EXPERIMENTS ON THE EFFECT OF DYES ON
INDUCTION AND RESPIRATION IN THE
AMPHIBIAN GASTRULA
BY R. A. BEATTY, S. DE JONG AND M. A. ZIELINSKI
From the Zoological and Biochemical Laboratories, Cambridge(Received 12 October 1938)
(With One Plate)
INTRODUCTION
FROM the work of experimental embryologists and biochemists on the gastrulation period in amphibian development, it appears that the cells of the blastula all contain the morphogenetic stimulating substance or substances which are later responsible for the neural induction. But they contain it in some inactive or masked form, from which it is liberated only at the beginning of imagination at the dorsal lip of the blastopore. In other regions of the gastrula, it may indeed be liberated, but only by artificial treatment; such as the denaturation of the proteins of the ventral ectoderm or yolk endoderm by heat or by treatment with organic solvents (Holtfreter, 1933). But it is not absolutely necessary to kill the inactive tissues in order to cause the appearance of the inductive power in them, for Waddington et al. (1936) showed that after pieces of ventral ectoderm had remained isolated for some time in dilute solutions of methylene blue, they would, when implanted into the blastocoele cavity of another embryo, perform neural inductions. These might be only on the part of the host, but the graft also in many cases showed considerable neuralization. In later experiments, which are reported in the present paper, it was found that neuralization of the isolated explant would occur without any implantation into another embryo.
proper-Effect of Dyes on Induction and Respiration in Amphibian Gastrula 151
ties of the isolated ventral ectoderm.1 On the other hand, methylene blue in dilute concentrations appears to have an acceleratory effect on the oxygen consumption of gastrula tissue, although it is not so marked as in other cases such as sea-urchin eggs.
Since the original discovery of the methylene blue effect by Waddington et al. [193 6] it has been generally assumed that the mechanism must be indirect, i.e. a liberation of the natural organizer substance contained in the ventral ectoderm; rather than direct, i.e. the action of the dye itself as an organizer substance. The latter possi-bility cannot yet, however, be excluded, and it would be interesting to implant into the blastocoele cavity of gastrulae graded doses of dyes, running down to very small concentrations, in order to determine the optimum concentration. In this connexion, it is interesting that Finkelstein & Schapiro (1937) implanted dinitro-phenol in agar into the blastocoele cavity, but obtained no neural differentiations. At the same time, there is evidence (Needham & Boell, 19396) that the anaerobic glycolysis of the ventral ectoderm is increased considerably by dinitro-o-cresol. It is clear that a great deal more work is required on these and similar phases of the problem.
METHODS
Isolation of the ventral ectoderm from over the blastocoele cavity was carried out by the usual Spemann technique of watchmaker's forceps and glass needles under strictly sterile conditions.2 Early gastrulae of Triton alpestris were used. The dye solutions were made up in sterile distilled water, but not themselves sterilized afterwards. After a variable period (from 1 to 3 days) in the dye solutions, the explants were removed, fixed in Michaelis solution, serially sectioned and stained. Measurement of the respiratory rate of the isolated pieces of ventral ectoderm, etc., was carried out by the Cartesian Diver technique of Linderstrem-Lang (1937) as applied to this purpose by Boell & Needham (1939). Full descriptions of this micro-manometric technique, which is about fifteen hundred times more sensitive than the standard Warburg manometers, will be found in the latter paper. The only modification used by us was the employment of Holtfreter solution containing methylene blue at the desired concentration, instead of ordinary Holtfreter solution. For obtaining the amounts of tissue used, the micro-Kjeldahl technique described by Needham & Boell (1939a) was used. Gastrulae of Rana esculenta and
Ambly-stoma mexicanum were used as material. The former were obtained by implantation
of pituitary glands of the same species (July) as described by Rugh (1937), and artificially fertilized; the latter were obtained by injection of Antuitrin S, and natur-ally fertilized. The main batch of eggs was kept in the ice chest and samples used as required. We always allowed some eggs from these samples to develop in order to make sure that their development was normal.
1
The experiments of Marston (1923) and Commoner (1938) on the formation of dye-protein complexes render the expectation that dyes could liberate the organizer substance from its inactive precursor by competitive complex-formation not without plausibility.
