EVALUATION OF THE DRUG THERAPY MANAGEMENT OF CKD
PATIENTS (STAGE 3-5) IN A TERTIARY CARE HOSPITAL
Dr. Parthasaradhi1, Dr. Mehruq Fatima2, Syeda Fareha Fatima*3, Umme Salma4, Nabeela Fathima5
1
Department of Nephrology, Owaisi Hospital and Research Centre, Hyderabad, Telangana,
India.
2
Assisstant Professor, Department of Pharmacy Practice, Deccan School of Pharmacy,
Hyderabad, India.
3,4,5
Doctor of Pharmacy Student, Department of Pharmacy Practice, Deccan School of
Pharmacy, Osmania University, Hyderabad, Telangana, India.
ABSTRACT
Background: Chronic kidney disease population can be distinguished
by its vulnerability as it is associated with multiple concurrent
co-morbidities requiring the use of numerous medications, ensuing
intricate therapeutic regimens, intermittent medication modifications
and restricted lifestyles resulting in a substantial risk for developing
actual and potential drug-related problems (DRPs) which are
associated with significant morbidity, mortality, and cost. Aim: To
evaluate the drug therapy regimens of CKD stage (3-5) patients for
determining the prevalence and identifying the types of DRPs.
Methods: Prospective observational 6-month study was conducted in
CKD (stage3-5) patients who were aged > 18 years and admitted in
owaisi hospital and research centre. Patients' data and drug therapies
were collected through direct patient interview, discussion with
nursing staff, assessment of patients' medication charts and medical records. DRPs were
identified and classified into 6 main categories (according to The PCNE V 5.01). Descriptive
analysis was done for demographic data, comorbidities, drug utilization, and DRPs' profiles
and Pearson's correlation test was used to assess the correlation between drugs and DRPs.
Results: Out of 30 patients in the study, 26 had DRPs. A total of 79 DRPs were identified
with an average of 2.63 + 2.0 DRPs per subject. Of which, 53.16% were actual DRPs and
Volume 8, Issue 13, 711-723. Research Article ISSN 2277– 7105
Article Received on 21 Sept. 2019,
Revised on 11 Oct. 2019, Accepted on 01 Nov. 2019
DOI: 10.20959/wjpr201913-16194
*Corresponding Author
Syeda Fareha Fatima
Doctor of Pharmacy
Student, Department of
pharmacy practice, Deccan
school of pharmacy,
Osmania university,
Hyderabad, Telangana,
46.83% were potential DRPs. Drug interactions (48.10%) and adverse drug reactions
(29.11%) were found to be the major DRPs followed by Drug choice problem (11.39%),
Dosing problem (10.12%), Other DRPs (1.26%) and Drug use problem (0%) respectively.
Conclusion: Our study reveals that DRPs among the CKD (stage3-5) patients were high.
Drug interactions and adverse drug reactions contributed to the majority of DRPs identified.
Timely appropriate monitoring, prevention, identification and resolution of DRPs are crucial
to optimize clinical outcomes in CKD patients.
KEYWORDS: Drug-Related Problems, Chronic Kidney Disease, PCNE, Adverse reactions,
drug interactions, dosing problems.
INTRODUCTION
Chronic kidney disease (CKD) is one of the most prevalent therapeutic problems and the
number of people affected by it is increasing globally each year. In the last decade, the U.S
has witnessed a 30% increase in the prevalence of CKD.[1] According to the World health
organization (WHO), approximately 8,00,000 mortality cases have been reported each year
worldwide.[2] Patients with CKD require early detection and appropriate treatment to prevent
its progression.
