ELECTRONIC STRUCTURE, SURFACE REACTIVITY A N D SITE ANALYSIS O F TRANSITION METAL COMPLEXES A N D METALLOPROTE INS BY X - R A Y P H O T O E L E C T R O N SPECTROSCOPY
Thesis By
Frank John Grunthaner
In Partial Fulfillment o f the Requirements "
f o r the Degree sf Doctor o f Philosophy
California I n s t i t u t e o f Technology Pasadena, California
I974
As t h i s p e r i o d of my l i f e draws t o a c l o s e , it i s a p p r o p r i a t e t o
remember t h o s e who have c o n t r i b u t e d s o much p r o f e s s i o n a l l y and p e r s o n a l l y during t h i s time and work. The understanding and p a t i e n c e of my t h e s i s a d v i s o r , D r . Harry B. Gray, i s g r a t e f u l l y acknowledged. H i s perception, c r e a t i v i t y , and imagination were a constant s t i m u l a t i o n and encouragement,
I am deeply indebted t o D r . Paul Pietrokowsky who introduced me t o e l e c t r o n spectroscopy and s t e a d f a s t l y r e s i s t e d my i n i t i a l pessimism w i t h h i s i n t u i t i v e b e l i e f i n t h e f u t u r e promise of t h i s f i e l d .
D r . J a y A. Young who i n i t i a t e d my education s o many years ago, must be remembered f o r t h e considerable i n f l u e n c e of h i s teachings on my p r e s e n t and p a s t .
My years a t Calitech have been memorable and deeply s a t i s f y i n g due i n l a r g e measure t o t h e wealth sf my f r i e n d s h i p s with Mr. John Swmson, D r . Jurg Waser, D r . Joe Gordon, and M r . Bob Nixon. P a r t i c u l a r mention must be.made of t h e e x h i l a r a t i n g experience sf having been a b l e t o teach
and work with t h a t unique breed of s c h o l a r , t h e Caltech undergraduate. My studencs i n Chem 1 and 3 t a u g h t me f a r more than 1 was a b l e to imparc -co them. The considerable a s s i s t a n c e of two o f t h e s e s t u d e n t s , M r . Lou S c h e f f e r and Miss Paula Clendening, who worked Bong and hard with m e during t h e l a s t hours of t h i s work, i s g r a t e f u l l y acknowledged,
iii
t o t h e p r o g r e s s of t h i s work.
My c o l l e a g u e s a t Caltech i n our r e s e a r c h group, Dana Powers, Bob Holwerda,
J i l l Rawlings, and D r . Diane Guttermann gave i n v a l u a b l e experimental
a s s i s t a n c e and endured t h e development o f t h e i d e a s p r o j e c t e d i n t h i s
t h e s i s . J i m Wurzback has been a s o u r c e of c o n s i d e r a b l e t e c h n i c a l a s s i s -
t a n c e and h a s t a u g h t me much.
D r . Sten Samson h a s labored f o r many long hours t r y i n g t o t r a n s l a t e my
crude w r i t i n g i n t o p a s s a b l e communication s o t h a t o t h e r s could r e a d t h i s
t h e s i s . H i s s e l f l e s s d e t e r m i n a t i o n and p a t i e n c e w i t h my i l l i t e r a c y have
p u t me deeply i n h i s d e b t .
L a s t l y my f e e l i n g s concerning t h e p a t i e n c e and u n d e r s t m d i n g of my mother
and f a t h e r through a l l t h e s e y e a r s of s t u d y cannot adequately be expressed.
Through t h e long hours sf a s s i s t a n c e i n t h e p r e p a r a t i o n sf t h i s t h e s i s ,
t h e y e a r s o f compassion, understanding, and s t r e n g t h she has given me,
i v ABSTRACT
High r e s o l u t i o n x-ray photoelectron core l e v e l s p e c t r a f o r a v a r i e t y of
t r a n s i t i o n metal complexes and m e t a l l o p r o t e i n s a r e p r e s e n t e d and r e l a t e d
t o q u e s t i o n s of s u r f a c e r e a c t i v i t y , e l e c t r o n i c s t r u c t u r e and metal i o n
valency. Core l e v e l s p e c t r a of VOSO 4 J 'JB2 9 'J203> 'J205> I J , VN, Na3V04, B203, B, H3B03, Na2B40,10H20, Au,Laccase, S t e l l a c y a n i n , P l a s t o c y a n i n ,
2 - Hemocyanin, Spinach Ferredoxin, High P o t e n t i a l Iron P r o t e i n , Fe S (SEt)4
,
4 4
CuC12 (n-Bu S (CH2) *S-n-Bu)
,
Cu(n-Pr(C4H4N20H) 5) a r e r e p o r t e d .E l e c t r o n binding e n e r g i e s c o r r e l a t e d by i n t e r n a l r e f e r e n c i n g a r e t a b u l a t e d
f o r t h e s p e c i e s s t u d i e d .
Charging e f f e c t s i n i n s u l a t i n g chemical s p e c i e s a r e q u a n t i f i e d and used
t o examine t h e e l e c t r o n i c and chemical p r o p e r t i e s of t h e compounds
s t u d i e d . I t is demonstrated t h a t charge n e u t r a l i z a t i o n i n e l e c t r o n spectroscopy i s p r i m a r i l y due t o capture of secondary e l e c t r o n s i n t h e sample chamber with average k i n e t i c e n e r g i e s below 6 eV.
Data handling methods f o r n o i s e removal based on F o u r i e r methods a r e
presented. Treatment of t h e observed photoelectron l i n e shape i s given
and s p e c t r a l deconvolution i s employed i n d a t a a n a l y s i s .
P a s s i v a t i o n r e a c t i o n s on vanadium d i b o r i d e i n carboxylic a c i d a t t a c k a r e
a t t r i b u t e d t o t h e formation of s u r f a c e vanadium oxides. Q u a n t i t a t i v e
a s p e c t s of t h e e l e c t r o n s p e c t r a a r e developed, t o g e t h e r with observed
charging phenomenon, t o show t h e unperturbed ebservazion of adsorbed s p e c i e s
v
The valency of copper i n Laccase i s s t u d i e d by means of new copper sulfo-complexes and t h e well-defined p r o t e i n s S t e l l a c y a n i n and P l a s t o -
cyanin. Type 1 and Type 2 copper i s observed and Type 3 copper i s described by a CUI-S-S-CUI model. Oxyhemocyanin i s a l s o s t u d i e d and assigned i n
terms of a c u p r i c model.
The ~ e ~ ~ ~ (c l u s t e r complex ~ e t ) ~ ~i s - examined and t h e mercaptyl and s u l f i d o s u l f u r s a r e assigned. Only one i r o n s t a t e i s observed, F ~ I I I ,
supporting t h e d e l o c a l i z e d model of t h i s s p e c i e s . I t i s suggested t h a t t h e oxidized and reduced c l u s t e r s p e c i e s can be s t a b i l i z e d i n t h e e l e c t r o n
spectrometer. By comparison of t h e p h o t o e l e c t r o n s p e c t r a , t h e c l u s t e r
complex i s shown t o be a reasonable model f o r 2-Fe and 4-Fe Ferredoxins and a d i s c u s s i o n of t h e s p e c t r a l d i f f e r e n c e s i s given.
The experimental parameters necessary f o r t h e s u c c e s s f u l s t u d y of m e t a l l o -
p r o t e i n s by p h o t o e l e c t r o n spectroscopy a r e developed i n terms of elemental
s e n s i t i v i t y , decompositioii p r o f i l i n g , photoreduction, energy r e f e r e n c e
TABLE OF CONTENTS
I . INTRODUCTION
P I . GENERAL THEORETICAL CONSIDERATIONS
A. Theory of P h o t o e l e c t r i c Emission 1. General E i n s t e i n i a n Equation 2 . Work Function Treatment 3 . VC E f f e c t s
B . Core Electron Binding Energy S h i f t s 1. Free Atom Description
2 . Approximate Molecular Models 3 . S o l i d S t a t e Complications 4. Reference Level
C. Second Order E f f e c t s 1. M u l t i p l e t S p l i t t i n g 2 . E l e c t r o s t a t i c S p l i t t i n g 3 . S a t e l l i t e T r a n s i t i o n s D. Q u a n t i t a t i v e Aspects
1. T r a n s i t i o n P r o b a b i l i t i e s 2 . Empirical Relationships
H P
I. EXPERIMENTAL METHODSA. General Experimental Description I. Basic System
2
.
