metal-organic papers
Acta Cryst.(2005). E61, m1295–m1297 doi:10.1107/S1600536805017599 Liuet al. [Cd
2L(ClO4)2(H2O)2][Cd2L2](ClO4)2
m1295
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
Diaquadiperchlorato[l-11,23-dimethyl-3,7,15,19-tetraazatricyclo[19.3.1.1
9,13]hexacosa-1(25),2,7,9,-
11,13(26),14,19,21,23-decaene-25,26-diolato-1j
2N
,
N
000;2j
2N
000000,
N
000000000;1:2j
2O
:
O
000]dicadmium(II)
diaqua[l-11,23-dimethyl-3,7,15,19-tetraazatri-cyclo[19.3.1.1
9,13]hexacosa-1(25),2,7,9,11,13(26),-14,19,21,23-decaene-25,26-diolato-1j
2N
,
N
000;-2j
2N
000000,
N
000000000;1:2j
2O
:
O
000]dicadmium(II) diperchlorate
Bo Liu,aHong Zhou,a,bZhi-Quan
Pan,a* Xue-Lei Huaand
Lei Chena
aSchool of Chemical Engineering and Pharmacy,
Wuhan Institute of Chemical Technology, Wuhan 430073, People’s Republic of China, andbCollege of Chemistry and Molecular Science of Wuhan University, Wuhan 430072, People’s Republic of China
Correspondence e-mail: zhiqpan@163.com
Key indicators
Single-crystal X-ray study
T= 293 K
Mean(C–C) = 0.009 A˚
Rfactor = 0.050
wRfactor = 0.119
Data-to-parameter ratio = 14.4
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
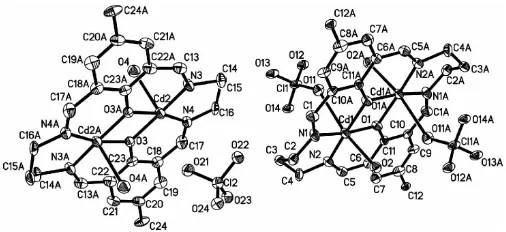
In the title complex, [Cd2(C24H26N4O2)(ClO4)2(H2O)2]-[Cd2(C24H26N4O2)(H2O)2](ClO4)2, the two distinct macro-cycles adopt essentially flat structures. They each encapsulate two Cd atoms. One Cd coordination polyhedron, completed by a water O atom and a perchlorate O atom, is distorted octahedral, while the other is best described as a CdN2O3 square-based pyramid, with water in the apical site. Both dinuclear complexes are centrosymmetric.
Comment
Since the first dinuclear macrocyclic complexes were synthe-sizedviatemplate condensation at the beginning of the 1970 s, these species, especially those containing Schiff base diphenol macrocyclic ligands, have attracted much attention (Pilkington & Robson, 1970; Mohanta et al., 1998; Wang et al., 1997; Brianeseet al., 1999; Gaoet al., 2001). Macrocyclic complexes synthesized by the cyclocondensation reaction between 2,6-diformyl-4-R-phenol and alkylenediamine have been obtained by a stepwise template reaction (R is CH3, n-butyl or Cl; Shangguanet al., 2000; Zhouet al., 2005; Wanget al., 1997).
In order to understand better the different metal–cavity and metal–metal interactions in such species, the title complex, (I), (Fig. 1), with the macrocycle derived from the cyclo-condensation reaction between 2,6-diformyl-4-R-phenol and polyamine, was synthesized and its structure is presented here. Selected bond distances and angles relevant to the CdII coordination spheres are listed in Table 1.
There are two distinct dinuclear complexes in (I), both generated from the atoms of the asymmetric unit by inversion symmetry. Each contains a pair of CdIIatoms bridged by the two endogenous phenolic O atoms of the macrocycle ligand, with Cd1 Cd1iand Cd2 Cd2iiseparations of 3.0882 (8) and 3.0880 (8) A˚ , respectively [symmetry codes: (i) 1x, 2 y, 1z; (ii) x, 1 y, z]. The phenolic bridging angles are 103.41 (17) and 103.79 (18)for the Cd1 and Cd2 complexes, respectively.
The CdIIcoordination is subtly different in the two mol-ecules. Atom Cd1 has a bound water molecule at 2.390 (4) A˚ and a close perchlorate O atom at 2.610 (4) A˚ . This results in a distorted CdN2O4 octahedron. Atom Cd2 also possesses a bound water molecule [Cd—O = 2.436 (5) A˚ ], but the perchlorate groups associated with the Cd2 complex cation are much further away, at 2.947 A˚ . Thus, the Cd2 coordination can be described as a CdN2O3square-based pyramid. All the atoms in each of the macrocycles, except the propylene groups, are approximately coplanar.
