organic papers
o3506
Yanet al. C22H29NO doi:10.1107/S1600536806021763 Acta Cryst.(2006). E62, o3506–o3507
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
3a,5b-Dimethyl-2,3,3a,4,5,5a,5b,6,7,13,13a,13b-dodecahydro-1
H
-8-azacyclopenta[
a
]chrysen-3-ol
Ji-Zhong Yan, Jian Li and Guo-Wu Rao*
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, People’s Republic of China
Correspondence e-mail: rgw@zjut.edu.cn
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.039
wRfactor = 0.112
Data-to-parameter ratio = 10.7
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 1 June 2006 Accepted 8 June 2006
#2006 International Union of Crystallography
All rights reserved
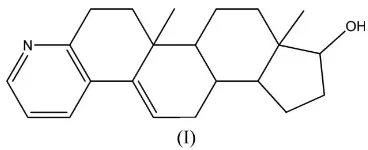
The title compound, C22H29NO, is built up from five fused
rings, four of which are six-membered and one five-membered. The ring adjacent to the five-membered ring adopts a chair conformation.
Comment
Testosterone derivatives have a high biological activity and have been widely used in the preparation of hormone-based drugs (Alvarez-Ginarteet al., 2005). As part of our continuing interest in the structure–activity relationship of testosterone derivatives, we have isolated the product, (I), from the reac-tion of propargylamine and 17-hydroxy-10,13-dimethyl-1,7,8,10,11,12,13,15,16,17-decahydro-2H-cyclopenta[a ]phen-anthren-3(4H,9H,14H)-one.
The molecular structure of (I) is illustrated in Fig. 1. It is built up from five fused rings, four of which are six-membered and one five-membered. The C4/C5/C17–C20 ring fused with the five-membered ring adopts a chair conformation (Cremer & Pople, 1975). The other rings have no obvious conforma-tions. There is an O—H N hydrogen-bond interaction, building a zigzag chain parallel to the a axis (Table 1 and Fig. 2).
Experimental
The title compound was prepared according to the procedure of Yan
et al. (2006). Crystals were obtained by slow evaporation of a methanol solution at room temperature.
Crystal data
C22H29NO
Mr= 323.46
Orthorhombic,P212121
a= 8.629 (2) A˚
b= 13.664 (5) A˚
c= 15.158 (4) A˚
V= 1787.2 (9) A˚3
Z= 4
Dx= 1.202 Mg m
3
MoKradiation
= 0.07 mm1
T= 298 (2) K Chunk, colorless 0.390.280.19 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
!scans
Absorption correction: none 17581 measured reflections
2326 independent reflections 1958 reflections withI> 2(I)
Rint= 0.031
[image:1.610.239.423.343.418.2]Refinement
Refinement onF2 R[F2> 2(F2)] = 0.039
wR(F2) = 0.113
S= 1.07 2326 reflections 218 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.074P)2
+ 0.0296P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001 max= 0.17 e A˚
3 min=0.14 e A˚
3
Extinction correction:SHELXL97
Extinction coefficient: 0.0045 (18)
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O1—H1 N1i
0.82 2.04 2.862 (2) 178
Symmetry code: (i)xþ1
2;yþ1;zþ 1 2.
H atoms were included in calculated positions and refined using a riding model, withUiso(H) = 1.2 (or 1.5 for methyl H atoms) times
Ueq(C). C—H distances were constrained to 0.96 A˚ for methyl H atoms, 0.97 A˚ for methylene H atoms, 0.98 A˚ for methine H atoms, and 0.93 A˚ for the remainder, and O—H = 0.82 A˚. In the absence of significant anomalous scattering, Friedel pairs were averaged, and the absolute configuration assigned arbitrarily.
Data collection: PROCESS-AUTO (Rigaku, 1998); cell refine-ment:PROCESS-AUTO; data reduction:CrystalStructure (Rigaku/ MSC, 2004); program(s) used to solve structure:SHELXS97 (Shel-drick, 1997); program(s) used to refine structure: SHELXL97
(Sheldrick, 1997); molecular graphics: ORTEP-3 for Windows
(Version 1.05; Farrugia, 1997); software used to prepare material for publication:WinGX(Farrugia, 1999).