Vacuum Requirements 3. X-ray Contributions4. Observed Line Width Contributions B. Instrumental C h a r a c t e r i s t i c s
Page 1
1.
a. Vacuum Level
b , Magnetic S h i e l d i n g
vii
2.
McPherson ESCA
36a.
High Resolution Analyzer
b
.
Sample Chmber Configuration
C. Voltaic Potential Effects
1. Flood Gun Experiments
2.Bias-Potential Method
1.
Observational Depth
a. Surface Effect
b.
Contamination
c. Oxidation-Reduction
d.
Success of Anaerobic Conditions
e.Reproducibility
2.
Mounting Methods
a. Metals
b.
Solids
1. Powders on Adhesive Tape
2.
Powder Pellatizing on Graphite Substrates
3 .
Physical Abrasion on Soft Coarse Metal Substrates
4.Compression on Metal Grids
5 .
Slurry Deposition
6 -
Thin Films from Solution Deposition
a. Amorphous
b.
Single Crystals
c.
Liquids
1.
Frozen Solutions
a. Freeze Drying
, b.
Thermal Variation of Work Function with
Temperatures
2.Liquid Beams
3 .
Particle Size Effects
DATA ANALYSIS
A. Description of Data Characteristics
I .
N(E) Distribution
2.Channel Sampling
3.Time-Averaging
v i i i
1. Moving Average
2 . T r i a n g u l a r Weighting Functions 3 . Least Squares Convolution Functions C. F o u r i e r Noise F i l t r a t i o n Methods
---"-
1 . Frcyucncy Analysis of Signal 2 . Power S p e c t r a l Density of Noise
3 . Frequency o f I n s t r u m e n t a l l y Generated Noise 4 . Truncation of Higher Order Terms
a . Sin ( x ) / x Convolution i n r e a l domain b . Ringing O s c i l l a t i o n s
5. Optimal Resolution F i l t r a t i o n
a. Estimation Methods
-
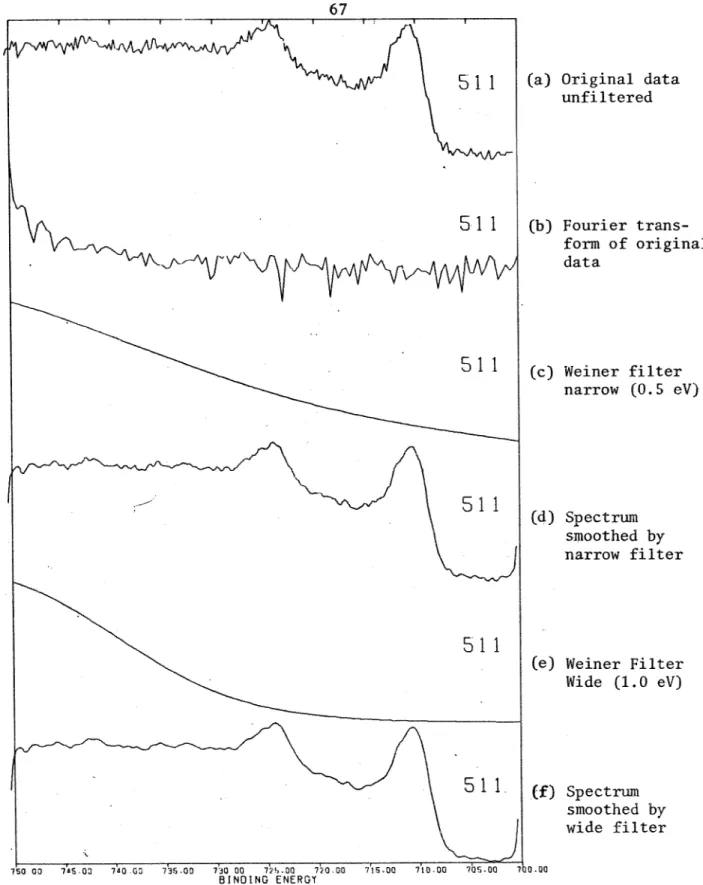
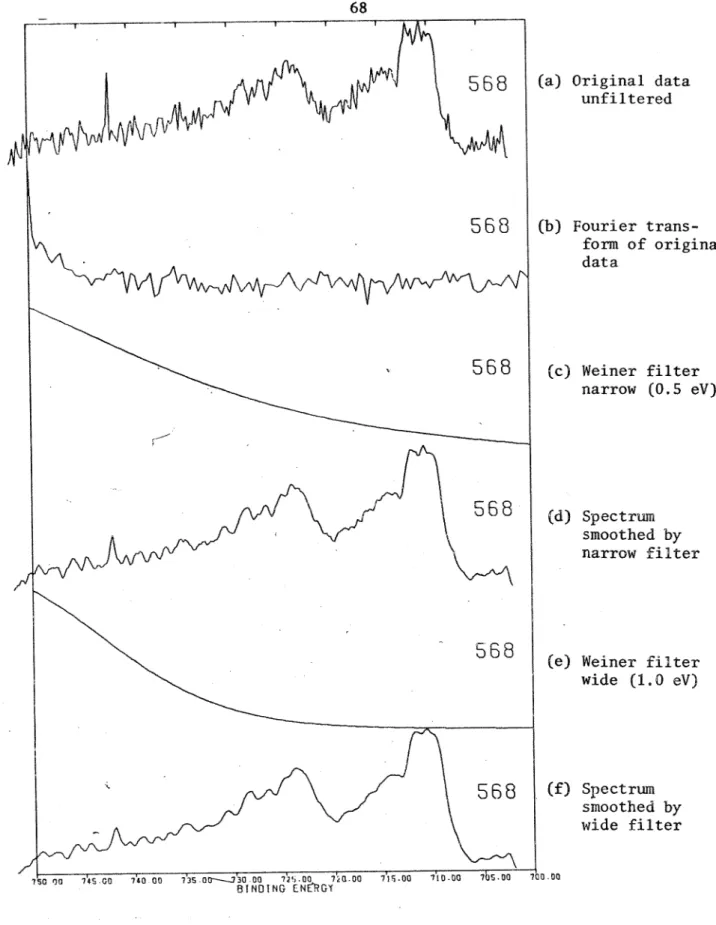
Wiener F i l t e r 1. Derivation2 , Application
3 . Moise/Signal Sample
b. Detection Methods
-
Matched F i l t e r D. DeconvolutionI . Experimental Basis
2. Instrumental Broadening Functions 3 . Preliminary R e s u l t s
SURFACE STUOIES OF 'v'ia4ADiL% OPBGRiDE
A. I n t r o d u c t i o n
1. P r e p a r a t i o n of Vanadium Carboxylate Dimers 2 . V O5 C a t a l y s i s
3 . C
2
arging E f f e c t Experiments8. Experimental
I . Source of Vanadium Compounds a. VB3 Samples
b. Vanadium Model Compounds 2 . Mounting of Samples
a. Graphite P e l l e t s
b . Abrasion i n t o Coarse A 1 P l a t e 3. Flood Gun Experiments
R e s u l t s and Discussion
, 1. S p e c t r a l Analysis of VB2 s p e c t r a
a. Charging Experiments b
.
Oxidation E f f e c t sc . P a s s i v a t i o n Differences
d. Q u a n t i t a t i v e Analysis o f Surface Composition e , D i f f e r e n t i a l Analysis of Sample Charging Behavior 2 . Model Compound S t u d i e s
a. This work
1. Oxide Overlayer 2. Charging V a r i a t i o n s b. Previous S t u d i e s
V I . COPPER METALLOPROTEINS 120
A. I n t r o d u c t i o n 124
1. General Problem o f Valency i n M e t a l l o p r o t e i n s 2. Previous ESCA P r o t e i n S t u d i e s
B. Experimental
I . Sample Source
a. Buffered S o l u t i o n s b. Freeze-dried Specimens 2. ESCA Sample P r e p a r a t i o n
a. A i r Dried Thin Films b. Frozen S o l u t i o n s
c . Variable Temperature
d . Charging
3 . Radiation Decomposition 4. Photo Reduction
5. Energy Reference Standards 6. Curve Analysis
@.