With a batch of Discoglossus pinctus embryos we had the curious experience that although their development seemed to be normal, their respiratory rate was extremely low. Dissection of the gastrulae was accomplished with Spemann glass needles in the ordinary way, but the stiff envelopes of Rana esculenta eggs made it necessary to open them with iridectomy scissors as described by Needham et al. (1939). It is important that in every experiment the pieces should come from the same gastrula and that they should be bilaterally symmetrical, i.e. left and right pieces, looking at the yolk-plug with the dorsal blastopore lip upwards.
RESULTS
The results of the explantations into dye solutions, from the laying seasons of 1937 and 1938, are given in Table I. Janus green and neutral red are the dyes which are not known to have any effect on the respiratory rate of cells, yet, as can be seen from the table and the illustrations (PI. I), two possibly neural tubes were obtained with the former, and a number of lesser neuralizations with both. Pyocyanin gave one tube, but phenazine methochloride, which Dickens (1936) found to increase the respiratory rate of tumour tissue 250%, gave no positive results.
Table uyc Methylene blue Pyocyanin Dinitro-o-cresol Janus green Neutral red Phenazine methochloride Control
I. Explantattons of ventral ectoderm into
Molar concentration
1-25 x io-» 1-25 x io-* 1-25 xio-" 1-25 x io~* 1-25 x io~* 1-25 x io~°
I X IO~* I X IO~*
1-25 x io~* 1-25 x io~5 1-25 x 10-* 1-25 x io~* 1-25 x io-1
0 No. ex-planted 1 3 4 3 19 3 7 4 2 2 9 25 5 13 3 No. cut 1 3 4 1 18 3 3 4 17 9 15 S 6 3 dye solutions Reaction 1 t np — 1 1 — — — — — — — — — — — t I I I I ? All B — 1 — 3 — 1 — 1 4 2 — — — D 1 2 1 1 15 2 2 4 15 4 13 5 6 3
Note. In the above table, the classification is made according to the plan of Waddington et al. (I93S)> save that all types of B + are included under B, and B— is included under D. np = neural
plate; t = neural tube. A, neural plate or tube inductions; B, all grades of palisade inductions; D, ectodermal proliferation.
Effect of Dyes on Induction and Respiration in Amphibian Gastrula 153
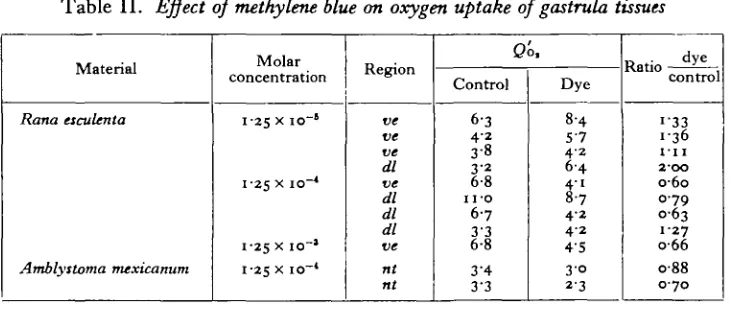
[image:4.451.45.410.232.387.2]great as that recorded for echinoderm eggs by Barron & Hoffman (1930), but more nearly resembles the increases found by Bodine & Boell (1936) on the grasshopper embryo. Here the increases were 2% on the pre-diapause embryos, 100% on the diapause embryos, and about 25 % on the post-diapause embryos. Whether there is any parallelism between the effects of methylene blue on respiratory rate here recorded, and the effect on induction, must be left for further work to determine. The only error which might be introduced into the experiments required for the results of Table II would be that the nitrogen content of the dye in the diver is included in the Kjeldahl nitrogen, thus giving too low values for the experimental values as opposed to the controls. However, it may be calculated that this error, even in the strongest concentrations of dye, cannot exceed 5 %.