Experimental
2,6-Diformyl-4-methylphenol was prepared by a modification of the literature method of Taniguchi (1984).N,N0
-Bis(3-formyl-5-methyl-salicylidene)propylenediimine was prepared by the literature method of Okawa & Kida (1972). A mixture of Cd(OAc)2(H2O)2(0.133 g, 0.5 mmol) and N,N0
-bis(3-formyl-5-methylsalicylidene)propylene-diimine (0.183 g, 0.5 mmol) in absolute methanol (30 ml) was added dropwise to a methanol solution (10 ml) containing 1,3-diamino-propane (0.037 g, 0.5 mmol). After stirring the mixture for 10 h at room temperature, a green–brown solution formed. A methanol solution (10 ml) containing cadmium perchlorate hexahydrate (0.210 g, 0.5 mmol) was added dropwise. A red–brown solid was obtained after stirring at room temperature for about 3 h. The product was filtered off, recrystallized from MeCN–Et2O (4:1), washed with diethyl ether and dried under a vacuum (yield 0.149 g, 35%). Red–brown block-like crystals of (I) suitable for X-ray diffraction were obtained by diffusion of ethyl acetate into an MeCN
solution over one month. Analysis calculated for
C24H30N4O12Cl2Cd2: C 33.43, H 3.50, N 6.49%; found: C 33.56, H 3.57, N 6.40. IR (KBr,, cm1): 3482 (O—H), 1638 (C N), 1096 and 621 (ClO4).
Crystal data
[Cd2(C24H26N4O2)(ClO4)2(H2O)2
]-[Cd2(C24H26N4O2
)(-H2O)2](ClO4)2 Mr= 1724.44
Monoclinic,P21=c a= 16.5483 (15) A˚
b= 12.3987 (11) A˚
c= 14.7440 (13) A˚
= 105.512 (1)
V= 2914.9 (5) A˚3
Z= 2
Dx= 1.965 Mg m 3
MoKradiation Cell parameters from 5603
reflections
= 2.2–22.9
= 1.71 mm1 T= 293 (2) K Block, red-brown 0.340.240.22 mm
Data collection
Bruker SMART Apex CCD area-detector diffractometer
’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 2000)
Tmin= 0.62,Tmax= 0.69
16225 measured reflections
5731 independent reflections 4747 reflections withI> 2(I)
Rint= 0.032 max= 26.0 h=20!10
k=15!15
l=18!18
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.050 wR(F2) = 0.119
S= 1.08 5731 reflections 399 parameters
H-atom parameters constrained
w= 1/[2
(Fo 2
) + (0.054P)2 + 1.6448P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001 max= 0.74 e A˚
3 min=0.99 e A˚
3
Table 1
Selected bond length (A˚ ).
Cd1—N1 1.962 (5)
Cd1—O1 1.964 (4)
Cd1—O1i
1.971 (4)
Cd1—N2 1.974 (5)
Cd1—O2 2.390 (4)
Cd1—O11 2.610 (4)
Cd2—O3 1.949 (4)
Cd2—N4 1.961 (5)
Cd2—O3ii
1.976 (4)
Cd2—N3 1.981 (5)
Cd2—O4 2.436 (5)
Symmetry codes: (i)xþ1;yþ2;zþ1; (ii)x;yþ1;z.
All H atoms were placed in calculated positions, with C—H distances in the range 0.93–0.97 A˚ and O—H equal to 0.85 A˚, and included in the refinement in the riding-model approximation, with
Uiso(H) = 1.2–1.5Ueq(C,O).
Data collection:SMART(Bruker, 2000); cell refinement:SAINT
(Bruker, 2000); data reduction:SAINT; program(s) used to solve structure: SHELXTL (Bruker, 2000); program(s) used to refine structure:SHELXTL; molecular graphics:SHELXTL; software used to prepare material for publication:SHELXTL.
The authors thank the National Science Foundation of China (grant No.20271039).
References
Brianese, N., Casellato, U., Tamburini, S., Tomasin, P. & Vigota, P. A. (1999).
Inorg. Chim. Acta,293, 178–194.
