484 (7) 0.063 (2)
H11 0.7768 0.3063 0.9691 0.075*
C13 0.9598 (8) 0.4349 (5) 0.8469 (8) 0.063 (2)
H13 1.0403 0.4084 0.8710 0.076*
C14 0.9494 (9) 0.5226 (5) 0.8636 (9) 0.075 (3)
H14 1.0235 0.5547 0.8998 0.090*
C15 0.8317 (9) 0.5613 (5) 0.8274 (8) 0.066 (2)
H15 0.8261 0.6198 0.8388 0.079*
C16 0.7208 (9) 0.5150 (5) 0.7742 (8) 0.061 (2)
H16 0.6406 0.5420 0.7493 0.073*
C17 0.7298 (7) 0.4288 (5) 0.7584 (7) 0.0540 (19)
H17 0.6552 0.3971 0.7232 0.065*
C18 0.8495 (7) 0.3881 (5) 0.7945 (6) 0.0474 (16)
C19 0.7579 (7) 0.2617 (5) 0.6080 (6) 0.0479 (17)
H19A 0.7657 0.2026 0.5865 0.058*
H19B 0.6679 0.2717 0.5998 0.058*
C20 0.7948 (7) 0.3201 (5) 0.5188 (6) 0.0523 (18)
H20A 0.8071 0.3780 0.5497 0.063*
H20B 0.8759 0.3007 0.5110 0.063*
C21 0.6913 (7) 0.3197 (5) 0.3967 (6) 0.0498 (17)
H21A 0.6080 0.3318 0.4058 0.060*
H21B 0.6868 0.2633 0.3613 0.060*
C22 0.8465 (7) 0.5315 (6) 0.4443 (7) 0.056 (2)
H22 0.9237 0.5023 0.4563 0.067*
C23 0.8467 (9) 0.6076 (6) 0.5033 (8) 0.066 (2)
H23 0.9239 0.6298 0.5545 0.079*
C24 0.7323 (10) 0.6506 (6) 0.4863 (9) 0.075 (3)
H24 0.7327 0.7022 0.5255 0.090*
C25 0.6174 (9) 0.6179 (6) 0.4120 (8) 0.070 (2)
H25 0.5403 0.6468 0.4023 0.084*
C26 0.6165 (6) 0.5428 (7) 0.3522 (6) 0.0576 (18)
H26 0.5387 0.5212 0.3014 0.069*
C27 0.7312 (7) 0.4981 (5) 0.3667 (7) 0.0487 (18)
C28 0.9389 (8) 0.4353 (6) 0.2361 (8) 0.064 (2)
H28 0.9113 0.4918 0.2317 0.077*
C29 1.0470 (9) 0.4150 (7) 0.2058 (9) 0.079 (3)
H29 1.0918 0.4573 0.1804 0.095*
C30 1.0877 (9) 0.3316 (8) 0.2137 (9) 0.079 (3)
H30 1.1616 0.3176 0.1952 0.095*
C31 1.0214 (10) 0.2694 (7) 0.2484 (9) 0.082 (3)
H31 1.0486 0.2130 0.2503 0.098*
C32 0.9140 (9) 0.2883 (6) 0.2808 (8) 0.060 (2)
H32 0.8711 0.2454 0.3075 0.072*
C33 0.8711 (7) 0.3728 (5) 0.2728 (6) 0.0482 (17)
C34 0.4815 (8) 0.3557 (5) 0.1431 (7) 0.058 (2)
H34 0.4677 0.3269 0.2067 0.070*
C35 0.3838 (8) 0.3580 (6) 0.0308 (8) 0.068 (2)
H35 0.3059 0.3297 0.0197 0.082*
C36 0.4031 (9) 0.4011 (6) −0.0604 (8) 0.069 (2)
supporting information
sup-4
Acta Cryst. (2007). E63, m227–m229
C37 0.5181 (9) 0.4403 (6) −0.0474 (8) 0.068 (2)
H37 0.5313 0.4679 −0.1122 0.081*