We are grateful to Professor Jian-Ming Gu of the Center of Analysis & Measurement of Zhejiang University for help with the X-ray diffraction experiment.
References
Alvarez-Ginarte, Y. M., Crespo, R., Montero-Cabrera, L. A., Ruiz-Garcia, J. A., Ponce, Y. M., Santana, R., Pardillo-Fontdevila, E. & Alonso-Becerra, E. (2005).QSAR Combin. Sci.24, 218–226.
Cremer, D. & Pople, J. A. (1975).J. Am. Chem. Soc.97, 1354–1358. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Rigaku (1998).PROCESS-AUTO. Rigaku Corporation, Tokyo, Japan. Rigaku/MSC (2004). CrystalStructure. Version 3.6.0. Rigaku/MSC, The
Woodlands, Texas, USA.
Sheldrick, G. M. (1997). SHELXL97 and SHELXS97. University of Go¨ttingen, Germany.
[image:2.610.48.297.73.187.2]Yan, J.-Z., Li, J. & Rao, G.-W. (2006).Acta Cryst.E62, o2773–o2774.
Figure 1
[image:2.610.315.561.78.331.2]The structure of (I), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2
View showing the O—H N hydrogen bonding (dashed lines) and the formation of the zigzag chain. For clarity, only H atoms involved in hydrogen bonding are shown. [Symmetry code: (i)x+1
supporting information
sup-1 Acta Cryst. (2006). E62, o3506–o3507
supporting information
Acta Cryst. (2006). E62, o3506–o3507 [https://doi.org/10.1107/S1600536806021763]
3a,5b-Dimethyl-2,3,3a,4,5,5a,5b,6,7,13,13a,13b-dodecahydro-1
H
-8-azacyclo-penta[
a
]chrysen-3-ol
Ji-Zhong Yan, Jian Li and Guo-Wu Rao
3a,5 b-Dimethyl-2,3,3a,4,5,5a,5 b,6,7,13,13a,13b-dodecahydro- 1H-8-azacyclopenta[a]chrysen-3-ol
Crystal data
C22H29NO
Mr = 323.46
Orthorhombic, P212121 Hall symbol: P 2ac 2ab
a = 8.629 (2) Å
b = 13.664 (5) Å
c = 15.158 (4) Å
V = 1787.2 (9) Å3
Z = 4
F(000) = 704
Dx = 1.202 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 14394 reflections
θ = 3.1–27.6°
µ = 0.07 mm−1
T = 298 K Chunk, colorless 0.39 × 0.28 × 0.19 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
17581 measured reflections 2326 independent reflections
1958 reflections with I > 2σ(I)
Rint = 0.031
θmax = 27.5°, θmin = 3.1°
h = −11→10
k = −17→17
l = −19→19
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.113
S = 1.07 2326 reflections 218 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.074P)2 + 0.0296P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.17 e Å−3 Δρmin = −0.14 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