CKD is characterized by abnormalities in kidney structure or decline in kidney function, for a
period of 3 months or longer.[3] Frequent complications associated with progression of CKD
include cardiovascular disease, sodium and water imbalance, anemia, metabolic acidosis,
hyperkalemia and renal osteodystrophy and, if left untreated may lead to an extended hospital
stay and increased cost. Moreover, the progression of CKD may lead to an increased number
of drugs to manage its complications and co-morbidities, thereby leading to increased
incidence of drug-related problems (DRPs). The decline in kidney function is associated with
alteration in the pharmacokinetics of the majority of drugs. Pharmacokinetic alteration of
drugs, such as a change in drug bioavailability, protein binding level, drug distribution and
elimination, may worsen the already deteriorating condition of CKD patients and make them
vulnerable to DRPs.[4]
DRP may be defined as an event involving drug therapy that actually or potentially interferes
with desired health outcomes.[5] In this context, a potential problem means a condition that
may cause drug-related morbidity or death if no action is undertaken. An actual problem is
health-related quality of life of patients. Hence, a systematic review of patients' drug use may
help health-care professionals in identifying DRPs, resolving actual DRPs and preventing
potential DRPs for effective drug therapy and better patient health outcomes.[4]
Unfortunately, there are limited prospective studies conducted on evaluating drug therapy
problems in CKD patients in India. Therefore, this study was conducted aiming to identify
and evaluate drug-related problems (DRPs) in patients with stages 3-5 chronic kidney disease
to optimize drug therapy and reduce drug-related morbidity and mortality.
MATERIALS AND METHODS
Study design: A prospective observational 6-month study was conducted in CKD (3-5)
patients who were admitted under the nephrology department of owaisi hospital and research
centre, Hyderabad, Telangana, after obtaining the approval from the institutional review
board of the hospital.
Study participants: The study's inclusion criteria were met by 30 patients, aged 18 years or
older, diagnosed with stages (3-5) of chronic kidney disease who were willing to participate,
expressed a desire to achieve its objectives and gave their written informed consent.
Data collection: Patient's data collection was carried out during hospitalization and at
discharge time. The questionnaire used in this study for data collection included date of
admission/discharge, patients' socio-demographic characteristics (including age, sex, weight,
height, smoking status, alcohol/substance abuse sleep and dietary pattern),
co-morbidities/past medical history, past medication history (including herbal and OTC
medicines), allergy/adverse drug reaction history, current medication profiles (drug name,
indication, dose, frequency and duration), discharge medications, physical and general
examination, clinical information, laboratory investigations and diagnostic examinations. For
the DRP: adverse drug reactions, Indian pharmacopeia commission (IPC) – suspected adverse
drug reaction reporting form was used.
Assessing the Drug-Related Problems: The Investigators identified the DRPs through direct
patient interview, discussion with nursing staff, assessment of patients' medication charts and
medical records with the help of recommendations from IBM Micromedex® Drug Ref and
IBM Micromedex® Drug Interactions software databases. The identified DRPs were
drug-related problems, which categorizes DRPs into six primary domains.[17] Further DRPs
were divided into actual and potential DRPs.
Data Analysis: Descriptive analysis was done for demographic data, drug utilization and
DRPs. The continuous variables were summarized as mean + SD; categorical variables were
summarized as frequencies and percentages. Pearson's correlation was used to assess the
correlation between drugs and DRPs. The statistical test was two-tailed and P<0.05 was used
to indicate statistical significance.
RESULTS
There were 30 patients who met the inclusion criteria for the six-month prospective
observational study and their demographic characteristics are summarised in Table 1. Male
patients (80%) were more than female patients. The mean age of the study population was
51.0 + 14.5 years and a high number of subjects was found in the age range 51-60 years
(36.7%). The data also reveals 27.67% of subjects were smokers or had a history of smoking
and 14% subjects were alcoholics or had a history of drinking. Majority of the patients were
middle class (60%); unemployed or retired (60%) and married (83%).
Table 1: Patients Socio-Demographic Characteristics.