R e s u l t s and DiscussionModel Complex S t u d i e s a. Binding Energies
b, S a t e l l i t e Observations c . Photo Reduction
Model Copper P r o t e i n s a. S t e l l a c y a n i n b. Plastocyanin
3 . Laccase
a. Binding Energies
b . Q u a n t i t a t i v e Analysis of Photo Reduction Products c. S a t e l l i t e Observation
d. Decomposition of P r o t e i n e . S u l f u r E l e c t r o n S p e c t r a
E.
Difference Analysis o f Model Complex Data g . Valency Assignments4 . Hemocyanin
a. Binding Energies
b. S a t e l l i t e Observations c. Copper S p e c t r a
d. Oxygen S p e c t r a
e . Valency Assignments D. Conclusions
VII. STUDIES OF THE IRON-SULFUR MOIETY I N 2-IRON
AND
4-IRON FERREWXINSA. I n t r o d u c t i o n
1. I r o n i n P r o t e i n s
2. E l e c t r o n Spectroscopy o f S u l f u r Containing Compounds B. Experimental
3; .S&mple S n u r c e
2. Anaerobic P r e p a r a t i o n 3. Energy Reference Standard 4. Curve Analysis
C. R e s u l t s and Discussions I . Model Complex S t u d i e s
a. C l u s t e r Complexes 1. Holm's Compound 2. Other
3 . Oxidat ion E f f e c t s 4. Difference Analysis b. Iron-Sulfur Complexes
1. Fe S p e c t r a 2 , S u l f u r 2p Region
3 . Other S p e c t r a l Regions 2. Perredoxin S p e c t r a
b.
Sulfur Spectra
1. Oxidation Effects
2.
Labile Sulfur Assignments
Conclusions
1.
Iron Spin State
2.
Sulfur
ProteinEnvironments
3.
Future work
VIII. CONCLUSIONS
A. Application of ESCA to a Variety of Fundamental Problems
B. Experimental Design Criterion
C .
Passivation Studies of VB2
D. Valency in Copper Metalloproteins
E. Studies of Sulfur Environments in Proteins
F. Iron Cluster Complexes as Models of 'Iron-Sulfur
Proteins
G.Data Handling Methods
IX.
APPENDICES
A. Index of Spectra
B. Electron Energy Diagrams
C.
General ESCA Data Analysis Progrm
1 I .
-
INTRODUCTIONThe a p p l i c a t i o n of s p e c t r o s c o p i c techniques, which p r o f i l e t h e i n t e r a c t i o n
of e l e c t r o m a g n e t i c r a d i a t i o n with m a t t e r has y i e l d e d information about
t h e e l e c t r o n i c s t r u c t u r e . Generally, t h e s e techniques measure r e l a t i o n -
s h i p s between s p e c i e s i n t h e ground s t a t e and i n t h e bound e x c i t e d s t a t e s .
A few years ago, K. Siegbahnf s ESCA monograph1 e x c i t e d new i n t e r e s t i n a
s p e c t r o s c o p i c method which made p o s s i b l e t h e d i r e c t study o f t h e energy
d i s t r i b u t i o n o f bound e l e c t r o n s i n atoms, molecules and s o l i d s .
This method r e q u i r e d t h e i l l u m i n a t i o n of a sample with electromagnetic
r a d i a t i o n and t h e a n a l y s i s o f t h e k i n e t i c energy o r momentum d i s t r i b u t i o n
of t h e p h o t o e l e c t r o n s emitted. A considerable number of reviews of t h e t h e o r y 2 > Y 4, experiment5, and a p p l i c a t i o n 7 , o f e l e c t r o n spectroscopy
have appeared s i n c e t h e p i o n e e r i n g work o f t h e Uppsala group.
1. Siegbahn, Kai; Nordling, Carl; Fahlman, Anders; Nordberg, Ragnar; Hamrin, K j e l l ; Hedman, J a n ; Johansson, Gunnilla; Bergmark, Tossten; Karlsson, Sven; Lindgren, Hngvar; Lindberg, Bernt; E l e c t r o n Spectros- copy f o r Chemical Analysis
-
Atomic, Molecular and S o l i d S t a t e Studies-
bv Means o f E l e c t r o n S ~ e c t r o s c o ~ v . , L Almavist G Wiksells ~ o k t r v c K r i AB, Stockholm, Sweden, 1967.
Hollander, J . M . , and S h i r l e y , D.A.,
-
Ann. Rev. NucP.Sci.,-
20, 435 (1970). Siegbahn, e t . aP., ESCA Applied t o Free Molecules, North Holland,Amsterdam, 1969,
Gelius, U., Molecular O r b i t a l s and Line I n t e n s i t i e s i n ESCA Spectra Uppsala U n i v e r s i t y , I n s t i t u t e of physics, P u b l i c a t i o n #753, November
1971.
Fadley, C.S., T h e s i s , U n i v e r s i t y of C a l i f o r n i a , Berkeley, 1970.
P o l l a k , R . A . , T h e s i s , U n i v e r s i t y of C a l i f o r r ~ i a , Berkeley, 1972,
Bremser, W . , Xray Photoelectron Spectroscopy, i n Topics i n Current Chemistry, 36,"Springer-Verlag, B e r l i n , 1973.
2
The e l e c t r o n s of i n t e r e s t escape from a depth of 4 t o 120 i n s i d e t h e s o l i d sample and a r e n o t perturbed by c o l l i s i o n . The observed energy of t h e photo-emitted e l e c t r o n i s r e l a t e d t o t h e atomic number o f t h e emitting '
atom as well a s t h e p r i n c i p a l quantum number of t h e bound e l e c t r o n , t h e angular momentum and t h e chemical environment. The discovery of t h e
chemical s h i f t of t h e core e l e c t r o n bindi.ng e n e r g i e s promised considerable information about chemical s t r u c t u r e
.
I n i t i a l enthusiasm f o r t h i s new form of spectroscopy was dampened because of experimental and instrumental d i f f i c u l t i e s : (1) The chemical s h i f t i s small i n r e l a t i o n t o t h e n a t u r a l X-ray l i n e widths and instrumental
r e s o l u t i o n . (2) Surface contamination of samples gave r i s e t o d a t a which was n o t r e p r e s e n t a t i v e of t h e bulk m a t e r i a l . (3) The sample o f t e n
deoomposed i n t h e vacuum o r s u f f e r e d r a d i a t i o n damage, 44) Charge b u i l t up on t h e sample a l t e r e d t h e energy of emitted e l e c t r o n s i n a non-repro- d u c i b l e manner.
Because of t h e g r e a t p o t e n t i a l of tk method a s an a n a l y t i c a l t o o l , t h e author decided t o overcome t h e s e d i f f i c u l t i e s and t o study t h e e l e c t r o n i c s t r u c t u r e of t r a n s i t i o n - m e t a l complexes. Also, it seemed important t o apply ESCA t o b i s - i n o r g a n i c chemistry, i n c o - q o r a t i n g t r a n s i t i o n metals. Here enzymatic a c t i v i t y a t s p e c i f i c s i t e s 9 ( o f t e n c a l l e d a c t i v e s i t e s )
can be s t u d i e d , e s p e c i a l l y a s regards oxygen and e l e c t r o n t r a n s p o r t .
9 . Gray, H . B . , @per P r o t e i n s Based on
3
Model complexes have been developed, which approximate t h e metal-protein
environment. To such systems s t a n d a r d s p e c t r o s c o p i c techniques a s well
a s magnetic and electrochemical methods have a l r e a d y been a p p l i e d g l l l
.
Metal ions a t a c t i v e s i t e s of enzymes have unusual ligand configurations
probably as a r e s u l t of t h e i r unique e l e c t r o n i c s t r u c t u r e . To devise
a model r e q u i r e s knowledge of t h e i d e n t i t y of l i g a n d s , t h e i r coordination,
and t h e i r physical a c c e s s i b i l i t y .
T h e p r e s e n t work has concerned i t s e l f with a s e r i e s of copper and i r o n
m e t a l l o p r o t e i n s f o r which w e l l - c h a r a c t e r i z e d model complexes were a v a i l -
a b l e . The valency and s i t e environment of t h e metal were d e t e m i n e d i n
l a c c a s e and hemocyanin. Concurrently, s u l f u r environments i n metallo-
p r o t e i n s were s t u d i e d . A s i m i l a r study of t h e i r o n - s u l f u r c l u s t e r s i n
f e r r e d o x i n s was c a r r i e d o u t .
In t h i s t h e s i s emphasis i s placed mainly on t h e experimental a s p e c t s a s
P O ,-gar& ESCA a ~ d metal l ~ p r e l e i n s
.