C9 0.1676 (2) 0.27327 (14) 0.34204 (11) 0.0445 (4)
N1 0.4125 (2) 0.20557 (12) 0.29556 (11) 0.0530 (4)
C17 0.0807 (2) 0.47017 (13) 0.51837 (11) 0.0408 (4)
H17 0.0714 0.4182 0.5626 0.049*
C8 0.0804 (2) 0.34584 (14) 0.39522 (12) 0.0450 (4)
C16 0.1743 (2) 0.42659 (13) 0.44047 (11) 0.0414 (4)
C13 0.3289 (2) 0.26753 (14) 0.34511 (12) 0.0462 (4)
C4 −0.1694 (2) 0.54042 (14) 0.57055 (12) 0.0440 (4)
H4 −0.1638 0.4899 0.6163 0.053*
C12 0.3368 (3) 0.14424 (16) 0.24245 (13) 0.0566 (5)
H12 0.3940 0.1014 0.2076 0.068*
C5 −0.08533 (19) 0.49838 (13) 0.49161 (11) 0.0423 (4)
H5 −0.0798 0.5488 0.4457 0.051*
O1 −0.20838 (17) 0.74994 (11) 0.72028 (10) 0.0630 (4)
H1 −0.1233 0.7612 0.7421 0.095*
C10 0.0935 (3) 0.20673 (17) 0.28549 (13) 0.0573 (5)
H10 −0.0140 0.2068 0.2810 0.069*
C6 −0.1711 (2) 0.41027 (18) 0.45463 (14) 0.0567 (5)
H6A −0.2139 0.3730 0.5034 0.068*
H6B −0.2572 0.4334 0.4191 0.068*
C1 −0.2098 (2) 0.65400 (15) 0.68498 (13) 0.0504 (5)
H1A −0.1938 0.6072 0.7331 0.060*
C20 −0.0934 (2) 0.63134 (14) 0.61063 (12) 0.0447 (4)
C19 0.0696 (2) 0.60122 (16) 0.63991 (15) 0.0565 (5)
H19A 0.0618 0.5541 0.6875 0.068*
H19B 0.1241 0.6582 0.6622 0.068*
C15 0.3222 (2) 0.37902 (16) 0.47660 (15) 0.0555 (5)
H15A 0.3832 0.4283 0.5068 0.067*
H15B 0.2939 0.3294 0.5194 0.067*
C11 0.1789 (3) 0.14169 (18) 0.23703 (14) 0.0630 (6)
H11 0.1300 0.0963 0.2008 0.076*
C18 0.1625 (2) 0.55627 (16) 0.56388 (15) 0.0579 (5)
H18A 0.2615 0.5339 0.5864 0.069*
supporting information
sup-3 Acta Cryst. (2006). E62, o3506–o3507
H14A 0.4693 0.3836 0.3704 0.071*
H14B 0.5023 0.2942 0.4325 0.071*
C7 −0.0743 (2) 0.34402 (17) 0.39993 (13) 0.0536 (5)
H7 −0.1255 0.2974 0.3661 0.064*
C21 0.2163 (3) 0.50424 (17) 0.37148 (15) 0.0613 (6)
H21A 0.2758 0.5552 0.3989 0.092*
H21B 0.1232 0.5315 0.3471 0.092*
H21C 0.2762 0.4746 0.3253 0.092*
C3 −0.3408 (2) 0.5679 (2) 0.56358 (16) 0.0680 (6)
H3A −0.3617 0.6022 0.5088 0.082*
H3B −0.4060 0.5102 0.5665 0.082*
C2 −0.3686 (2) 0.6345 (2) 0.64291 (16) 0.0674 (7)
H2A −0.4370 0.6028 0.6849 0.081*
H2B −0.4160 0.6954 0.6242 0.081*
C22 −0.0873 (4) 0.71779 (16) 0.54672 (15) 0.0700 (7)
H22A −0.0396 0.7728 0.5753 0.105*
H22B −0.1907 0.7349 0.5291 0.105*