Bruker (2000).SMART(Version 6.22),SAINT(Version 6.22) andSHELXTL
(Version 6.10). Bruker AXS Inc., Madison, Wisconsin, USA.
Gao, J., Martell, A. E. & Motekaitis, R. J. (2001).Inorg. Chim. Acta,325, 164– 170.
Mohanta, S., Baitalik, S., Dutta, S. K. & Adhikary, B. (1998).Polyhedron,17, 2669–2677.
Okawa, H. & Kida, S. (1972).Bull. Chem. Soc. Jpn,45, 1759–1770. Pilkington, N. H. & Robson, R. (1970).Aust. J. Chem.23, 2226–2236. Shangguan, G. Q., Matrell, A. E., Zhang, Z. R. & Reibenspies, J. H. (2000).
Inorg. Chim. Acta,299, 47–58.
metal-organic papers
m1296
Liuet al. [Cd [image:2.610.44.297.70.186.2]2L(ClO4)2(H2O)2][Cd2L2](ClO4)2 Acta Cryst.(2005). E61, m1295–m1297 Figure 1
Taniguchi, S. (1984).Bull. Chem. Soc. Jpn,57, 2683–2689.
Wang, Z., Reibenspies, J. & Martell, A. E. (1997).Inorg. Chem.36, 629–636.
Zhou, H., Peng, Z. H., Pan, Z. Q., Liu, B. & Liu, Y. Q. (2005).J. Coord. Chem. 58, 443–451.
metal-organic papers
Acta Cryst.(2005). E61, m1295–m1297 Liuet al. [Cd
supporting information
sup-1
Acta Cryst. (2005). E61, m1295–m1297
supporting information
Acta Cryst. (2005). E61, m1295–m1297 [https://doi.org/10.1107/S1600536805017599]
Diaquadiperchlorato[
µ
-11,23-dimethyl-3,7,15,19-tetraazatricyclo-[19.3.1.1
9,13]hexacosa-1(25),2,7,9,11,13(26),14,19,21,23-decaene-25,26-diolato-1
κ
2N
,
N
′
;2
κ
2N
′′
,
N
′′′
;1:2
κ
2O
:
O
′
]dicadmium(II) diaqua[
µ
-11,23-di-
methyl-3,7,15,19-tetraazatricyclo-[19.3.1.1
9,13]hexacosa-1(25),2,7,9,11,13(26),14,19,21,23-decaene-25,26-diolato-1
κ
2N
,
N
′
;2
κ
2N
′′
,
N
′′′
;1:2
κ
2O
:
O
′
]dicadmium(II) diperchlorate
Bo Liu, Hong Zhou, Zhi-Quan Pan, Xue-Lei Hu and Lei Chen
Bis(aqua)bis(diperchlorato)(3,7,15,19-tetraaza-25,26-dihydroxy-11,23-
dimethyltricyclo[19,3,1,19,13]hexacosa-1(25),2,7,9,11,13 (26),14,19,21,23-
decaene-1κ2N,N′;2κ2N′′,N′′′;1:2κ2O:O′)dicadmium(II) bis(aqua)(3,7,15,19-tetraaza-25,26-dihydroxy-11,23-
dimethyltricyclo[19,3,1,19,13]hexacosa-1(25),2,7,9,11,13 (26),14,19,21,23-
decaene-1κ2N,N′;2κ2N′′,N′′′;1:2κ2O:O′)dicadmium(II) diperchlorate
Crystal data
[Cd2(ClO4)2(C24H26N4O2)(H2O)2]
[Cd2(C24H26N4O2)(H2O)2]·2ClO4
Mr = 1724.44
Monoclinic, P21/c
Hall symbol: -P 2ybc
a = 16.5483 (15) Å
b = 12.3987 (11) Å
c = 14.7440 (13) Å
β = 105.512 (1)°
V = 2914.9 (5) Å3
Z = 2
F(000) = 1712
Dx = 1.965 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5603 reflections
θ = 2.2–22.9°
µ = 1.71 mm−1