Radizt ioz dw.age, d e h y d r a t i o n i nvacuum and thermal decomposition were monitored. An energy r e f e r e n c i n g
system was devised f o r t h e comparison of model complex and p r o t e i n
s p e c t r a .
10. T s i b r i s , J . C . M . , and Woody, R.W., Coordination Chemistry Reviews, E l s e v i e r Publishing, Amsterdam, 1970.
4
S p e c i a l s e c t i o n s of t h i s t h e s i s a r e devoted t o t h e d e s c r i p t i o n o f
experiments concerning charging e f f e c t s and t o removal'of n o i s e through
d a t a - h a n d l i n g methods.
X-ray p h o t o e l e c t r o n spectroscopy i s l i m i t e d t o t h e examination of only t h e f i r s t few angstroms of t h e s u r f a c e o f a s o l i d and seems t o be an i d e a l t o o l f o r t h e s t u d y o f t h e p a s s i v a t i o n of vanadium d i b o r i d e . I n
t h i s c a s e , t h e s t u d y o f charging e f f e c t s l e a d s t o i n c r e a s e d s t r u c t u r a l
i n f o r m a t i o n . The r e s u l t s o f t h i s s t u d y a r e compared with d a t a r e c e n t l y
o b t a i n e d elsewhere on c a t a l y s t s c o n s i s t i n g o f vanadium oxide and vanadium-
1 1 . GENERAL THEORETICAL
-
CONSIDERATIONS A. Theory of Photoelectron EmissionThe work r e p o r t e d i n t h i s t h e s i s concerns t h e i n t e r p r e t a t i o n of photo emission s p e c t r a r e s u l t i n g from X-ray adsorption by core l e v e l s . Several d e t a i l e d reviews of t h e o r e t i c a l a s p e c t s of e l e c t r o n spectroscopy a r e given i n t h e l i t e r a t ~ r e l , ~ > ~ , ~ . The r e c e n t treatment by ~ a d l e ~ l ~ i s t h e most r i g o r o u s one. The energy conservation equation f o r a f r e e atom o r molecule i s
i
hv + E = EKIN + Ef(k)
,
wherehv z photon energy
Ei E t o t a l i n i t i a l energy of t h e adsorber E~~~ r K i n e t i c energy of t h e photo-electron
Ef[k) 5 t o t a l f i n a l energy of t h e adsorber a f t e r t h e
e j e c t i o n o f an e l e c t r o n from t h e ~ t h o r b i t a l a s a photo-electron
For atomic s t a t e s , t h e index k s t a n d s f o r t h e quantum numbers nP o r n l j .
For molecules, k i s determined by t h e o v e r a l l symmetry of t h e adsorbing
i f
molecular o r b i t a l . E and E (k)s may i n c l u d e c o n t r i b u t i o n s from e l e c t r o n i c , v i b r a t i o n a l , r o t a t i o n a l and t r a n s l a t i o n a l e n e r g i e s .
The energy p e r t u r b a t i o n caused by f a c t o r s o t h e r than X-ray adsorption [thermal e x c i t a t i o n , secondary e l e c t r o n c s l i i s i o n ) a r e small compared t o t h e r e s o l v i n g power of t h e instrument, which i s 0.55 eV. Thus E~ i s assumed t o be unique o r single-valued.
6
Relaxation processes can l e a d t o u n c e r t a i n t y i n t h e f i n a l energy o f
f
t h e s t a t e (E [ k ) ) . The p h o t o e l e c t r o n v e l o c i t i e s a r i s i n g from i r r a d i a t i o n by s o f t X-rays a r e approximately n o n - r e l a t i v i s t i c . Considering momentum
f
'
conservation E (k) can be w r i t t e n as t h e sum of a r e c o i l energy E p l u s r a term containing t h e e n e r g i e s corresponding t o a l l o t h e r modes o f
mot ion
I f
ef
(k) = E + E (k).
rFor a given hv and E E i n c r e a s e s with decreasing atomic o r molecular K I N ' r
mass I . The r e c o i l e n e r g i e s f o r d i f f e r e n t atoms c a l c u l a t e d by Siegbahn
a r e : H
-
0 . 9 eV, L i-
0 . 1 eV, Na-
0.04 eV, K-
0.02 eV, Rb-
0.01 eV (AlKa, EKIN = 1486 eV). E can be n e g l e c t e d with r e s p e c t t o instrumentalr
r e s o l u t i o n , and equation (1) reduces t o
The binding energy o f an e l e c t r o n i s defined a s t h e p o s i t i v e energy .
required t o remove it t o i n f i n i t y with zero k i n e t i c energy. ~ e ~ r e s e n t i n ~ t h e binding energy of t h e k c e l e c t r o n by Eb(k), t h i s r e l a t i o n s h i p i s
f i
Eb(k)
=
E (k)-
E (4)and equation 3 can be r e w r i t t e n a s
hv = E + Eb(k)
.
KIN (5)
FIGURE 1
8 ~t a b s o l u t e zero, t h e Fermi l e v e l ,
E~
'
f o r a metal i s t h e h i g h e s t occupied l e v e l . This i n t e r p r e t a t i o n of E i s approximately c o r r e c tF
a t room temperatures. The work f u n c t i o n , 9 , f o r a s o l i d i s defined a s t h e energy s e p a r a t i o n between t h e vacuum and t h e Fermi l e v e l s . When t h e specimen and spectrometer a r e i n thermodynamic e q u i l i b r i u m , t h e two Fermi l e v e l s w i l l r e s t a t t h e same p o t e n t i a l . The r e s p e c t i v e vacuum l e v e l s need n o t be e q u i l i v a n t ; s e e Figure 1. When t h e photo e l e c t r o n p a s s e s from t h e sample t o t h e a n a l y z e r , it w i l l experience a p o t e n t i a l
equal t o $
-
where @ i s t h e work f u n c t i o n of t h e sample.s 'spectrometer s
The k i n e t i c energy,
E ~ o f t h e e l e c t r o n i n s i d e t h e analyzer ~ ~ ' i s r e l a t e d t o t h e escaping k i n e t i c energy, E i I N , a t t h e sample s u r f a c e by
Binding e n e r g i e s i n a m e t a l l i c s o l i d can be measured e a s i l y r e l a t i v e t o t h e Fermi Bevels o f sample and spectrometer. For s o l i d s , equation (5) becomes
where t h e s u p e r s c r i p t F r e f e r s t o r e f e r e n c i n g a t t h e F e m i l e v e l .
In semiconductors and i n s u l a t o r s , t h e F e m i l e v e l i s n o t e a s i l y defined, s i n c e it l i e s somewhere between t h e valence bands (predominately f i l l e d ) and t h e conduction bands Qpredominately unoccupied) 3 . Themodynmic e q u i l i b r i u m does n o t occur when t h e specimen impedes r e p l e n i s h i n g of t h e e l e c t e d e l e c t r o n s .
9
Charging e f f e c t s r e s u l t i n a n e t r e t a r d a t i o n of t h e photoelectrons b e f o r e they e n t e r t h e spectrometer. The magnitudes of t h e s e e f f e c t s vary from 1 t o 100 e l e c t r o n v o l t s and a r e observed f o r both s o l i d s 5 and g a s e s 3 . The charging s h i f t depends upon t h e photon f l u x , t h e secondary e l e c t r o n f l u x from spectrometer s u r f a c e s , t h e temperature, m d t h e s u r f a c e and bulk c o n d u c t i v i t i e s of s o l i d samples.
I f t h e charge i s uniformly d i s t r i b u t e d over t h e s u r f a c e of t h e sample (of magnitude, Vc) then, equation 7 becomes
hv = E
KIN + E~ (k) + 'c 'spectrometer
.
(8) I f V i s presumed t o be zero, t h e binding e n e r g i e s would appear t o beC
h i g h e r than t h e y a c t u a l l y a r e . A non-uniform charging p o t e n t i a l would r e s u l t i n a broadening and d i s t o r t i o n of t h e peaks.