H22C −0.0279 0.7000 0.4956 0.105*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C9 0.0461 (10) 0.0456 (10) 0.0420 (8) −0.0059 (8) −0.0091 (8) 0.0029 (8)
N1 0.0551 (10) 0.0469 (9) 0.0571 (9) 0.0014 (8) −0.0029 (8) 0.0009 (7)
C17 0.0365 (8) 0.0416 (9) 0.0443 (8) −0.0043 (7) −0.0072 (8) 0.0032 (7)
C8 0.0422 (9) 0.0480 (10) 0.0449 (9) −0.0071 (8) −0.0103 (8) 0.0027 (8)
C16 0.0330 (7) 0.0418 (9) 0.0494 (9) −0.0043 (7) −0.0052 (8) 0.0009 (8)
C13 0.0467 (10) 0.0421 (10) 0.0497 (9) −0.0032 (8) −0.0058 (9) 0.0031 (8)
C4 0.0353 (8) 0.0509 (11) 0.0458 (9) −0.0023 (8) −0.0031 (8) 0.0070 (8)
C12 0.0688 (13) 0.0489 (11) 0.0523 (10) 0.0017 (10) −0.0020 (11) −0.0007 (9)
C5 0.0353 (8) 0.0491 (10) 0.0424 (8) −0.0033 (7) −0.0053 (7) 0.0083 (8)
O1 0.0480 (8) 0.0605 (9) 0.0806 (9) 0.0084 (7) 0.0044 (8) −0.0093 (8)
C10 0.0550 (11) 0.0643 (13) 0.0528 (10) −0.0078 (10) −0.0144 (10) −0.0067 (10)
C6 0.0381 (9) 0.0740 (14) 0.0579 (11) −0.0111 (10) −0.0061 (9) −0.0079 (10)
C1 0.0427 (9) 0.0553 (12) 0.0532 (10) 0.0022 (8) 0.0011 (8) 0.0028 (9)
C20 0.0379 (8) 0.0451 (10) 0.0511 (9) −0.0013 (8) 0.0010 (8) 0.0073 (8)
C19 0.0399 (9) 0.0603 (12) 0.0693 (12) 0.0006 (9) −0.0115 (10) −0.0170 (10)
C15 0.0402 (9) 0.0564 (12) 0.0700 (12) 0.0014 (9) −0.0202 (10) −0.0140 (10)
C11 0.0689 (13) 0.0647 (13) 0.0553 (11) −0.0050 (12) −0.0122 (11) −0.0153 (10) C18 0.0335 (8) 0.0614 (12) 0.0788 (13) −0.0061 (9) −0.0048 (10) −0.0193 (11)
C14 0.0379 (9) 0.0592 (12) 0.0812 (15) 0.0000 (9) −0.0103 (10) −0.0167 (11)
C7 0.0445 (10) 0.0644 (13) 0.0519 (10) −0.0162 (9) −0.0061 (9) −0.0083 (10)
C21 0.0588 (12) 0.0544 (12) 0.0708 (12) −0.0024 (10) 0.0166 (11) 0.0089 (10)
C3 0.0375 (9) 0.0932 (18) 0.0732 (14) 0.0025 (11) −0.0078 (10) −0.0121 (13)
C2 0.0394 (10) 0.0920 (18) 0.0709 (14) 0.0031 (11) 0.0035 (10) −0.0071 (13)
Geometric parameters (Å, º)
C9—C13 1.395 (3) C1—C2 1.535 (3)
C9—C10 1.404 (3) C1—C20 1.541 (3)
C9—C8 1.483 (3) C1—H1A 0.9800
N1—C12 1.333 (3) C20—C22 1.529 (3)
N1—C13 1.342 (3) C20—C19 1.532 (3)
C17—C18 1.536 (2) C19—C18 1.532 (3)
C17—C5 1.538 (2) C19—H19A 0.9700
C17—C16 1.549 (3) C19—H19B 0.9700
C17—H17 0.9800 C15—C14 1.518 (3)
C8—C7 1.336 (3) C15—H15A 0.9700
C8—C16 1.531 (2) C15—H15B 0.9700
C16—C21 1.533 (3) C11—H11 0.9300