T = 293 K Block, red-brown 0.34 × 0.24 × 0.22 mm
Data collection
Bruker SMART Apex CCD area-detector diffractometer
Radiation source: sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 2000)
Tmin = 0.62, Tmax = 0.69
16225 measured reflections 5731 independent reflections 4747 reflections with I > 2σ(I)
Rint = 0.032
θmax = 26.0°, θmin = 2.1°
h = −20→10
k = −15→15
supporting information
sup-2
Acta Cryst. (2005). E61, m1295–m1297
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.050
wR(F2) = 0.119
S = 1.08 5731 reflections 399 parameters 15 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.054P)2 + 1.6448P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.74 e Å−3
Δρmin = −0.99 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Cd1 0.46250 (3) 0.88597 (3) 0.47745 (3) 0.03797 (13)
Cd2 0.06482 (3) 0.59085 (3) 0.03415 (3) 0.03932 (14)
C1 0.2854 (4) 0.9012 (5) 0.3867 (5) 0.0493 (16)
H1 0.2343 0.8683 0.3590 0.059*
C2 0.3265 (4) 0.7232 (5) 0.4194 (4) 0.0409 (13)
H2A 0.2689 0.7120 0.3831 0.049*
H2B 0.3322 0.6982 0.4832 0.049*
C3 0.3856 (4) 0.6606 (5) 0.3757 (4) 0.0399 (13)
H3A 0.3606 0.5913 0.3542 0.048*
H3B 0.3921 0.6997 0.3211 0.048*
C4 0.4715 (4) 0.6423 (5) 0.4428 (5) 0.0509 (16)
H4A 0.4653 0.5951 0.4930 0.061*
H4B 0.5062 0.6051 0.4091 0.061*
C5 0.5919 (4) 0.7284 (5) 0.5343 (4) 0.0401 (13)
H5 0.6088 0.6566 0.5413 0.048*
C6 0.6551 (3) 0.8043 (4) 0.5796 (4) 0.0339 (12)
C7 0.7323 (4) 0.7603 (6) 0.6258 (4) 0.0470 (15)
H7 0.7378 0.6857 0.6298 0.056*
C8 0.8020 (4) 0.8246 (6) 0.6665 (5) 0.0482 (15)
C9 0.7900 (3) 0.9337 (5) 0.6573 (4) 0.0416 (14)
H9 0.8358 0.9781 0.6825 0.050*
C10 0.7153 (3) 0.9823 (5) 0.6137 (4) 0.0326 (11)
C11 0.6415 (3) 0.9187 (4) 0.5732 (4) 0.0303 (11)
C12 0.8852 (4) 0.7735 (5) 0.7149 (4) 0.0354 (12)
supporting information
sup-3
Acta Cryst. (2005). E61, m1295–m1297
H12B 0.8768 0.6987 0.7266 0.053*
H12C 0.9090 0.8096 0.7736 0.053*
C13 −0.0208 (4) 0.7940 (5) 0.0248 (5) 0.0465 (14)
H13 −0.0203 0.8681 0.0346 0.056*
C14 0.1179 (4) 0.8159 (5) 0.1140 (5) 0.0474 (15)
H14A 0.1245 0.8054 0.1808 0.057*
H14B 0.1039 0.8910 0.0992 0.057*
C15 0.1991 (4) 0.7885 (6) 0.0904 (5) 0.0536 (18)
H15A 0.1891 0.7932 0.0226 0.064*
H15B 0.2398 0.8436 0.1179 0.064*
C16 0.2384 (4) 0.6801 (5) 0.1213 (4) 0.0447 (15)
H16A 0.2893 0.6744 0.1010 0.054*
H16B 0.2544 0.6781 0.1895 0.054*
C17 0.2277 (4) 0.4990 (6) 0.1044 (5) 0.0506 (16)
H17 0.2849 0.5068 0.1325 0.061*