Patient demographics Category No. Of patients n (%)
Age (Mean + SD)
51.0 + 14.5 18-40 41-60 61-80 - 7 (23.3%) 15 (50%) 8 (26.7%) Gender Male Female 24(80%) 06(20%) Addiction Smokers Ex-smokers Non-smokers Alcoholics Ex-alcoholics Non-alcoholics 3 (10%) 5 (17.67%) 22 (73.33%) 2 (7%) 2 (7%) 26 (86%) Educational Status Educated Uneducated 17(57%) 13(43%) Employment Status Employed Unemployed 12(40%) 22(60%) Economic Status Low Middle Upper middle 09(30%) 18(60%) 03(10% Marital Status Married Unmarried
Clinical characteristics
Table 2 outlines clinical characteristics of the study population that depicts the majority of
patients (97%) were diagnosed with CKD stage 3-5 with a mean glomerular filtration rate
(GFR) of 10.56 + 3.64 mL/min. Mean hospital stay of the study population was 8.73 +5.71
days. The average number of co-morbidities per subject was found to be 6.06 + 2.49, the
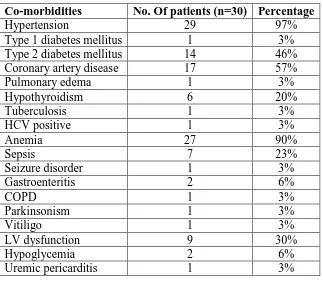
major co-morbidity being hypertension (97%), Anaemia (90%), coronary artery disease
(57%) followed by Diabetes mellitus (49%).
Table 2: Clinical Characteristics of The Study Population.
Variables Mean + SD
BMI (kg/m2) 22.58 + 6.17
SBP (mm Hg) DBP(mm Hg)
130 + 24.49 79.6 + 11.29
Haemoglobin (g/dL) 7.94 + 1.92
Serum creatinine (mg/dL) 7.76 + 4.43
Glomerular filtration rate (ml/min/1.73m2) 10.56 + 3.64 Serum sodium(mmol/L)
Serum potassium (mmol/L) Serum calcium (mmol/L) Serum phosphorus (mmol/L)
134.04 + 5.02 4.46 + 0.93 7.42 + 1.65 5.17 + 2.24
Blood Urea (mg/dL) 173.8 + 87.89
Serum Uric Acid (mg/dL) 7.95 + 1.46
Mean hospital stay (days) 8.73 +5.71
CKD STAGE n(%)
STAGE 3a STAGE 3b STAGE 4 STAGE 5
0% 0% 1(3%) 29(97%)
Presence of co-morbiditiesn (%)
<5 9 (30%)
5-10 20 (66.7%)
>10 1 (3.33%)
Average no. of co-morbidity per patient (Mean + SD) 6.06 + 2.49
Haemodialysis n (%)
Yes No
[image:5.595.122.474.242.637.2]Table 3: Co-Morbid Conditions in The Study Population.
Co-morbidities No. Of patients (n=30) Percentage
Hypertension 29 97%
Type 1 diabetes mellitus 1 3%
Type 2 diabetes mellitus 14 46%
Coronary artery disease 17 57%
Pulmonary edema 1 3%
Hypothyroidism 6 20%
Tuberculosis 1 3%
HCV positive 1 3%
Anemia 27 90%
Sepsis 7 23%
Seizure disorder 1 3%
Gastroenteritis 2 6%
COPD 1 3%
Parkinsonism 1 3%
Vitiligo 1 3%
LV dysfunction 9 30%
Hypoglycemia 2 6%
Uremic pericarditis 1 3%
Drug utilization profile and DRPs
Total numbers of medication orders issued to 30 subjects were calculated as 538 with an
average of 16.73 + 6.11 drugs per patient. Half of the study population was prescribed > 16
drugs.
Table 4 depicts commonly used therapeutic class of drugs for the study population, of which
the most common were cardiovascular drugs (18.9%), followed by the class of drugs acting
on blood and blood-forming organs (15.9%). Further, the 10 most prevalent drugs prescribed
were gastrointestinal drugs (15%), other drugs (9.8%), anti-infectives’ (9.6%),vitamins
(9.6%), respiratory drugs (5.3%), analgesics, antipyretics and NSAIDs (4%), CNS agents
(3.1%), hormones and endocrinal agents (3.1%), fluids, electrolytes and parenteral nutrition
[image:6.595.138.461.89.370.2]Table 4: Therapeutic Classes of Drugs Prescribed for the Study Population.