C38 0.6148 (8) 0.4389 (5) 0.0621 (6) 0.0543 (19)
H38 0.6924 0.4670 0.0713 0.065*
C39 0.5981 (6) 0.3961 (4) 0.1588 (6) 0.0416 (15)
C40 0.5950 (7) 0.9012 (6) 0.4523 (8) 0.058 (2)
C41 0.6554 (8) 0.9516 (5) 0.5693 (7) 0.057 (2)
H10D 0.439 (6) 0.197 (5) 0.322 (8) 0.100*
H10E 0.375 (9) 0.260 (6) 0.367 (6) 0.100*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Mo1 0.0506 (3) 0.0450 (3) 0.0630 (4) −0.0057 (3) 0.0255 (3) −0.0096 (3)
P1 0.0493 (11) 0.0413 (10) 0.0436 (10) 0.0002 (9) 0.0170 (9) 0.0015 (8)
P2 0.0447 (10) 0.0432 (10) 0.0442 (10) 0.0009 (8) 0.0122 (8) 0.0028 (8)
O1 0.060 (3) 0.057 (3) 0.052 (3) −0.008 (3) 0.017 (3) −0.002 (3)
O2 0.074 (4) 0.058 (4) 0.109 (5) −0.017 (3) 0.032 (4) 0.005 (3)
O3 0.092 (5) 0.105 (5) 0.068 (4) 0.005 (4) 0.042 (4) 0.020 (4)
O4 0.063 (3) 0.057 (4) 0.058 (3) −0.004 (3) 0.012 (3) −0.011 (2)
O5 0.058 (4) 0.066 (4) 0.106 (5) 0.001 (3) 0.023 (4) −0.026 (4)
O6 0.087 (5) 0.092 (5) 0.105 (6) −0.013 (4) 0.052 (4) −0.029 (4)
O7 0.108 (5) 0.082 (5) 0.066 (4) −0.009 (4) 0.026 (4) 0.012 (3)
O8 0.073 (4) 0.067 (4) 0.081 (4) 0.004 (3) 0.024 (3) 0.006 (3)
O9 0.059 (4) 0.055 (4) 0.103 (5) −0.011 (3) 0.039 (3) −0.028 (3)
O10 0.090 (5) 0.081 (5) 0.116 (6) 0.007 (4) 0.049 (5) 0.003 (4)
C1 0.054 (5) 0.050 (5) 0.062 (5) −0.005 (4) 0.016 (4) 0.000 (4)
C2 0.062 (5) 0.066 (6) 0.073 (6) −0.010 (4) 0.031 (4) −0.014 (5)
C3 0.058 (5) 0.061 (6) 0.095 (7) 0.008 (4) 0.025 (5) −0.010 (5)
C4 0.063 (5) 0.062 (6) 0.083 (7) 0.009 (4) 0.012 (5) 0.007 (5)
C5 0.059 (5) 0.048 (5) 0.072 (5) 0.008 (4) 0.025 (4) 0.017 (4)
C6 0.050 (4) 0.045 (4) 0.051 (4) −0.004 (3) 0.017 (3) 0.001 (3)
C7 0.059 (5) 0.048 (5) 0.052 (4) −0.006 (4) 0.021 (4) −0.005 (3)
C8 0.069 (5) 0.054 (5) 0.066 (5) −0.019 (4) 0.018 (4) 0.004 (4)
C9 0.084 (7) 0.085 (7) 0.068 (6) −0.030 (6) 0.034 (5) 0.001 (5)
C10 0.110 (8) 0.068 (6) 0.066 (5) −0.020 (6) 0.057 (6) −0.002 (5)
C11 0.089 (6) 0.045 (5) 0.061 (5) −0.015 (4) 0.034 (5) −0.006 (4)
C12 0.054 (4) 0.048 (4) 0.041 (4) −0.002 (3) 0.016 (3) −0.008 (3)
C13 0.055 (5) 0.049 (5) 0.083 (6) −0.001 (4) 0.019 (4) −0.007 (4)
C14 0.070 (6) 0.053 (7) 0.102 (7) −0.013 (4) 0.028 (5) −0.022 (5)