B. S h i f t s i n Binding Energy of Core Electrons
Molecular formation and r e a c t i o n causes r e d i s t r i b u t i o n s of valence e l e c t r o n s compared t o t h e f r e e atom, These r e d i s t r i b u t i o n s a f f e c t t h e p o t e n t i a l of t h e i n n e r e l e c t r o n s a d r e s u l t i n changes i n t h e i r binding e n e r g i e s ,
In SiegbahnPs modelY f o r p r e d i c t i n g chemical s h i f t s , t h e valence e l e c t r o n s a r e r e p r e s e n t e d by a s p h e r i c a l s h e l l of n e g a t i v e charge. The p o t e n t i a l e x e r t e d by t h i s s h e l l on t h e core e l e c t r o n s w i t h i n i t i s given by
where 6 i s t h e number of valence s h e l l e l e c t r o n s and r i s t h e r a d i u s of t h e s h e l l . I f one e l e c t r o n i s l o s t , t h e screening p o t e n t i a l seen by t h e core e l e c t r o n s i s reduced by
2
10
which i s e q u i v a l e n t t o t h e chemical s h i f t . This model c o r r e c t l y pre-
d i c t s t h e order of magnitude of t h e chemical s h i f t b u t overestimates
t h e e x t e n t of charge t r a n s f e r .
The chemical s h i f t s of a l l core l e v e l s should be t h e same by t h i s f r e e
atom model. Fadley, e t a1. 14, i n an i n v e s t i g a t i o n o f iodine compohds,
found t h a t t h e s h i f t s i n t h e 2s1/2, 2 ~ 1 / 2 ' 2P3/2' 3d3/2' 3d5/29 4s1/2' and 4p l e v e l s of i o d i n e were equal w i t h i n experimental e r r o r . Oxida-
3/ 2
t i o n number was t h e f i r s t empirical q u a n t i t y used t o p r e d i c t t h e chemical
s h i f t . This parameter, however, gives a poor i l l u s t r a t i o n of t h e a c t u a l
charge d i s t r i b u t i o n i n molecules. Here, t h e d i s t r i b u t i o n i s b e t t e r
approximated by a number of o t h e r models. The most important of t h e s e
a r e :
1. The Pauling Valence Bond (PVB) model
2 . The extended Huckel Molecular O r b i t a l model
3 . The CNDO molecular o r b i t a l method.
The PVB method e m p i r i c a l l y c o r r e l a t e s bond lengths and e l e c t r o n e g a t i v i t i e s t o e s t i m a t e t h e d i s t r i b u t i o n of atomic charge1 5. The atom charge i s
t h e sum sf t h e formal charge on atom A and t h e charge t r a n s f e r r e d from bonds of p a r t i a l i o n i c c h a r a c t e r , Expressing t h i s aPgebraicaIly,
s~
= Q, f .EB+AnI where14. Fadley, C.S., Hagstrom, S.M.M., Klein, M.P., and S h i r l e y , D.A., J. Chem. Phys.,
-
48, 3779 (1968).15. Pauling,
L.,
,
Ithaca, New York, Cornelland
11
A r formal charge on c o v a l e n t l y bonded atom A
C r summation over a l l bonds t o atom A n average bond number
r p a r t i a l i o n i c c h a r a c t e r o f bond I
- -0.25 (x. - X ) 2
= l - e A B
X~
-
xBf
e l e c t r o n e g a t i v i t y d i f f e r e n c e between A and 0.The p r e d i c t a b i l i t y of t h e s e models was e v a l u a t e d by Hollander and s h i r 1 e y 2 . The CNDO method gave t h e b e s t c o r r e l a t i o n s with experiment, while t h e
e m p i r i c a l PVB model gave b e t t e r r e s u l t s than t h e extended HuckeP t r e a t - ment.
C. Second Order E f f e c t s
I f t h e i n i t i a l s t a t e has a non-zero angular momentum 9 , t h e n t h e h o l e
c r e a t e d by t h e e j e c t i o n s f a core e l e c t r o n can couple t o J t o form .,
two OP more f i n a l s t a t e s . This e f f e c t has been termed r n u l t i p l e t s p l i t
-
zing5, U n r e s t r i c t e d Hartree-Fock c a l c u l a t i o n s p r e d i c t s p l i t t i n g s ofup t o I 2 eV. The Eree-atom model p r e d i c t s t h a t t h e r e l a t i v e i n t e n s i t i e s of t h e m u l t i p l e t peaks a r e given by t h e s t a t i s t i c a l weight s f t h e two f i n a l s t a t e s .
5
For example, i n t h e spectrum from t h e Mi2' i o n , (d high s p i n )
t h e f i n a l s t a t e s p i n (J) can be 5/2 t 1/2 and t h e m u l t i p l e t i n t e n s i t i e s would be i n t h e r a t i o o f 2 5 + 1 o r 7:s. The s p l i t t i n g observed f o r a
s e r i e s of i r o n compounds5 was 6 eV o r about h a l f t h e s p l i t t i n g p r e d i c t e d .
12
Processes involving more than one e l e c t r o n were f i r s t s t u d i e d i n d e t a i l by Kraus, Carlson and c o - ~ o r k e r s ~ ~ , ~ ~ ~ ~ ~ . These s t u d i e s of neon and
argon used photon e n e r g i e s of 270 eV t o 1 . 5 keV. Two and t h r e e - e l e c t r o n
t r a n s i t i o n s were p r e d i c t e d i n photon adsorption with p r o b a b i l i t i e s a s
h i g h a s 20% f o r each adsorbed photon.
Two types of two-electron t r a n s i t i o n s can be d i s t i n g u i s h e d depending on
whether t h e second e l e c t r o n i s e x c i t e d t o a higher energy bound s t a t e (shake-up) o r t o an unbound continuum s t a t e (shake-off). These t r a n s i -
t i o n s can be w r i t t e n
(121 Shake-up :
( " ~ ) L , s ( n ' l ' p P
hy
( n l I q - I ( n ) ( n l + PhotoelectronShake-off : . (13)
( 1 1
3
TI^)^-'
( n v lflP-l(~KINIt~w)' + Photoelectron,where ( n l B P ) P r e p r e s e n t s some o u t e r s u b s h e l l from which t h e second e l e c - t r o n i s e x c i t e d .
Both processes lower t h e k i n e t i c energy of ehe primary phofoelectron, which leads t o s a t e l l i t e s t r u c t u r e ow t h e low-kinetic energy (high binding energy) s i d e of t h e peak.
17. Kraus, M.Q.; V e s t a l , M . E . ; Johnston, W.H.; and CarPson, T.A.; Phys Rev,
-
133, A385 (1964)18. Carlson, T.A., and Kraus, M.O., Phys Rev,
-
137, A1655 (1965)1 4
I n i t i a l work a l s o demonstrated t h a t f o r AlKa,photons shake-off s t r u c t u r e should be seen f o r binding e n e r g i e s l e s s than 500 eV.
M u l t i p l e - e l e c t r o n t r a n s i t i o n s have been p r e d i c t e d f o r metals20 ,21. These processes a r e complicated by glasmon e x c i t a t i o n .
D. S p e c t r a l Lines and I n t e n s i t y D i s t r i b u t i o n s
X-ray photon adsorption can r e s u l t i n t h e s t i m u l a t i o n of f o u r d i f f e r e n t processes as d e t a i l e d i n Fig. 2 * .
The i n i t i a l X-ray capture r e s u l t s i n promotion of an e l e c t r o n t o e i t h e r a bound s t a t e (X-ray adsorption) o r t o t h e vacuum continuum (X-ray photoelectron emission). In e i t h e r c a s e , a K s t a t e vacancy
i s c r e a t e d .
P a r t of t h e energy of t h e photon s t o r e d i n t h e system i s d i s s i p a t e d by e l e c t r o n r e l a x a t i o n which can r e s u l t i n e i t h e r X-ray fluorescence o r
d
Auger-electron emission. 'l'he p o t e n t i a l emission of Auger e l e c t r o n s complicates t h e i n t e r p r e t a t i o n of t h e observed k i n e t i c energy d i s t r i b u - tion,N(E),because t h e apparent binding energies corresponding t o t h e peaks a r e independent of t h e energy of t h e i n c i d e n t X-ray-photons.