Therapeutic Classes n (%)
Cardiovascular drugs 102 (18.9%)
Fluids, Electrolytes and Parenteral Nutrition 16 (2.9%)
Gastrointestinal drugs 81 (15%)
Anti-Infectives 52 (9.6%)
Drugs acting on blood or Blood Forming organs 86 (15.9%)
Vitamins and minerals 52 (9.6%)
Hormones and Endocrine drugs 17 (3.1%)
Respiratory drugs 29 (5.3%)
CNS agents 17 (3.1%)
Anti-hyperuricemic agents 11 (2%)
Analgesics, antipyretic and NSAIDs 22 (4%)
Others 53 (9.8%)
Total no. of drugs prescribed during the study 538
Drugs prescribed per patient
≤10 11-15 ≥16 2 13 15
Average no. of drugs prescribed per patient (Mean + SD) 16.73 + 6.11
86.66% of the study population experienced DRPs. On average, DRPs per patient was found
to be 2.25 + 1.42. Drug interactions (48.10%) and adverse drug reactions (29.11%) were
found to be the major drug therapy problems followed by Drug choice problem (11.39%),
Dosing problem (1012%), Other DRPs (1.26%) and Drug use problem (0%) respectively.
Potential DRPs accounted for the majority of the cases as opposed to actual/manifest DRPs.
Table 5 summarizes the DRPs identified in the participants. The study showed that there was
an increase in the number of drug-related problems with an increase in the number of drugs
[image:7.595.114.483.89.365.2]per prescription and the association was statistically significant (P=0.008).
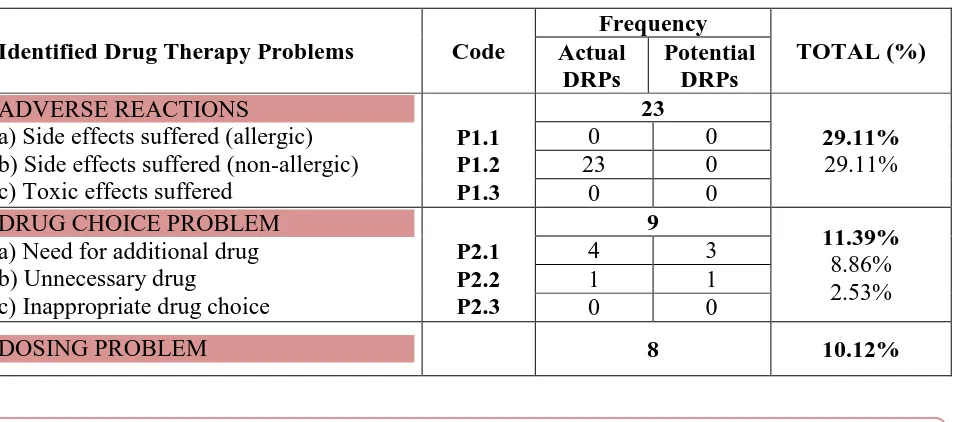
Table 5: Summary of DRPs Identified in The Study Population.
Identified Drug Therapy Problems Code
Frequency TOTAL (%) Actual DRPs Potential DRPs ADVERSE REACTIONS a) Side effects suffered (allergic) b) Side effects suffered (non-allergic) c) Toxic effects suffered
P1.1 P1.2 P1.3 23 29.11% 29.11%
0 0
23 0
0 0
DRUG CHOICE PROBLEM a) Need for additional drug b) Unnecessary drug
c) Inappropriate drug choice
P2.1 P2.2 P2.3 9 11.39% 8.86% 2.53%
4 3
1 1
0 0
[image:7.595.63.541.573.784.2]a)Drug dose too low or dosage regime not frequent enough
b) Drug dose too high or dosage regime too frequent (need dosage adjustment according to CKD stage)
c) Duration of treatment too short d) Duration of treatment too long
P3.1
P3.2
P3.3 P3.4
0 2
2.53%
6.32%
1.26%
0 5
0 0
0 1
DRUG USE PROBLEM
a) Drug not taken/administered at all b) Wrong drug taken/administered
P4.1 P4.2
0
0%
0 0
0 0
DRUG INTERACTIONS a) Potential interaction
b) Actual/Manifest interaction
P5.1 P5.2
38 48.10%
31.64% 16.45%
0 25
13 0
OTHER DRPs
a) Patient dissatisfied with therapy despite taking drug(s) correctly
b) Insufficient awareness of health and diseases (possibly leading to future problems)
c) Unclear complaints. Further clarification necessary
d) Therapy failure (reason unknown)
P6.1 P6.2 P6.3 P6.4 1 1.26% 1.26%
0 0
0 0
0 0
1 0
TOTAL =79
[image:8.595.64.542.69.425.2]DRPs- Drug related problems
Table 6: Drugs V/s Drug Related Problems.