C15 0.085 (6) 0.036 (5) 0.090 (6) −0.007 (4) 0.046 (5) −0.005 (4)
C16 0.065 (5) 0.049 (5) 0.071 (5) 0.007 (4) 0.025 (4) 0.004 (4)
C17 0.048 (4) 0.043 (4) 0.071 (5) 0.005 (3) 0.020 (4) 0.000 (4)
C18 0.053 (4) 0.039 (4) 0.051 (4) −0.004 (3) 0.017 (3) 0.001 (3)
C19 0.056 (4) 0.041 (4) 0.051 (4) −0.001 (3) 0.023 (3) 0.003 (3)
C20 0.049 (4) 0.058 (5) 0.049 (4) −0.006 (4) 0.015 (3) 0.005 (3)
C21 0.060 (4) 0.046 (4) 0.045 (4) 0.002 (4) 0.019 (4) −0.001 (3)
C23 0.061 (5) 0.061 (5) 0.070 (6) −0.007 (4) 0.014 (4) −0.016 (4)
C24 0.088 (7) 0.067 (6) 0.079 (6) −0.003 (5) 0.038 (6) −0.021 (5)
C25 0.054 (5) 0.076 (6) 0.081 (6) 0.003 (4) 0.022 (5) −0.023 (5)
C26 0.045 (3) 0.059 (4) 0.067 (4) 0.006 (5) 0.016 (3) −0.020 (5)
C27 0.050 (4) 0.048 (4) 0.048 (4) −0.004 (3) 0.016 (3) 0.005 (3)
C28 0.052 (5) 0.061 (5) 0.085 (6) 0.007 (4) 0.029 (4) 0.007 (4)
C29 0.060 (5) 0.091 (8) 0.097 (8) 0.000 (5) 0.041 (5) 0.004 (6)
C30 0.058 (6) 0.113 (9) 0.067 (6) 0.021 (6) 0.022 (5) −0.004 (6)
C31 0.089 (7) 0.078 (7) 0.089 (7) 0.042 (6) 0.045 (6) 0.016 (6)
C32 0.071 (6) 0.057 (5) 0.058 (5) 0.015 (4) 0.029 (4) 0.009 (4)
C33 0.047 (4) 0.051 (4) 0.047 (4) 0.002 (3) 0.016 (3) 0.006 (3)
C34 0.065 (5) 0.066 (5) 0.043 (4) −0.011 (4) 0.017 (4) −0.001 (4)
C35 0.054 (5) 0.079 (6) 0.059 (5) −0.018 (4) 0.001 (4) −0.004 (5)
C36 0.071 (5) 0.075 (6) 0.050 (5) 0.002 (5) 0.003 (4) 0.003 (4)
C37 0.072 (6) 0.078 (6) 0.051 (5) 0.010 (5) 0.016 (4) 0.011 (4)
C38 0.056 (4) 0.058 (5) 0.047 (4) −0.005 (4) 0.013 (4) 0.011 (4)
C39 0.046 (4) 0.041 (4) 0.037 (3) −0.003 (3) 0.013 (3) 0.000 (3)
C40 0.041 (4) 0.056 (5) 0.075 (5) 0.003 (4) 0.017 (4) 0.002 (4)
C41 0.054 (5) 0.061 (5) 0.058 (5) 0.018 (4) 0.020 (4) 0.005 (4)
Geometric parameters (Å, º)
Mo1—O9 1.657 (6) C14—H14 0.9300
Mo1—O6 1.890 (7) C15—C16 1.376 (11)
Mo1—O7 1.890 (7) C15—H15 0.9300
Mo1—O5 1.943 (6) C16—C17 1.370 (10)
Mo1—O8 1.945 (7) C16—H16 0.9300
Mo1—O4 2.043 (6) C17—C18 1.392 (10)
Mo1—O1 2.213 (5) C17—H17 0.9300
P1—C12 1.775 (7) C19—C20 1.544 (9)
P1—C6 1.781 (8) C19—H19A 0.9700
P1—C18 1.787 (8) C19—H19B 0.9700