C16—C15 1.534 (3) C18—H18A 0.9700
C13—C14 1.496 (3) C18—H18B 0.9700
C4—C5 1.513 (3) C14—H14A 0.9700
C4—C3 1.530 (3) C14—H14B 0.9700
C4—C20 1.530 (3) C7—H7 0.9300
C4—H4 0.9800 C21—H21A 0.9600
C12—C11 1.365 (3) C21—H21B 0.9600
C12—H12 0.9300 C21—H21C 0.9600
C5—C6 1.520 (3) C3—C2 1.526 (3)
C5—H5 0.9800 C3—H3A 0.9700
O1—C1 1.416 (3) C3—H3B 0.9700
O1—H1 0.8200 C2—H2A 0.9700
C10—C11 1.369 (3) C2—H2B 0.9700
C10—H10 0.9300 C22—H22A 0.9600
C6—C7 1.485 (3) C22—H22B 0.9600
C6—H6A 0.9700 C22—H22C 0.9600
C6—H6B 0.9700
C13—C9—C10 116.0 (2) C4—C20—C1 100.04 (14)
C13—C9—C8 121.73 (17) C19—C20—C1 116.15 (17)
C10—C9—C8 122.26 (18) C20—C19—C18 111.72 (17)
C12—N1—C13 118.09 (19) C20—C19—H19A 109.3
C18—C17—C5 110.76 (15) C18—C19—H19A 109.3
C18—C17—C16 113.41 (15) C20—C19—H19B 109.3
C5—C17—C16 112.38 (14) C18—C19—H19B 109.3
C18—C17—H17 106.6 H19A—C19—H19B 107.9
C5—C17—H17 106.6 C14—C15—C16 112.90 (17)
C16—C17—H17 106.6 C14—C15—H15A 109.0
C7—C8—C9 121.56 (18) C16—C15—H15A 109.0
C7—C8—C16 121.18 (18) C14—C15—H15B 109.0
C9—C8—C16 117.18 (15) C16—C15—H15B 109.0
C8—C16—C21 108.56 (15) H15A—C15—H15B 107.8
supporting information
sup-5 Acta Cryst. (2006). E62, o3506–o3507
C8—C16—C17 110.04 (14) C10—C11—H11 120.4
C21—C16—C17 112.16 (15) C19—C18—C17 113.88 (16)
C15—C16—C17 108.94 (14) C19—C18—H18A 108.8
N1—C13—C9 123.59 (18) C17—C18—H18A 108.8
N1—C13—C14 115.40 (18) C19—C18—H18B 108.8
C9—C13—C14 121.00 (18) C17—C18—H18B 108.8
C5—C4—C3 120.15 (18) H18A—C18—H18B 107.7
C5—C4—C20 114.64 (15) C13—C14—C15 112.72 (17)
C3—C4—C20 104.01 (18) C13—C14—H14A 109.0
C5—C4—H4 105.6 C15—C14—H14A 109.0
C3—C4—H4 105.6 C13—C14—H14B 109.0
C20—C4—H4 105.6 C15—C14—H14B 109.0
N1—C12—C11 122.8 (2) H14A—C14—H14B 107.8
N1—C12—H12 118.6 C8—C7—C6 125.48 (18)
C11—C12—H12 118.6 C8—C7—H7 117.3
C4—C5—C6 111.03 (15) C6—C7—H7 117.3
C4—C5—C17 109.47 (14) C16—C21—H21A 109.5
C6—C5—C17 110.63 (16) C16—C21—H21B 109.5
C4—C5—H5 108.5 H21A—C21—H21B 109.5
C6—C5—H5 108.5 C16—C21—H21C 109.5
C17—C5—H5 108.5 H21A—C21—H21C 109.5
C1—O1—H1 109.5 H21B—C21—H21C 109.5
C11—C10—C9 120.2 (2) C2—C3—C4 104.11 (18)
C11—C10—H10 119.9 C2—C3—H3A 110.9
C9—C10—H10 119.9 C4—C3—H3A 110.9
C7—C6—C5 114.48 (16) C2—C3—H3B 110.9
C7—C6—H6A 108.6 C4—C3—H3B 110.9
C5—C6—H6A 108.6 H3A—C3—H3B 109.0