Mean + SD P-Value DRUGS (average no. Of drugs per patient) 17.93 + 6.11
0.008
DRPs (average no. Of DRPs per patient) 2.63 + 2.0
Note: P < 0.05 is statistically significant
DISCUSSION
During the 6 month study, patients diagnosed with chronic kidney disease of stages 3-5
including patients on maintenance hemodialysis of a tertiary care hospital were included to
evaluate their drug therapy to identify the associated drug-related problems.
Several co-morbidities were found among the study population and the major co-morbid
conditions were hypertension (97%), Anaemia (90%), Coronary artery disease (57%) and
Diabetes mellitus (49%). This is concordant to the findings of N.Vanitha Rani et.al.[4] in
diabetes mellitus, and Coronary artery disease. The mean number of comorbidities per patient
was 6.06 + 2.49.
This study has revealed a very high proportion (86.66%) of CKD (stage 3-5) patients with
DRPs. Almost all the patients (97%) in the study were diagnosed with stage 5 CKD and only
3% were diagnosed with stage 4 CKD and no patient had stage 3 CKD.
Total numbers of 538 medication orders were issued to 30 subjects with an average of 16.73
+ 6.11 drugs per patient, which eminently indicates polypharmacy. This is a little high
compared to the studies conducted by Car done et.al.[10] which stated that dialysis patients
were prescribed an average of 12 medications and Rani et al.[4] where the average number of
drugs per prescription was found to be 8.93 + 3.26.
A total of 79 Drug-related problems were identified in 86.66% of the study population while
13.33% had no DRPs, which is similar to the study conducted by Maxwell O Adibeet.al.[25]
where they reported that 70.03% of CKD patients in their study had DRPs. Average no. Of
DRPs per patient was found to be 2.63 + 2.0. When comparing the rate of DRPs experienced
by each patient, Patel HR et.al.[11] reported a mean of 3.2 DRPs per patient.
The drug-related problems (DRPs) identified in the study were as follows:
Adverse Drug Reactions All the ADRs identified were non-allergic, which is in line with the
study conducted by Hesty U. Ramadaniati et.al.[2] where they reported non-allergic adverse
drug events as one of the major DRPs. The most common ADR found in the study population
was nausea (26%). Majority of the ADRs observed were falling under the gastrointestinal
system category (65.2%). On the WHO-UMC scale, the most frequent category was
‘possible’ (79.41%), followed by ‘probable’ (11.76%), ‘unlikely’ (8.82%). 0% ADRs were
assessed as ‘certain’, ‘conditional’ and ‘unclassifiable.
Drug choice problems 9 DRPs (11.39%) were identified as problems of drug choice. 7
problems were found under the sub-category ‘Need for additional drug’, and 2 problems were
found under the category ‘Unnecessary drug’.
Dosing problems 8 DRPs (10.12%) were identified to be problems of drug dosing. 5 dosing
problems were found under the sub-category, ‘Drug dose too high or dosage regime too
frequent’; 2 dosing problems were found under the sub-category, ‘Drug dose too low or
‘Duration of treatment too long’; however no dosing problem was found under the sub- category, ‘Duration of treatment too short’. All the dosing problems identified in this study
are potential DRPs. No Drug use problems (0%) were identified in this study.