P1—C19 1.789 (7) C20—C21 1.515 (10)
P2—C27 1.762 (8) C20—H20A 0.9700
P2—C39 1.775 (6) C20—H20B 0.9700
P2—C33 1.781 (7) C21—H21A 0.9700
P2—C21 1.781 (7) C21—H21B 0.9700
O1—C40 1.262 (9) C22—C23 1.379 (12)
O2—C40 1.246 (10) C22—C27 1.396 (10)
O3—C41 1.221 (9) C22—H22 0.9300
O4—C41 1.293 (10) C23—C24 1.377 (12)
O5—O6 1.456 (10) C23—H23 0.9300
O7—O8 1.451 (9) C24—C25 1.376 (12)
O10—H10D 0.86 (8) C24—H24 0.9300
O10—H10E 0.86 (8) C25—C26 1.369 (12)
C1—C2 1.380 (12) C25—H25 0.9300
C1—C6 1.392 (11) C26—C27 1.396 (10)
supporting information
sup-6
Acta Cryst. (2007). E63, m227–m229
C2—C3 1.373 (13) C28—C33 1.380 (11)
C2—H2 0.9300 C28—C29 1.380 (11)
C3—C4 1.369 (13) C28—H28 0.9300
C3—H3 0.9300 C29—C30 1.371 (14)
C4—C5 1.390 (12) C29—H29 0.9300
C4—H4 0.9300 C30—C31 1.354 (15)
C5—C6 1.385 (10) C30—H30 0.9300
C5—H5 0.9300 C31—C32 1.381 (12)
C7—C8 1.380 (10) C31—H31 0.9300
C7—C12 1.392 (10) C32—C33 1.396 (11)
C7—H7 0.9300 C32—H32 0.9300
C8—C9 1.380 (12) C34—C39 1.380 (10)
C8—H8 0.9300 C34—C35 1.406 (11)
C9—C10 1.352 (12) C34—H34 0.9300
C9—H9 0.9300 C35—C36 1.345 (12)
C10—C11 1.372 (11) C35—H35 0.9300
C10—H10 0.9300 C36—C37 1.363 (12)
C11—C12 1.398 (10) C36—H36 0.9300
C11—H11 0.9300 C37—C38 1.378 (11)
C13—C18 1.373 (10) C37—H37 0.9300
C13—C14 1.396 (11) C38—C39 1.386 (9)
C13—H13 0.9300 C38—H38 0.9300
C14—C15 1.360 (12) C40—C41 1.540 (12)
O9—Mo1—O6 103.3 (3) C17—C16—H16 120.4
O9—Mo1—O7 101.8 (3) C15—C16—H16 120.4
O6—Mo1—O7 89.4 (4) C16—C17—C18 120.7 (8)
O9—Mo1—O5 99.7 (3) C16—C17—H17 119.6
O6—Mo1—O5 44.6 (3) C18—C17—H17 119.6
O7—Mo1—O5 132.7 (3) C13—C18—C17 119.7 (7)
O9—Mo1—O8 99.0 (3) C13—C18—P1 122.4 (6)
O6—Mo1—O8 132.1 (3) C17—C18—P1 117.8 (6)
O7—Mo1—O8 44.4 (3) C20—C19—P1 114.5 (5)
O5—Mo1—O8 161.1 (3) C20—C19—H19A 108.6
O9—Mo1—O4 90.7 (3) P1—C19—H19A 108.6
O6—Mo1—O4 132.7 (3) C20—C19—H19B 108.6
O7—Mo1—O4 132.0 (3) P1—C19—H19B 108.6
O5—Mo1—O4 88.9 (3) H19A—C19—H19B 107.6
O8—Mo1—O4 88.1 (3) C21—C20—C19 111.5 (6)
O9—Mo1—O1 165.5 (3) C21—C20—H20A 109.3
O6—Mo1—O1 86.9 (3) C19—C20—H20A 109.3
O7—Mo1—O1 88.4 (3) C21—C20—H20B 109.3
O5—Mo1—O1 80.2 (3) C19—C20—H20B 109.3