In g e n e r a l , photoelectron and, Auger-electron l i n e s can be s e p a r a t e d i f a p p r o p r i a t e t a r g e t m a t e r i a l s a r e used ( t h u s s e l e c t i n g t h e most s u i t a b l e c h a r a c t e r i s t i c r a d i a t i o n ) . In most cases it r e q u i r e s s u b s t a n t i a l e f f o r t t o change t h e t a r g e t ,
28. Hedin, L . , and Lundqvist, S . , S o l i d S t a t e Physics,
-
2 3 ,P
(19693PHOTOE 1,ECTRON AND AUGER LINES
-
(A1 Xrays)(A = Auger l i n e ; 1 = sub 1/2; 3 = sub 3/2; 5 = sub 5/2; 7 = sub 7/2)
Te4p Bels Rb 3d Ce4d Ni3s Pr4d h12s Nd4d BiSpl T14f7 Cu3s HgS s
In4s Ge3p3 T14f5 I4P Ge3pl Sm4d Sr3d Eu4d P ~ P Zn3s Sn4s T1Ss Pb4f7 Gd4d As3p3 Pb4f5 As3pB Tb4d PbSs S i 2 s 3 3 s W4d Ga3s Bi4f7 Y3d BiSs Ho4d Se3p3 Cs4p3 Bi4f5 S ~ P Se3pl l'e4s Er4d5 Cs4pl Er4d3 Zr3dS Ba493 Tm4d Ge4s Br3p3 ThSp3 Zr3d3 Yb4d5 14s B l s P2s Br3pl Ba4pl La4p3 Lu4dS Yb4d3 C12p As 3s Lu4d3 Nb3dS Ea4p1
Nb 3d3 Ce4p3 Hf4dS Pr4p3 Ce4pP Hf4d3 Nd4p3 Mo3dS S2s ThSpP Evlo3d3 Ta4dS Cs4s Se3s Pr4p3. Rb3p3 Ta4d3 Nd4p 1 W4dS Rb 3p 1 Sm4p3 Ba4s Br3s Eu4p3 W4d3 U5pl Re4d5 Sm4pP .
Sr3p3 C12s La4s
271 Gd4p3
273 * Os4d5
2 74 Re4d3
279 * Ru3d5
280 S r 3 p l
284 Ru3d3
284 Eu4pl
284 * Cls
289 Gd4pl
290 Ce4s
290 Os4d3
290 ThSs
294 * K2p3
295 * Tr4dS
297 K2pl
301 * T3p3
305 Pr4s
306 Ho4p3
307 * Rh3d5
311 Tb4pl
312 Rh3d3
312 I r 4 d 3
313 Y3pl
314 * Pt4d5
316 Nd4s
320 Er4p3
322 Rb3s
32 2 Geh
324 USs
331 * Z ~ 3 p 3
331 Pt4d3
3 32 D Y ~ P ~
334 * A u 4 G
335 = Th4f7
335 * Pd3dS
337 Tin4p3
34 8 Pd3d3
343 Ho4p1
343 Yb4p3
344 Th4fS
345 Zr3pl
347 Sm4s
347 * Ca2p3
350 Ca2pl
352 Au4d3
354 * GeA
358 Sr3s
359 Lu4p3
360 Eu4s
TASLE 1
PHOTOELECTRON AND AUGER LINES (CONTINUED) -
363 * Nb3p3 469 Os4p3
366 Er4pl 472 Tm4 s
367 * Ag3d5 479 Cua
374 Ag3d3 480 Zn A
376 Gd4s 483 Ru3pl ,
377 * K2s 485 * Sn3d5
379 Nb3pl 487 Yb4s
379 Hg4d3 492 W4pl
380 Hf4p3 493 G a A
381 * U4f7 494 Sn3d3
386 Tm4pl 495 I r 4 p 3
386 * Tk4d5 496 * Rh3p3
392 V4fS 500 * Sc2s
393 * F.103~3 500 * N a A
395 Y3s 503 * ZnA
396 Yb4pl 505 Mo3s
398 Tb4s 506 Lu4s
399 * Nls 511 G a A
400 G a A 513 * V2p3
402 * Sc2p3 518 Re4pl
404 * Cd3d5 519 Pt4p3
405 Ta4p3 520 V2pl
407 Sc2pl 521 Rh3pl
407 T14d3 522 G a A
410 Mo3pP 528 * Sb3d5
410 Lu4pl 531 * Pd3p3
411 Cd3d3 532 * 81s
413 * Pb4d5 537 Sb3d3
416 iDy4s 538 Hf4s
425 GeA 5 30 N a i l
426 W4p3 546 Au4p3
427 * G a A 547 BsJpl
431 Zr3s 547 CuA
435 Pb4d3 559 Bd3p1
436 Ho4s 564 * T i 2 s
437 Hf4pl 566 Ta4s
438 * C22s 567 Zn A
440 * Bi4d5 568 * CuA
443 * In3d5 571 H g 4 ~ 3
444 deA 571 * Ag3p3
445 Re4p3 572 * Te3d5
449 Er4s 575 * Cr2p3
451 In3d3 577 I r 4 p l
454 GeA 581 Zn A
455 * Ti2p3 582 Te3d3
461 T i 2 p l 584 Cr2pl
461 * Ru3p3 585 Ru3s
464 Bi4d3 590 Zn A
465 Ta4pl 595 W4s
469 Nb3s 602 &3pP
* S t r o n g e s t Auger Line o r one element spectrum, n o t i n c l u d i n
608 ~ t 4 p l 779 * C02p3 978 * Nd3d5
609 T14p3 780 U4d3 981 * CA
617 * Cd3p3 781 * Ba3d5 984 * IA
620 * I3d5 782 Co A 998 Cs3p3
625 Re4s 784 Fc A 1000 Nd3d3
62 7 Rh3s 794 Co2pl 1001 OA
62 8 '42s 796 Ba3d3 1005 * TeA
629 CuA 797 * P r A 1006 Te3s
631 I3d3 800 Hg4s 1007 C r A
632 La4d 806 Bi4pl 1007 Ni2s
640 Cua 812 Sb3pl 1015 * TeA
640 * N i A 819 Te3p3 1020 Ya
641 * Mn2p3 826 In3s 1021 * Zn2p3
644 AuJpl 827 * CeA 1032 Sba
645 Pb4p3 832 * La3d5 1040 * Sba
648 CuA 835 * FA 1044 Zn2pB
65 1 Cd3pl 838 Co A 1045 U4p3
652 Mn2pl 843 Co A 1055 SnA
655 Os4s 845 T14s 1060 * VA
664 * Hn3p3 845 * FeA 1063 * SnA
670 Pd3s 846 Fe2s 1063 Ba3p3
675 * SmA 848 La3d3 1065 Cs2pP
677 Hg4pl 855 * Ni2p3 1072 13s
677 * Th4d5 861 FA 1072 * Na1s
679 Bi4p3 865 * E a A 1077 TiA
686 * F l s 870 Te3pl 1080 HnA
690 I r 4 s 873 Ni2pl 9081 * Sm3dP
695 Cr2s 875 I3p3 1087 * InA
702 lin3p1 884 Sn3s 1096 Cu2s
? ~ n* F-?-.I
I I V I - G L ~ ~ $84 * ~ e ~ d a 1107 Sin363
712 CuA 890 H3 aA 1107 * Fla
712 N i A 89 4 Pb4s 1109 . CdA
714 %h4d3 896 Fe A 1113 * TiA
715 * Sn3p3 902 Ce3d3 1 1 1 6 " CdA
715 CoA 903 * BaA 1116 * Ga2p3
717 Ag3s 908 * MnA PI24 Ea3p3
72 2 'h"l4pP 315 Cs A 1131 AgA
723 Fe2pl 926 Co2s 1131 * Eu3d5
724 P t 4 s 927 * C s A 1136 * AgA
726 * Cs3d5 931 P3pl 1137 Ba3pl
738 * U4d5 931 * Pr3d5 1143 Ga2pP
740 Cs3d3 931 * Cu2p3 1157 * ScA*
755 * NdA 9 39 Bi4s 1158 * PdA
757 Sn3pl 944 Sb3s 1190 * *A
759 Au4s 951 Cu2p1 1198 * C a A
764 Pb4pl 951 Pr3d3 1215 * RuA
766 Sb3p3 952 MnA 1230 * CA
769 Mn2s 967 * C r A 1239 * KA
770 Cd35 968 Th4p3 I307 * C l A
778 N i A 972 I A 1315 * BA
Figurc 2a
PRINCIPAL
PHOTO ELECTRON ENERGIES
9500 1400 1300 1200 I100 1000 900 800 700 600 500 400 300 200 100 0
.t--- Binding Energy i n BV
18
Figure 2b
P R I N C I P A L
A U G E R
E L E C T R O N E N E R G I E S
1500 1400 1300 1200 1100 9000 900 800 700 800 BfKl 46M 300 Mil 100 0
c-- Binding Energy in eV
19
The Hewlett-Packard 5950A spectrometer has no provision f o r changing t h e t a r g e t . As most photoelectron s p e c t r a presented i n t h i s t h e s i s were
taken with t h i s spectrometer, observed x-ray photoelectron l i n e s and major Auger-electron l i n e s have been catalogued f o r a n a l y s i s of t h i s d a t a
(Table 1). The s t r o n g e s t photoelectron and Auger l i n e s a r e marked by an a s t e r i s k ( * ) . The e n e r g i e s given i n t h a t t a b l e a r e compiled from obser- v a t i o n s by siegbahnl, and ~ a ~ n e r ~ ~ .