Drug interactions The assessment of the drug therapies on the software program
MICROMEDEX revealed a total of 38 (48.10%) drug interactions in the study population
and all of them were found to be drug-drug interactions (DDIs). DDIs is the major DRP
identified in this study which is concordant to the findings of N.vanitha rani et.al.[4] in which
they have reported Drug interactions as the most frequent DRP observed in their study. Out
of the total DDIs identified, 65.78% are potential DRPs and 34.21% are actual DRPs, which
is in line with the study conducted by Alessandra batista marquito[13] where potential drug
interactions were majorly reported. According to the severity classification, the DDIs are
classified as follows in the descending order of their occurrence– 47.36% were major, 45%
were moderate, 7.89% were contraindicated and minor drug interactions are 0%. By using the
Drug interaction probability scale (DIPS)[18] the DDIs are classified as follows: Highly
probable (0%), probable (2.63%), possible (31.57%) and doubtful (66%). Only 1 problem
(1.26%) is identified under the category ‘Other DRP’
Out of the total DRPs identified, 53.16% are actual DRPs and 46.83% are potential DRPs. In
a study conducted by Hesty U. Ramadaniati et.al.[2] potential DRPs accounted for the
majority of the DRPs as opposed to actual/manifest DRPs. The findings of this study conform
to the reports of studies by Rani et al.[4] and St. Peter WL[16] that there is an upward tendency
of incidence of DRP in accordance to the increased number of medications. Drug interactions
(48.10%) and adverse drug reactions (29.11%) were the major sources of DRPs in this study.
These findings were similar with Hesty u. Ramadaniati et.al.[2] where they concluded adverse
drug reactions and sub-optimal effect of treatment as the major DRPs and also with the study
findings of Levey AS et.al.[14] and Sarnak MJ et.al.[15] in which the common DRPs in CKD
are adverse events, drug interactions and improper doses. A study carried out by Grabe D.W
et.al[12] also reported drug interactions as the most common DRP identified.
It has been well defined that reducing the occurrence of DRPs will bring about better
outcomes for patients, and alleviate the financial burden.[6-8] In addition, there may also be
great personal costs to those involved and may result in time away from work, low patient
satisfaction and lessened public trust toward health care.[7-9] Hence, it is significant to
CONCLUSION
Our study reveals that the prevalence of drug-related problems among the CKD (stage3-5)
patients was high. 79 DRPs were identified in thirty study participants. Drug interactions and
adverse drug reactions were the most common DRPs. Actual DRPs accounted for the
majority of the DRPs identified as compared to the potential DRPs. The nature of prospective
observation of our study provides better opportunities to capture actual and potential DRPs.
DRPs have profound ramifications in CKD patients already burdened by multimodal therapy.
It has been well defined that the consequences of DRPs result in extended hospitalization,
readmissions to the hospital, higher cost and premature death. Our study advocates the
importance of timely appropriate monitoring, prevention, identification, intervention and
resolution of Drug-related problems to optimize clinical outcomes and reduce drug-related
morbidity and mortality in CKD patients and implores the need for further studies on a larger
study population.
ACKNOWLEDGEMENT
All authors contributed to the design of the study, interpretation of data and writing of the
manuscript. The authors would like to extend sincere gratitude to their respective family
members for their great support.
Conflict of Interests
The authors have none to declare.
Abbreviations
ADR: Adverse drug reaction; BMI: Body mass index; CCBs: Calcium channel blockers;
CNS: Central nervous system; COPD: Chronic obstructive pulmonary disease; CKD: Chronic
kidney disease; ClCr: creatinine clearance; DBP: Diastolic blood pressure; DRPs: Drug
related problems; DDIs: Drug-drug interactions; DIPS: Drug interaction probability scale;
ESA: Erythropoietin stimulating agent; ESRD: End stage renal stage disease; FDA: Food and
Drug Administration; GI: Gastrointestinal; GFR: Glomerular filteration rate; HD:
Hemodialysis; IPC: Indian pharmacopoeia commission; IV: Intravascular; MBD: Mineral
and Bone Disorder; NSAIDs: Non-steroidal Anti-Inflammatory Drugs; PCNE:
Pharmaceutical care network europe; SBP: Systolic blood pressure; SCr: Serum creatinine;
REFERENCES
1. Varma PP. Prevalence of chronic kidney disease in India - Where are we heading?. Indian
Journal of Nephrology, 2015; 25(3): 133-135.