O8—Mo1—O1 81.0 (3) H20A—C20—H20B 108.0
O4—Mo1—O1 74.8 (2) C20—C21—P2 111.6 (5)
C12—P1—C6 109.0 (3) C20—C21—H21A 109.3
C12—P1—C18 111.0 (3) P2—C21—H21A 109.3
C12—P1—C19 107.2 (3) P2—C21—H21B 109.3
C6—P1—C19 111.6 (3) H21A—C21—H21B 108.0
C18—P1—C19 107.6 (3) C23—C22—C27 120.5 (8)
C27—P2—C39 109.7 (3) C23—C22—H22 119.8
C27—P2—C33 111.0 (4) C27—C22—H22 119.8
C39—P2—C33 107.7 (3) C24—C23—C22 119.7 (8)
C27—P2—C21 107.9 (3) C24—C23—H23 120.1
C39—P2—C21 110.1 (3) C22—C23—H23 120.1
C33—P2—C21 110.6 (4) C25—C24—C23 120.6 (9)
C40—O1—Mo1 115.2 (5) C25—C24—H24 119.7
C41—O4—Mo1 120.8 (5) C23—C24—H24 119.7
O6—O5—Mo1 65.7 (4) C26—C25—C24 119.9 (8)
O5—O6—Mo1 69.6 (4) C26—C25—H25 120.0
O8—O7—Mo1 69.8 (4) C24—C25—H25 120.0
O7—O8—Mo1 65.8 (4) C25—C26—C27 120.8 (7)
H10D—O10—H10E 109 (10) C25—C26—H26 119.6
C2—C1—C6 120.9 (8) C27—C26—H26 119.6
C2—C1—H1 119.6 C26—C27—C22 118.4 (8)
C6—C1—H1 119.6 C26—C27—P2 119.1 (6)
C3—C2—C1 119.9 (8) C22—C27—P2 122.2 (6)
C3—C2—H2 120.1 C33—C28—C29 120.8 (9)
C1—C2—H2 120.1 C33—C28—H28 119.6
C4—C3—C2 119.9 (8) C29—C28—H28 119.6
C4—C3—H3 120.0 C30—C29—C28 119.2 (10)
C2—C3—H3 120.0 C30—C29—H29 120.4
C3—C4—C5 120.7 (8) C28—C29—H29 120.4
C3—C4—H4 119.6 C31—C30—C29 120.7 (9)
C5—C4—H4 119.6 C31—C30—H30 119.7
C6—C5—C4 119.8 (8) C29—C30—H30 119.7
C6—C5—H5 120.1 C30—C31—C32 121.2 (10)
C4—C5—H5 120.1 C30—C31—H31 119.4
C5—C6—C1 118.7 (7) C32—C31—H31 119.4
C5—C6—P1 120.9 (6) C31—C32—C33 118.8 (9)
C1—C6—P1 120.4 (6) C31—C32—H32 120.6
C8—C7—C12 119.7 (7) C33—C32—H32 120.6
C8—C7—H7 120.1 C28—C33—C32 119.3 (7)
C12—C7—H7 120.1 C28—C33—P2 121.0 (6)
C7—C8—C9 119.7 (8) C32—C33—P2 119.5 (6)
C7—C8—H8 120.2 C39—C34—C35 120.0 (7)
C9—C8—H8 120.2 C39—C34—H34 120.0
C10—C9—C8 121.2 (8) C35—C34—H34 120.0
C10—C9—H9 119.4 C36—C35—C34 119.9 (8)
C8—C9—H9 119.4 C36—C35—H35 120.1
C9—C10—C11 120.1 (8) C34—C35—H35 120.1
C9—C10—H10 119.9 C35—C36—C37 121.1 (8)
C11—C10—H10 119.9 C35—C36—H36 119.5
C10—C11—C12 120.2 (8) C37—C36—H36 119.5
supporting information
sup-8
Acta Cryst. (2007). E63, m227–m229
C12—C11—H11 119.9 C36—C37—H37 120.1
C7—C12—C11 119.1 (7) C38—C37—H37 120.1
C7—C12—P1 119.4 (5) C37—C38—C39 120.9 (8)