The i n t e n s i t y of t h e photoelectron l i n e i s p r o p o r t i o n a l t o t h e concentra- t i o n of t h e element t o be s t u d i e d i n t h e region of observation. l4enkez3 has given a phenomenoPogical model f o r t h e i n t e n s i t y of a photoelectron
Pine o r i g i n a t i n g from a homogeneous sample with a smooth s u r f a c e . For '
very t h i c k samples, h i s equation reduces t o
W
@i = Q A T . N . A B I ~ ' where
@
-
z
i n t e n s i t y of t h e photoelectron Pine Q photon f l u xA : e f f e c t i v e sample a r e a
w I s o l i d angle of acceptance f o r t h e s p e c t r o m e t e r .
T E p h o t o e l e c t r i c c r o s s s e c t i o n i
N . r number d e n s i t y of e m i t t i n g atoms
P
A . E mean f r e e path f o r i photoelectrons i n t h e sample. B
'When a parameter f o r t h e e l e c t r o n emission of s u r f a c e contaminants i s included, t h e i n t e n s i t y becomes
W
Qi= Q A s i n
-
yiNiAiTi 4n2 2 . -a~ Anal Chem, 44, 967 r1972)
2 3 . Ilenke, B . L .
,
X-ray o p t i c s a n d X-ray MicroanaPysis, Academic Press,2 0
a f t e r s i m p l i f y i n g f o r t h e uniform i n t e n s i t y of t h e e x c i t i n g photons over
t h e i 1 luminated region.
R e g r e t t a b l y , experimental v a l u e s f o r X and T a r e l a c k i n g , and only
i i
r e l a t i v e c o r r e l a t i o n s can be made w i t h i n t h e same sample. An e x t e n s i v e
s e r i e s o f photoemission s e n s i t i v i t i e s have been given by ~ a ~ n e r ~ ~ . These a r e referenced t o t h e F l u o r i n e 1s l i n e (A1K e x c i t a t i o n ) . Additional
a
d a t a have appeared r e c e n t l y 2 4 a 9 2 s b . These provide r e l a t i v e e s t i m a t e s of
p h o t o e l e c t r i c c r o s s - s e c t i o n s , s c a t t e r i n g c o e f f i c i e n t s and mean-free
p h o t o e l e c t r o n p a t h s f o r a s e r i e s of elements and t h e i r major photoelectron
l i n e s . P l o t t i n g t h e d a t a on Log Log s c a l e has permitted enhanced e s t i -
mation of t r u e s e n s i t i v i t i e s by l i n e a r r e g r e s s i o n . This e m p i r i c a l
c o r r e l a t i o n o f elemental s e n s i t i v i t i e s i s displayed i n Figure 3 t o f a c i l i t a t e r e d u c t i o n of d a t a given i n t h i s t h e s i s .
24. Wagner, C . D . , Anal @hem,
-
44, PO50 (1972)24a. Jorgensen,C.K., and Berthou, H., Farad. Disc. Chern. Soc.,
2,
269 (1972).111. EXPERIMENTAL METHODS
A . General Experimental Description
The experiments described i n t h i s t h e s i s a r e based on an a n a l y s i s of t h e k i n e t i c energy d i s t r i b u t i o n of e l e c t r o n s photo emitted from s o l i d specimens upon i r r a d i a t i o n with X-rays. The b a s i c experimental arrange- ment c o n s i s t s of a sample chamber, a mechanism f o r sample i n t r o d u c t i o n , x-ray photon source, e l e c t r o n k i n e t i c energy monochromator, e l e c t r o n p u l s e d e t e c t o r , magnetic-field compensator, and supporting vacuum system.
Low p r e s s u r e s , t o r r , a r e r e q u i r e d t o avoid l i n e broadening o t h e r - wise caused by c o l l i s i o n s with gas molecules. I n commercial e l e c t r o n spectrometers, t h e vacuum i s produced by d i f f u s i o n pumps, turbo-molecular pumps o r ion pumps. Each of t h e s e gives r i s e t o s p e c i f i c problems.
The d i f f u s i o n and turbomolecular pumps pro&uce cracking products of pump o i l s t h a t may d e p o s i t on t h e sample s u r f a c e . The composition o f t h e s e products a r e i l l - d e f i n e d , although they appear t o be o x i d a t i o n products?5
The ion pmp/vac s o r b method r e s u l t s not only i n Power p r e s s u r e s b u t a l s o i n a hydrocarbon-free environment, containing e s s e n t i a l l y CO, C02 and H20 ( a t t o r r ) . With t h e s e lowered p r e s s u r e s , t h e s i g n a l s t r e n g t h i s increased s i g n i f i c a n t l y .
V i r t u a l l y each modern e l e c t r o n spectrometer has an e l e c t r o s t a t i c energy a n a l y ~ e r * ~ , of t h e kind f i r s t described by ~ u r c e l l ~ ~ i n 1938. This analyzer c o n s i s t s of c o n c e n t r i c s p h e r i c a l segments with t h e outermost
--
2 5 . Riggs, Wm. M , , P r i v a t e Communication
25a. Brundle, C . R . , and Roberts, M.W., Proc R Soc Eond, A 331, 383 (1972) 26. Luccesi, C . A . , and L e s t e r , J . E . , J . Chem Ed,
-
50, A205 (1973) '2 4
s p h e r e n e g a t i v e l y charged. P o t e n t i a l s a r e a d j u s t e d t o give a V = 0 plane along t h e c e n t r a l r a d i a l dimension. The u s e of i n l e t and-exkt s l i t s ,
p l a c e d a t 180' s e p a r a t i o n , t o govern e l e c t r o n pathways through t h e mono- chromator r e s u l t s i n maximum transmission through t h e analyzer. The
a n a l y z e r r e s o l u t i o n i s determined by t h e r a d i u s of t h e spheres and r e l a t i v e
s l i t widths. Commercial spectrometers employ c e n t r a l r a d i i ranging from
15.5 cm. t o 36.0 cm.
The s e v e r a l commercial and experimental approaches vary considerably i n t h e choice of a source of X-ray photons, Siegbahn's i n i t i a l work
d e s c r i b e d a demountable X-ray tube which used an aluminum o r magnesium t a r g e t . These sources emit considerable energy i n t h e Ka doublet.
P ,2
Additional X-ray energy i s emitted as Bremstrahlung, which i s p l o t t e d i n Figure 4 , a s a f u n c t i o n of a c c e l e r a t i n g v o l t a g e . In a d d i t i o n t o t h e
c h a r a c t e r i s t i c emission (Ka), a s e r i e s of s a t e l l i t e s are generated. The s t r o n g e s t of t h e s e i s t h e Kag 4 l i n e , which g i v e s two peaks i n . t h e
t
photoelectron spectrum a t Bower binding energy. Magnesium r a d i a t i o n g i v e s s e p a r a t i o n s of 8.412 eV a t 9.48% primary peak i n t e n s i t y f o r t h e a
3
l i n e and 10.142 eV a t 4.54% r e l a t i v e i n t e n s i t y 5 f o r t h e ct t r a n s i t i o n 5 . 4
The linewidth of t h e Ka doublet i s 0.8 eV f o r magnesium and 1.0 eV
1,2
f o r Aluminum.
An x-ray monochromator o f f e r s an a l t e r n a t i v e t o t h e "raw" x-ray source, I t reduces t h e n e t linewidth t o 0.1 eV and e l i m i n a t e s t h e complications
, . . . . . . . . .. ( L. . . .
25
The e l e c t r o n d e t e c t o r s a r e u s u a l l y d i s c r e t e e l e c t r o n m u l t i p l i e r s .