C18 0.1987 (4) 0.3884 (5) 0.0870 (4) 0.0413 (13)
C19 0.2622 (4) 0.3096 (6) 0.1213 (5) 0.0514 (16)
H19 0.3158 0.3310 0.1544 0.062*
C20 0.2441 (4) 0.2008 (5) 0.1056 (5) 0.0470 (15)
C21 0.1633 (4) 0.1724 (5) 0.0576 (5) 0.0443 (14)
H21 0.1504 0.0999 0.0457 0.053*
C22 0.0995 (4) 0.2504 (5) 0.0258 (5) 0.0465 (15)
C23 0.1160 (3) 0.3601 (5) 0.0407 (4) 0.0323 (11)
C24 0.3108 (5) 0.1164 (6) 0.1419 (4) 0.0524 (18)
H24A 0.3643 0.1508 0.1647 0.079*
H24B 0.3126 0.0677 0.0918 0.079*
H24C 0.2978 0.0769 0.1922 0.079*
Cl1 0.48841 (10) 0.88061 (12) 0.22993 (9) 0.0411 (3)
Cl2 0.09854 (11) 0.55513 (12) 0.31998 (11) 0.0474 (4)
N1 0.3475 (3) 0.8366 (4) 0.4203 (4) 0.0457 (13)
N2 0.5165 (3) 0.7430 (4) 0.4861 (4) 0.0407 (11)
N3 0.0497 (3) 0.7456 (4) 0.0589 (4) 0.0453 (11)
N4 0.1868 (3) 0.5864 (4) 0.0871 (4) 0.0427 (12)
O1 0.5695 (2) 0.9605 (3) 0.5301 (3) 0.0362 (7)
O2 0.4371 (3) 0.8821 (3) 0.6296 (3) 0.0442 (9)
H2D 0.4631 0.9436 0.6652 0.066*
H2E 0.4604 0.8172 0.6617 0.066*
O3 0.0570 (2) 0.4358 (3) 0.0120 (3) 0.0357 (8)
O4 0.0635 (3) 0.6278 (4) −0.1285 (3) 0.0539 (11)
H4D 0.0188 0.6768 −0.1561 0.081*
H4E 0.0554 0.5614 −0.1634 0.081*
O11 0.4766 (3) 0.9297 (3) 0.3092 (3) 0.0464 (9)
O12 0.5442 (3) 0.9389 (3) 0.1944 (3) 0.0450 (10)
O13 0.4149 (3) 0.8689 (4) 0.1584 (3) 0.0463 (11)
O14 0.5220 (3) 0.7793 (3) 0.2560 (3) 0.0410 (10)
O21 0.0647 (3) 0.5228 (4) 0.2257 (3) 0.0515 (11)
O22 0.1088 (3) 0.6675 (3) 0.3173 (3) 0.0490 (11)
supporting information
sup-4
Acta Cryst. (2005). E61, m1295–m1297
O24 0.0431 (3) 0.5297 (4) 0.3722 (3) 0.0518 (11)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Cd1 0.0367 (2) 0.0281 (2) 0.0446 (2) 0.00035 (16) 0.00310 (18) −0.00108 (17)
Cd2 0.0358 (2) 0.0321 (2) 0.0473 (3) −0.00281 (17) 0.00626 (19) −0.00108 (17)
C1 0.045 (4) 0.038 (3) 0.059 (4) 0.013 (3) 0.003 (3) 0.005 (3)
C2 0.029 (3) 0.052 (4) 0.039 (3) −0.003 (3) 0.004 (2) −0.002 (3)
C3 0.039 (3) 0.033 (3) 0.046 (3) 0.002 (2) 0.009 (3) 0.002 (2)
C4 0.053 (4) 0.047 (4) 0.043 (3) −0.008 (3) −0.003 (3) −0.016 (3)
C5 0.036 (3) 0.038 (3) 0.047 (3) 0.002 (2) 0.012 (3) 0.004 (3)
C6 0.029 (3) 0.035 (3) 0.038 (3) 0.006 (2) 0.010 (2) 0.000 (2)
C7 0.051 (4) 0.048 (4) 0.044 (3) 0.007 (3) 0.015 (3) −0.001 (3)
C8 0.039 (3) 0.064 (4) 0.047 (4) 0.012 (3) 0.022 (3) 0.010 (3)
C9 0.038 (3) 0.050 (3) 0.037 (3) 0.008 (3) 0.011 (3) 0.012 (3)
C10 0.030 (3) 0.044 (3) 0.025 (2) 0.002 (2) 0.009 (2) 0.003 (2)