C11—C12—P1 121.5 (6) C37—C38—H38 119.5
C18—C13—C14 119.1 (8) C39—C38—H38 119.5
C18—C13—H13 120.4 C34—C39—C38 118.3 (6)
C14—C13—H13 120.4 C34—C39—P2 121.8 (5)
C15—C14—C13 120.4 (8) C38—C39—P2 119.8 (5)
C15—C14—H14 119.8 O2—C40—O1 126.7 (8)
C13—C14—H14 119.8 O2—C40—C41 118.8 (8)
C14—C15—C16 120.8 (8) O1—C40—C41 114.5 (7)
C14—C15—H15 119.6 O3—C41—O4 124.9 (8)
C16—C15—H15 119.6 O3—C41—C40 121.3 (8)
C17—C16—C15 119.2 (8) O4—C41—C40 113.7 (7)
O9—Mo1—O1—C40 −8.2 (14) C16—C17—C18—C13 0.3 (12)
O6—Mo1—O1—C40 127.2 (6) C16—C17—C18—P1 −175.9 (6)
O7—Mo1—O1—C40 −143.3 (6) C12—P1—C18—C13 114.2 (7)
O5—Mo1—O1—C40 82.8 (6) C6—P1—C18—C13 −6.8 (8)
O8—Mo1—O1—C40 −99.2 (6) C19—P1—C18—C13 −128.8 (7)
O4—Mo1—O1—C40 −8.7 (5) C12—P1—C18—C17 −69.8 (7)
O9—Mo1—O4—C41 −175.8 (6) C6—P1—C18—C17 169.3 (6)
O6—Mo1—O4—C41 −66.9 (7) C19—P1—C18—C17 47.3 (7)
O7—Mo1—O4—C41 77.6 (6) C12—P1—C19—C20 172.5 (5)
O5—Mo1—O4—C41 −76.1 (6) C6—P1—C19—C20 −68.2 (6)
O8—Mo1—O4—C41 85.3 (6) C18—P1—C19—C20 53.0 (6)
O1—Mo1—O4—C41 4.1 (5) P1—C19—C20—C21 −166.0 (5)
O9—Mo1—O5—O6 −99.1 (4) C19—C20—C21—P2 172.6 (5)
O7—Mo1—O5—O6 17.0 (6) C27—P2—C21—C20 −58.9 (6)
O8—Mo1—O5—O6 89.4 (10) C39—P2—C21—C20 −178.5 (5)
O4—Mo1—O5—O6 170.4 (4) C33—P2—C21—C20 62.6 (6)
O1—Mo1—O5—O6 95.6 (4) C27—C22—C23—C24 0.5 (13)
O9—Mo1—O6—O5 90.4 (4) C22—C23—C24—C25 0.7 (15)
O7—Mo1—O6—O5 −167.6 (4) C23—C24—C25—C26 −1.3 (15)
O8—Mo1—O6—O5 −154.1 (4) C24—C25—C26—C27 0.7 (15)
O4—Mo1—O6—O5 −13.2 (5) C25—C26—C27—C22 0.5 (13)
O1—Mo1—O6—O5 −79.2 (4) C25—C26—C27—P2 174.1 (7)
O9—Mo1—O7—O8 −90.9 (4) C23—C22—C27—C26 −1.1 (12)
O6—Mo1—O7—O8 165.7 (4) C23—C22—C27—P2 −174.4 (7)
O5—Mo1—O7—O8 153.8 (4) C39—P2—C27—C26 36.1 (7)
O4—Mo1—O7—O8 10.9 (5) C33—P2—C27—C26 154.9 (6)
O1—Mo1—O7—O8 78.7 (4) C21—P2—C27—C26 −83.8 (7)
O9—Mo1—O8—O7 97.7 (4) C39—P2—C27—C22 −150.6 (6)
O6—Mo1—O8—O7 −19.5 (6) C33—P2—C27—C22 −31.7 (7)
O5—Mo1—O8—O7 −90.8 (10) C21—P2—C27—C22 89.5 (7)
O4—Mo1—O8—O7 −171.9 (4) C33—C28—C29—C30 −0.7 (15)
O1—Mo1—O8—O7 −97.0 (4) C28—C29—C30—C31 1.4 (16)
C1—C2—C3—C4 −1.7 (13) C30—C31—C32—C33 2.6 (15)
C2—C3—C4—C5 2.2 (14) C29—C28—C33—C32 0.9 (13)