2. Hesty U. Ramadaniati, Yusi Anggriani, Vonny M. Wowor; Drug-related problems in
chronic kidneys disease patients in an Indonesian hospital: do the problems really matter?
International Journal of Pharmacy and Pharmaceutical Sciences, 2016; 8(12): 298-30.
3. DiPiro J, Talbert R, Yee G, Matzke G, Wells B, Posey L. Pharmacotherapy: A
Pathophysiologic Approach, 9th edition. New York: McGraw-Hill Publishing,
1479-1535.
4. Rani N, Thomas R, Rohini E, Kannan S, Thennarasu P. A study of drug related problems
in chronic kidney disease patients of a tertiary care teaching hospital in south India.World
Journal of Pharmaceutical Research, 2014; 3(4): 1403-1417.
5. Ruths S, Viktil K., Blix S. Classification of drug-related problems. Tidsskr Nor
Legeforen, 2007; 127: 3073-6.
6. Classen DC, Pesstotnik SL, Evans ES, Lloyd JF, Burke JP. Adverse drug events in
hospitalised patients: excess length of stay, extra costs and attributable mortality. JAMA,
1997; 277: 301-6.
7. Bates DW, Spell N, Cullen DJ. The costs of adverse drug events in hospitalised patients.
JAMA, 1997; 277: 307-11.
8. IBM Micromedex® Drug Interactions. MICROMEDEX (Application version 2.8.0b483).
http://www.micromedexsolutions.com/micromedex2/4.14.0/WebHelp/Tools/Interactions/
Drug_Interactions_iP.htm [ONLINE]
9. Kaushal R. Medication Errors and Adverse Drug Events in Pediatric Inpatients. Jama,
2001; 285(16): 2114.
10.Cardone KE, Bacchus S, Assimon MM, Pai AB, Manley HJ. Medication-related
Problems in CKD. Advances in Chronic Kidney Disease, 2010; 17(5): 404–12.
11.Patel HR, Pruchnicki MC, Hall LE. Assessment for chronic kidney disease service in
high-risk patients at community health clinics. The Annals of pharmacotherapy, Jan,
2005; 39(1): 22-7.
12.Grabe DW, Low CL, Bailie GR, Eisele G. Evaluation of drug-related problems in an
outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol, 1997;
13.Marquito AB, Fernandes NMDS, Colugnati FAB, Paula RBD. Identifying potential drug
interactions in chronic kidney disease patients. Jornal Brasileiro de Nefrologia, 2014;
36(1): 26–34.
14.Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and
classification of chronic kidney disease: A position statement from Kidney Disease:
Improving Global Outcomes (KDIGO). Kidney International, 2005; 67(6): 2089–100.
15.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney
disease as a risk factor for development of cardiovascular disease: a statement from the
American Heart Association councils on kidney in cardiovascular disease, high blood
pressure research, clinical cardiology, and epidemiology and prevention. Hypertension,
2003; 42: 1050–65.
16.St. Peter WL. Improving Medication Safety in Chronic Kidney Disease Patients on
Dialysis through Medication Reconciliation. Advances in Chronic Kidney Disease, 2010;
17(5): 413-19.
17.Pharmaceutical Care Network Europe Foundation: The PCNE Classification V 5.01,
2006.
18.Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction
cases. Ann Pharmacother, 2007; 41(4): 674-80.
19.IBM Micromedex® Drug Ref (Application version 1.17.0b783).
http://www.micromedexsolutions.com/micromedex2/4.127.0/WebHelp/Document_help/