C11 0.026 (3) 0.031 (3) 0.030 (3) 0.001 (2) 0.003 (2) −0.002 (2)
C12 0.042 (3) 0.039 (3) 0.027 (3) 0.002 (2) 0.012 (2) 0.005 (2)
C13 0.053 (3) 0.041 (2) 0.053 (4) 0.008 (2) 0.026 (3) 0.006 (3)
C14 0.047 (4) 0.050 (4) 0.047 (3) −0.009 (3) 0.015 (3) −0.024 (3)
C15 0.045 (4) 0.064 (4) 0.051 (4) −0.031 (3) 0.010 (3) −0.012 (3)
C16 0.049 (4) 0.051 (4) 0.033 (3) −0.014 (3) 0.008 (3) −0.021 (3)
C17 0.029 (3) 0.056 (4) 0.063 (4) 0.006 (3) 0.006 (3) −0.007 (3)
C18 0.035 (3) 0.045 (3) 0.045 (3) 0.008 (3) 0.014 (3) 0.001 (3)
C19 0.041 (4) 0.062 (4) 0.056 (4) 0.009 (3) 0.021 (3) 0.004 (3)
C20 0.051 (4) 0.039 (3) 0.052 (4) 0.011 (3) 0.017 (3) 0.012 (3)
C21 0.042 (3) 0.038 (3) 0.059 (4) 0.008 (3) 0.025 (3) 0.009 (3)
C22 0.053 (4) 0.041 (3) 0.053 (4) 0.008 (3) 0.026 (3) 0.006 (3)
C23 0.030 (3) 0.0401 (15) 0.031 (3) 0.0105 (19) 0.016 (2) 0.004 (2)
C24 0.054 (4) 0.066 (4) 0.043 (3) 0.036 (3) 0.024 (3) 0.023 (3)
Cl1 0.0546 (9) 0.0452 (8) 0.0307 (7) 0.0092 (6) 0.0239 (7) 0.0102 (6)
Cl2 0.0573 (10) 0.0413 (8) 0.0419 (8) −0.0004 (7) 0.0102 (7) −0.0016 (6)
N1 0.030 (3) 0.041 (3) 0.060 (3) −0.009 (2) 0.000 (2) −0.008 (2)
N2 0.040 (3) 0.030 (2) 0.051 (3) 0.000 (2) 0.011 (2) 0.000 (2)
N3 0.039 (2) 0.0334 (12) 0.060 (3) −0.0080 (18) 0.009 (2) −0.006 (2)
N4 0.043 (3) 0.040 (3) 0.043 (3) −0.009 (2) 0.008 (2) −0.005 (2)
O1 0.0284 (16) 0.0297 (19) 0.0495 (17) 0.0013 (13) 0.0087 (13) 0.0026 (16)
O2 0.042 (2) 0.044 (2) 0.0488 (15) −0.0059 (18) 0.0157 (18) 0.0010 (18)
O3 0.036 (2) 0.0321 (9) 0.037 (2) −0.0028 (13) 0.0063 (17) −0.0011 (15)
O4 0.050 (3) 0.050 (3) 0.060 (3) 0.000 (2) 0.012 (2) 0.003 (2)
O11 0.050 (2) 0.047 (2) 0.0451 (12) 0.011 (2) 0.0190 (16) −0.0054 (13)
O12 0.061 (3) 0.048 (2) 0.033 (2) 0.010 (2) 0.023 (2) 0.0057 (18)
O13 0.060 (3) 0.052 (3) 0.034 (2) 0.009 (2) 0.026 (2) 0.0132 (19)
O14 0.056 (3) 0.043 (2) 0.031 (2) 0.0102 (19) 0.0239 (19) 0.0080 (16)
O21 0.065 (3) 0.047 (2) 0.043 (2) 0.003 (2) 0.015 (2) −0.0018 (19)
O22 0.062 (3) 0.040 (2) 0.046 (2) −0.002 (2) 0.016 (2) −0.0037 (19)
supporting information
sup-5
Acta Cryst. (2005). E61, m1295–m1297
O24 0.062 (3) 0.050 (3) 0.043 (2) −0.002 (2) 0.012 (2) 0.002 (2)
Geometric parameters (Å, º)
Cd1—N1 1.962 (5) C13—C22ii 1.427 (10)
Cd1—O1 1.964 (4) C13—H13 0.9300
Cd1—O1i 1.971 (4) C14—N3 1.486 (7)
Cd1—N2 1.974 (5) C14—C15 1.513 (9)
Cd1—O2 2.390 (4) C14—H14A 0.9700