C3—C4—C5—C6 −0.5 (14) C29—C28—C33—P2 −174.4 (7)
C4—C5—C6—C1 −1.6 (13) C31—C32—C33—C28 −1.8 (13)
C4—C5—C6—P1 −179.6 (7) C31—C32—C33—P2 173.5 (7)
C2—C1—C6—C5 2.1 (13) C27—P2—C33—C28 −38.1 (8)
C2—C1—C6—P1 −179.9 (6) C39—P2—C33—C28 81.9 (7)
C12—P1—C6—C5 −5.4 (8) C21—P2—C33—C28 −157.8 (7)
C18—P1—C6—C5 116.8 (7) C27—P2—C33—C32 146.7 (6)
C19—P1—C6—C5 −123.6 (7) C39—P2—C33—C32 −93.3 (7)
C12—P1—C6—C1 176.7 (6) C21—P2—C33—C32 27.0 (8)
C18—P1—C6—C1 −61.1 (7) C39—C34—C35—C36 −1.3 (14)
C19—P1—C6—C1 58.4 (8) C34—C35—C36—C37 2.4 (15)
C12—C7—C8—C9 2.6 (13) C35—C36—C37—C38 −2.6 (15)
C7—C8—C9—C10 −2.3 (15) C36—C37—C38—C39 1.7 (13)
C8—C9—C10—C11 1.2 (17) C35—C34—C39—C38 0.5 (12)
C9—C10—C11—C12 −0.5 (15) C35—C34—C39—P2 178.8 (7)
C8—C7—C12—C11 −1.9 (12) C37—C38—C39—C34 −0.6 (12)
C8—C7—C12—P1 178.6 (6) C37—C38—C39—P2 −179.0 (6)
C10—C11—C12—C7 0.8 (12) C27—P2—C39—C34 −104.5 (7)
C10—C11—C12—P1 −179.6 (7) C33—P2—C39—C34 134.6 (6)
C6—P1—C12—C7 −72.3 (7) C21—P2—C39—C34 14.0 (7)
C18—P1—C12—C7 165.9 (6) C27—P2—C39—C38 73.8 (7)
C19—P1—C12—C7 48.7 (7) C33—P2—C39—C38 −47.0 (7)
C6—P1—C12—C11 108.1 (7) C21—P2—C39—C38 −167.6 (6)
C18—P1—C12—C11 −13.6 (8) Mo1—O1—C40—O2 −171.5 (7)
C19—P1—C12—C11 −130.9 (7) Mo1—O1—C40—C41 11.2 (8)
C18—C13—C14—C15 −0.6 (14) Mo1—O4—C41—O3 179.3 (6)
C13—C14—C15—C16 0.3 (14) Mo1—O4—C41—C40 0.2 (8)
C14—C15—C16—C17 0.3 (13) O2—C40—C41—O3 −4.6 (12)
C15—C16—C17—C18 −0.6 (13) O1—C40—C41—O3 172.8 (7)
C14—C13—C18—C17 0.3 (13) O2—C40—C41—O4 174.6 (7)
C14—C13—C18—P1 176.3 (7) O1—C40—C41—O4 −8.0 (10)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O10—H10D···O8i 0.86 (8) 2.06 (8) 2.916 (10) 174 (9)
O10—H10E···O2ii 0.86 (8) 2.32 (4) 3.131 (11) 158 (9)
C7—H7···O4i 0.93 2.60 3.484 (9) 159
C19—H19A···O9i 0.97 2.36 3.159 (9) 140
C16—H16···O8ii 0.93 2.59 3.511 (11) 170
C19—H19B···O2ii 0.97 2.31 3.243 (9) 161
C26—H26···O3ii 0.93 2.55 3.257 (11) 133
C34—H34···O10 0.93 2.34 3.224 (11) 158
C36—H36···O6iii 0.93 2.56 3.408 (11) 152
supporting information
sup-10
Acta Cryst. (2007). E63, m227–m229
C22—H22···O5iv 0.93 2.42 3.280 (10) 153
C23—H23···O9iv 0.93 2.55 3.225 (10) 130