Table II. Effect of methylene blue on oxygen uptake of gastrula tissues
Material
Rana esculenta
Amblystoma mexicattum
Molar concentration
1-25 x io~*
1-25 x io~~*
1-25 x io"J
1-25 x io~*
Region ve ve ve dl tie dl dl dl ve nt nt Q'o, Control 6-3 4-2 3-8 3-2 6-8 n o 6-7 3 3 6-8 3'4
3-3
Dye 8 4 5 7 4'2 6 4 4 1 8 7 4 2 4 2 4'5 3 O 2'3
Ratio d y C control
i ' 3 3 1 3 6 i n
2-OO
0 6 0
0 7 9 0 6 3
1 2 7
0 6 6 0 8 8
0 7 0
Note. J2o, = c.mm. oxygen uptake by tissue corresponding to i mg. nitrogen of Kjeldahl per hour. dl=donal lip region; u« = ventral ectoderm; nt=just closed neural tube.
SUMMARY
1. It is shown that pieces of presumptive epidermis (ventral ectoderm of the gastrula), when isolated into weak solutions of several dyes, will undergo neural differentiation. Dyes such as Janus green and neutral red, which are not known to accelerate cell respiration, appear to have this effect, as well as methylene blue, the accelerating action of which on cell respiration is well known.
2. Measurements of the oxygen consumption of isolated pieces of the gastrula by the Cartesian Diver method show that methylene blue, if in weak concentration, has an accelerating action of about 45 %. In stronger concentrations it is inhibitory.
ACKNOWLEDGEMENTS
indebted for help and advice. The statistics of explantation include a few isolations made by the former in the summer of 1937. We also wish to thank Dr F. Dickens for the gift of a specimen of phenazine methochloride, and the Government Grant Committee of the Royal Society for a grant to Dr Needham which partially defrayed the cost of the work.
REFERENCES
BARRON, E. S. G. (1929). J. biol. Chem. 81, 445. (1930). J. exp. Med. 52, 447.
BARRON, E. S. G. & HARROP, G. A. (1928). J. biol. Chem. 79, 65. (1928). J. exp. Med. 48, 207.
BARRON, E. S. G. & HOFFMAN, L. A. (1930). J. gen. Physiol. 13, 483.
BODINE, J. H. & BOBLL, E. J. (1936). PTOC. SOC. Exp. Biol., N.Y., 34, 629. BOKLL, E. J. & NEEDHAM, J. (1939). PTOC. roy. Soc. B (in the Press).
COMMONER, B. (1938). J. cell. comp. Physiol. 12, 171. DICKENS, F. (1936). Biochem.J. 30, 1064.
ELLIOTT, K. A. C. & BAKER, Z. (1935). Biochem. J. 29, 2396.
FINKELSTEIN, E. M. & SCHAPIRO, E. M. (1937). Exp. Med. {Ukraine), 3, 5.
HOLTFRETER, J. (1933). Arch. EntwMech. Org. 128, 584. LINDERSTR0M-LANO, K. (1937). Nature, Lond., 140, 108. MARSTON, H. R. (1923). Biochem. J. 17, 851.
NEEDHAM, J. & BOELL, E. J. (1939a). Biochem. J. (in the Press). (i939fr). PTOC. roy. Soc. B (in the Press).
NEEDHAM, J., BOELL, E. J. & ROGERS, V. (1939). Proc. roy. Soc. B (in the Press).
RUGH, R. (1937). Science, 85, 588.
WADDINGTON, C. H., NEEDHAM, J. & BRACHET, J. (1936). Proc. roy. Soc. B, 120, 173.
WADDINGTON, C. H., NEEDHAM, J., NOWINSKI, W. W. & LEMBERG, R. (1935). Proc. roy. Soc. B, 117,
289.
E X P L A N A T I O N O F P L A T E I
Fig. 1. RA3—e. Methylene blue, M 1-25 x io~5. A well-formed neural tube (A), x 235. Fig. 2. RA 3—e (another piece). Methylene blue, same concentration. A palisade of neural cells (B).
x 140.
Fig. 3. RA5— d. Janus green, M 1-25 x io~*. A large cartwheel-shaped neural (?) induction (A). x 100.
Fig. 4. RA5— q. Janus green, M 1-25 x io~*. A neural plate of unusual form (B). x 100. Fig. 5. JG5 — 2. Janus green, M 1-25 x 1 o~6. Chaotic neuralization (B). x i o o .
JOURNAL OF EXPERIMENTAL BIOLOGY, XVI, 2. PLATE I
Fig. 1. Fig. 2.
Fig. 5- Fig. 6.