Cd1—O11 2.610 (4) C14—H14B 0.9700
Cd1—Cd1i 3.0882 (8) C15—C16 1.509 (10)
Cd2—O3 1.949 (4) C15—H15A 0.9700
Cd2—N4 1.961 (5) C15—H15B 0.9700
Cd2—O3ii 1.976 (4) C16—N4 1.450 (7)
Cd2—N3 1.981 (5) C16—H16A 0.9700
Cd2—O4 2.436 (5) C16—H16B 0.9700
Cd2—Cd2ii 3.0880 (8) C17—N4 1.267 (8)
C1—N1 1.293 (8) C17—C18 1.453 (9)
C1—C10i 1.444 (8) C17—H17 0.9300
C1—H1 0.9300 C18—C23 1.402 (8)
C2—N1 1.447 (8) C18—C19 1.425 (9)
C2—C3 1.520 (8) C19—C20 1.387 (10)
C2—H2A 0.9700 C19—H19 0.9300
C2—H2B 0.9700 C20—C21 1.381 (9)
C3—C4 1.515 (9) C20—C24 1.511 (8)
C3—H3A 0.9700 C21—C22 1.415 (9)
C3—H3B 0.9700 C21—H21 0.9300
C4—N2 1.507 (7) C22—C23 1.393 (8)
C4—H4A 0.9700 C22—C13ii 1.427 (10)
C4—H4B 0.9700 C23—O3 1.338 (6)
C5—N2 1.273 (8) C24—H24A 0.9600
C5—C6 1.431 (8) C24—H24B 0.9600
C5—H5 0.9300 C24—H24C 0.9600
C6—C7 1.389 (8) Cl1—O11 1.378 (4)
C6—C11 1.436 (7) Cl1—O12 1.381 (5)
C7—C8 1.399 (10) Cl1—O14 1.386 (4)
C7—H7 0.9300 Cl1—O13 1.388 (5)
C8—C9 1.369 (9) Cl2—O23 1.381 (5)
C8—C12 1.510 (8) Cl2—O24 1.384 (5)
C9—C10 1.371 (7) Cl2—O22 1.405 (5)
C9—H9 0.9300 Cl2—O21 1.411 (5)
C10—C11 1.443 (7) O1—Cd1i 1.971 (4)
C10—C1i 1.444 (8) O2—H2D 0.9600
C11—O1 1.298 (6) O2—H2E 0.9600
C12—H12A 0.9600 O3—Cd2ii 1.976 (4)
C12—H12B 0.9600 O4—H4D 0.9600
C12—H12C 0.9600 O4—H4E 0.9600
supporting information
sup-6
Acta Cryst. (2005). E61, m1295–m1297
N1—Cd1—O1 170.12 (19) N3—C13—H13 115.5
N1—Cd1—O1i 93.57 (19) C22ii—C13—H13 115.5
O1—Cd1—O1i 76.59 (17) N3—C14—C15 109.5 (5)
N1—Cd1—N2 96.8 (2) N3—C14—H14A 109.8
O1—Cd1—N2 92.98 (18) C15—C14—H14A 109.8
O1i—Cd1—N2 168.83 (18) N3—C14—H14B 109.8
N1—Cd1—O2 90.2 (2) C15—C14—H14B 109.8
O1—Cd1—O2 90.28 (15) H14A—C14—H14B 108.2
O1i—Cd1—O2 87.74 (16) C16—C15—C14 117.9 (6)
N2—Cd1—O2 96.39 (18) C16—C15—H15A 107.8
N1—Cd1—O11 88.8 (2) C14—C15—H15A 107.8
O1—Cd1—O11 88.70 (16) C16—C15—H15B 107.8
O1i—Cd1—O11 80.59 (15) C14—C15—H15B 107.8
N2—Cd1—O11 95.41 (18) H15A—C15—H15B 107.2
O2—Cd1—O11 168.20 (14) N4—C16—C15 116.3 (5)
N1—Cd1—Cd1i 131.77 (15) N4—C16—H16A 108.2
O1—Cd1—Cd1i 38.38 (11) C15—C16—H16A 108.2
O1i—Cd1—Cd1i 38.20 (11) N4—C16—H16B 108.2
N2—Cd1—Cd1i 131.24 (15) C15—C16—H16B 108.2
O2—Cd1—Cd1i 88.73 (10) H16A—C16—H16B 107.4
O11—Cd1—Cd1i 83.18 (10) N4—C17—C18 129.6 (6)
O3—Cd2—N4 93.08 (19) N4—C17—H17 115.2
O3—Cd2—O3ii 76.21 (18) C18—C17—H17 115.2
N4—Cd2—O3ii 168.40 (19) C23—C18—C19 122.1 (6)
O3—Cd2—N3 168.60 (19) C23—C18—C17 123.8 (5)