C7—C6—H6B 108.6 C3—C2—C1 106.89 (17)
C5—C6—H6B 108.6 C3—C2—H2A 110.3
H6A—C6—H6B 107.6 C1—C2—H2A 110.3
O1—C1—C2 109.00 (18) C3—C2—H2B 110.3
O1—C1—C20 117.19 (16) C1—C2—H2B 110.3
C2—C1—C20 104.07 (16) H2A—C2—H2B 108.6
O1—C1—H1A 108.8 C20—C22—H22A 109.5
C2—C1—H1A 108.8 C20—C22—H22B 109.5
C20—C1—H1A 108.8 H22A—C22—H22B 109.5
C22—C20—C4 112.98 (17) C20—C22—H22C 109.5
C22—C20—C19 111.09 (19) H22A—C22—H22C 109.5
C4—C20—C19 106.88 (16) H22B—C22—H22C 109.5
C22—C20—C1 109.30 (17)
C13—C9—C8—C7 −172.29 (19) C5—C4—C20—C22 62.4 (2)
C10—C9—C8—C7 8.0 (3) C3—C4—C20—C22 −70.7 (2)
C13—C9—C8—C16 11.1 (3) C5—C4—C20—C19 −60.0 (2)
C10—C9—C8—C16 −168.67 (17) C3—C4—C20—C19 166.80 (18)
C7—C8—C16—C21 −98.6 (2) C5—C4—C20—C1 178.53 (14)
C7—C8—C16—C15 142.84 (19) O1—C1—C20—C22 −41.7 (2)
C9—C8—C16—C15 −40.5 (2) C2—C1—C20—C22 78.8 (2)
C7—C8—C16—C17 24.5 (2) O1—C1—C20—C4 −160.47 (16)
C9—C8—C16—C17 −158.79 (15) C2—C1—C20—C4 −40.1 (2)
C18—C17—C16—C8 −176.38 (15) O1—C1—C20—C19 85.0 (2)
C5—C17—C16—C8 −49.79 (19) C2—C1—C20—C19 −154.62 (19)
C18—C17—C16—C21 −55.4 (2) C22—C20—C19—C18 −68.5 (2)
C5—C17—C16—C21 71.16 (19) C4—C20—C19—C18 55.2 (2)
C18—C17—C16—C15 66.4 (2) C1—C20—C19—C18 165.79 (18)
C5—C17—C16—C15 −166.99 (14) C8—C16—C15—C14 59.9 (2)
C12—N1—C13—C9 −2.0 (3) C21—C16—C15—C14 −57.8 (2)
C12—N1—C13—C14 178.34 (18) C17—C16—C15—C14 178.94 (16)
C10—C9—C13—N1 2.4 (3) N1—C12—C11—C10 2.3 (4)
C8—C9—C13—N1 −177.33 (16) C9—C10—C11—C12 −1.9 (3)
C10—C9—C13—C14 −178.00 (18) C20—C19—C18—C17 −54.3 (2)
C8—C9—C13—C14 2.3 (3) C5—C17—C18—C19 51.5 (2)
C13—N1—C12—C11 −0.4 (3) C16—C17—C18—C19 178.90 (17)
C3—C4—C5—C6 −53.3 (3) N1—C13—C14—C15 −163.74 (18)
C20—C4—C5—C6 −178.40 (15) C9—C13—C14—C15 16.6 (3)
C3—C4—C5—C17 −175.76 (18) C16—C15—C14—C13 −49.1 (3)
C20—C4—C5—C17 59.2 (2) C9—C8—C7—C6 176.87 (18)
C18—C17—C5—C4 −51.9 (2) C16—C8—C7—C6 −6.6 (3)
C16—C17—C5—C4 −179.88 (14) C5—C6—C7—C8 13.1 (3)
C18—C17—C5—C6 −174.56 (15) C5—C4—C3—C2 −163.08 (18)
C16—C17—C5—C6 57.4 (2) C20—C4—C3—C2 −33.1 (2)
C13—C9—C10—C11 −0.4 (3) C4—C3—C2—C1 7.5 (3)
C8—C9—C10—C11 179.36 (19) O1—C1—C2—C3 146.4 (2)
C4—C5—C6—C7 −159.24 (16) C20—C1—C2—C3 20.6 (2)
C17—C5—C6—C7 −37.5 (2)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O1—H1···N1i 0.82 2.04 2.862 (2) 178