N4—Cd2—N3 97.0 (2) C19—C18—C17 114.1 (6)
O3ii—Cd2—N3 93.30 (19) C20—C19—C18 120.2 (7)
O3—Cd2—O4 92.16 (15) C20—C19—H19 119.9
N4—Cd2—O4 97.70 (18) C18—C19—H19 119.9
O3ii—Cd2—O4 87.30 (15) C21—C20—C19 118.0 (6)
N3—Cd2—O4 91.78 (19) C21—C20—C24 121.3 (6)
O3—Cd2—Cd2ii 38.41 (11) C19—C20—C24 120.7 (7)
N4—Cd2—Cd2ii 131.35 (15) C20—C21—C22 121.9 (6)
O3ii—Cd2—Cd2ii 37.79 (11) C20—C21—H21 119.0
N3—Cd2—Cd2ii 130.95 (15) C22—C21—H21 119.0
O4—Cd2—Cd2ii 89.64 (11) C23—C22—C21 121.2 (6)
N1—C1—C10i 128.8 (6) C23—C22—C13ii 124.8 (6)
N1—C1—H1 115.6 C21—C22—C13ii 113.9 (6)
C10i—C1—H1 115.6 O3—C23—C22 122.7 (5)
N1—C2—C3 108.6 (5) O3—C23—C18 120.9 (5)
N1—C2—H2A 110.0 C22—C23—C18 116.4 (5)
C3—C2—H2A 110.0 C20—C24—H24A 109.5
N1—C2—H2B 110.0 C20—C24—H24B 109.5
C3—C2—H2B 110.0 H24A—C24—H24B 109.5
H2A—C2—H2B 108.4 C20—C24—H24C 109.5
C4—C3—C2 113.4 (5) H24A—C24—H24C 109.5
supporting information
sup-7
Acta Cryst. (2005). E61, m1295–m1297
C2—C3—H3A 108.9 O11—Cl1—O12 110.6 (3)
C4—C3—H3B 108.9 O11—Cl1—O14 107.6 (3)
C2—C3—H3B 108.9 O12—Cl1—O14 108.7 (3)
H3A—C3—H3B 107.7 O11—Cl1—O13 113.4 (3)
N2—C4—C3 115.1 (5) O12—Cl1—O13 107.8 (3)
N2—C4—H4A 108.5 O14—Cl1—O13 108.7 (3)
C3—C4—H4A 108.5 O23—Cl2—O24 112.1 (3)
N2—C4—H4B 108.5 O23—Cl2—O22 108.8 (3)
C3—C4—H4B 108.5 O24—Cl2—O22 110.2 (3)
H4A—C4—H4B 107.5 O23—Cl2—O21 110.0 (3)
N2—C5—C6 130.6 (6) O24—Cl2—O21 109.7 (3)
N2—C5—H5 114.7 O22—Cl2—O21 105.9 (3)
C6—C5—H5 114.7 C1—N1—C2 115.7 (5)
C7—C6—C5 115.6 (5) C1—N1—Cd1 123.5 (4)
C7—C6—C11 121.9 (5) C2—N1—Cd1 120.7 (4)
C5—C6—C11 122.5 (5) C5—N2—C4 114.7 (5)
C6—C7—C8 122.1 (6) C5—N2—Cd1 121.6 (4)
C6—C7—H7 118.9 C4—N2—Cd1 123.6 (4)
C8—C7—H7 118.9 C13—N3—C14 114.6 (5)
C9—C8—C7 116.1 (6) C13—N3—Cd2 121.7 (4)
C9—C8—C12 123.5 (6) C14—N3—Cd2 123.7 (4)
C7—C8—C12 120.4 (6) C17—N4—C16 112.5 (6)
C8—C9—C10 124.7 (5) C17—N4—Cd2 122.8 (4)
C8—C9—H9 117.6 C16—N4—Cd2 124.3 (4)
C10—C9—H9 117.6 C11—O1—Cd1 128.1 (3)
C9—C10—C11 120.7 (5) C11—O1—Cd1i 127.6 (3)
C9—C10—C1i 116.5 (5) Cd1—O1—Cd1i 103.41 (17)
C11—C10—C1i 122.7 (5) Cd1—O2—H2D 109.4
O1—C11—C6 122.3 (5) Cd1—O2—H2E 109.4
O1—C11—C10 123.3 (5) H2D—O2—H2E 109.5
C6—C11—C10 114.4 (5) C23—O3—Cd2 129.0 (4)
C8—C12—H12A 109.5 C23—O3—Cd2ii 125.6 (4)
C8—C12—H12B 109.5 Cd2—O3—Cd2ii 103.79 (18)
H12A—C12—H12B 109.5 Cd2—O4—H4D 109.8
C8—C12—H12C 109.5 Cd2—O4—H4E 109.2
H12A—C12—H12C 109.5 H4D—O4—H4E 109.5
H12B—C12—H12C 109.5 Cl1—O11—Cd1 141.6 (3)
N3—C13—C22ii 129.0 (6)