Spectrometers u s i n g monochromated sources employ multi-channel d e t e c t o r s t o r e c o v e r l o s t i n t e n s i t y . The combination of e l e c t r o n m u l t i p l i e r a r r a y s
with a phosphor videocon can provide 128 element d e t e c t o r s (MCA).
The l i n e width i n an e l e c t r o n spectrum i s g e n e r a l l y r e p r e s e n t e d a s t h e f u l l width of t h e observed peak a t h a l f t h e maximum i n t e n s i t y (FWHM). The c o n t r i b u t i o n s t o t h e l i n e width can be summarized a s
62 = A;
+
82+
A;+
X 5;
,
(16)where
Ag
"
t h e n a t u r a l l i n e widthAX f width of t h e e x c i t i n g x-ray l i n e
AK : broadening by t h e analyzer
Aw
r broadening due t o s o l i d s t a t e e f f e c t s i n t h e sample.With a non-monochromatized x-ray source, A predominates, whereas Ag and , X
A a r e dominant when monochromated x-rays a r e used. W
B . InstrtunentaP C h a r a c t e r i s t i c s
S e v e r a l spectrometers were employed i n t h i s work:
1. The J e t Propulsion Laboratory-Utah S t a t e U n i v e r s i t y spectrometer
2 , The McPherson ESCA 36 spectrometer 3. The Hewlett-Packard 5950A spectrometer 4. The DuPont 650 e l e c t r o n spectrometer.
26
u n i t 2 8 a l s o uses a supplementary cryopump f o r s t i l l lower chamber p r e s s u r e s .
During t h e development of t h e JPL-USU spectrometer, we experienced t h a t a very high degree of accuracy and superb workmanship i s r e q u i r e d f o r t h e c o n s t r u c t i o n of t h e e l e c t r o n analyzer. Misalignment of t h e analyzer spheres by p a r t s p e r thousand gave m u l t i p l e images and broadenings of gold 4f s p e c t r a t o 2.2 eV from 1.6 eV F W H M ~ ~ .
7/ 2
The JPL-USU spectrometer i s s h i e l d e d a g a i n s t t h e e a r t h ' s and o t h e r magnetic f i e l d s by means of Helmholtz c o i l s . In a d d i t i o n , t h e analyzer s e c t i o n i s surrounded by a paramagnetic s h i e l d . The McPherson ESCA 36 spectrometer employs a s e r i e s of t h r e e c o n c e n t r i c paramagnetic s h i e l d s t o prevent inhomogeneities i n t h e r e s i d u a l f i e l d .
The JPL-USU spectrometer was operated a t t o t o n r and gave about 3
2 x 10 counts p e r second (cps) f o r a f r e s h l y cleaned gold sample (Au 4 f
7/ 2 l i n e , FWHM ranging from 2.8 t o 1.6 eV). The McPherson ESCA 36,operated a t a gauge reading of l e s s than o r equal t o 1 0 ' ~ t o r r , gave about 2 x 10 4 cps with t h e same sample i n p l a c e Q F M 1 . 8 t o 1.5 eV)
.
In both cases, a single-channel d e t e c t o r and Mg Ka r a d i a t i o n was used.1,2
Most of t h e q u a n t i t a t i v e work r e p o r t e d i n t h i s t h e s i s was performed with t h e Hewlett-Packard 5950A spectrometer. This instrument employs a bent q u a r t z - c r y s t a l x-ray monochromator, an e l e c t r o s t a t i c electron-beam
monochromator, d i s p e r s i o n compensation e l e c t r o n o p t i c s , r e t a r d i n g p o t e n t i a l
2 8 . Rendina, J . , American Laborat-,
-
-
6, 27 (1972)2 8
e l e c t r o n l e n s , a multichannel d e t e c t o r and a multi-channel a n a l y z e r 3 0 ( s e e Fig. 5)
.
The di-spersion monochromators p a s s an energy packet E
-
AE t o E + AE(AE i s t h e d i s p e r s i o n o f t h e a n a l y z e r ) . In t h i s d e s i g n , t h e e l e c t r o n a n a l y z e r i s arranged s o t h a t i t s energy d i s p e r s i o n i s a n t i p a r a l l e l t o t h a t of t h e photon monochromator. If t h e d i s p e r s i o n AE1 of t h e e l e c t r o n a n a l y z e r i s set equal t o t h e d i s p e r s i o n AE2.0f t h e x-ray monochromator t h e t r a n s m i t t e d l i n e widths n e a r l y c a n c e l each o t h e r .
I n t h e o r y , n a t u r a l l i n e w i d t h s could be observed i n t h i s d i s p e r s i o n compensation system, b u t second and t h i r d o r d e r a b b e r a t i o n s l i m i t t h e minimum widths t o 0.5 eV.
The monochromator i l l u m i n a t e s a well-defined r e g i o n of t h e sample ( 1 x 5 mm) b u t reduces t h e t o t a l x-ray f l u x by about two o r d e r s of magnitude. The sample chamber and x-ray source a r e i s o l a t e d from each o t h e r by an aluminm. window and c a s e i s being taken t o avoid i r r a d i a t i n g t h e chamber walls. Thus, t h e number o f secondary s t r a y e l e c t r o n s
impinging on t h e sample i s v e r y low and an aeaxijllfary e l e c t r o n gwt (flood
gun) h a s t o be used to n e u t r a l i z e t h e charge t h a t b u i l d s up on t h e sample.
The vacuum i s produced by noble ion pumps g i v i n g an o p e r a t i n g p r e s s u r e of 3 x 1 0 ' ~ i n t h e main a n a l y z e r chamber. During normal o p e r a t i o n of t h e unbaked s p e c t r o m e t e r , t h e r e s i d u a l gas i n t h e main chamber c o n s i s t s
primarily of w a t e r , CO and H2, and small q u a n t i t i e s of hydrocarbons and
3 0 , Siegbahn, K . , Hammond, D., PeIPner-Feldigg, H., B a r n e t t , B.F., Science, 176, 245 (19721.
3 0
r a r e g a s e s . A t y p i c a l mass spectrum6 t a k e n w i t h t h e EAI Quad 250 quadrupole r e s i d u a l - g a s a n a l y z e r a t t a c h e d t o t h e main chamber i s shown
i n F i g . 6 .
The p o t e n t i a l of t h e r e t a r d i n g l e n s i s swept t o t r a c e t h e spectrum (Fig. 5 ) and t h e a n a l y z e r i s o p e r a t e d a t c o n s t a n t energy (110 eV). The analy- z e r t r a n s m i t s a 10 eV band which i s d e t e c t e d by a 128 element multichannel a r r a y . A phosphor/vidiocon system i s used t o d e t e c t t h e e l e c t r o n count r a t e . The i n f o r m a t i o n i s s t o r e d i n t h e multichannel d e t e c t o r (MCD).
The spectrum i s scanned over a s e l e c t e d v o l t a g e range s o t h a t each element i n t h e a r r a y "sees" each e n e r g y t w i c e d u r i n g a s i n g l e scan. Data a r e r e c o r d e d by a paper t a p e punch and x-y p l o t t e r .
Signal-to-background r a t i o s observed i n t h i s work v a r i e d from $2 t o B63:4 and s i g n a l - t o - n o i s e r a t i o s from 1 2 to P700:l. The background i s d e f i n e d a s t h e s i g n a l measured a t a p o i n t 7 eV Power i n b i n d i n g energy than t h e peak o f i n t e r e s t , The n o i s e i s approximated by t h e s t a n d a r d d e v i a t i o n o f each measurement which i s given by t h e s q u a r e r o o t of t h e number of counts p e r channel i n t h e background, Observed FWHM o f Au 4 f was
7/2
4
8.72 eV w i t h count r a t e s o f about 8.5 t o 9 . 7 x PO counts per second. Dispersion compensation changes t h e i n h e r e n t l i n e shape of t h e photo- e l e c t r o n peaks from an asymmetric t o a symmetrically broadened (by 0.55 eV) Lorentzian form5. This symmetry s i m p l i f i e s d e c o n v s l u t i o n a s i s d i s - cussed i n Chapter IV,
u s e a s a reference.
C. V o l t a i c P o t e n t i a l E f f e c t s
The r e f e r e n c i n g of binding e n e r g i e s f o r metals i s s t r a i g h t f o r w a r d , but p r e s e n t s some d i f f i c u l t i e s f o r i n s u l a t o r s . In t h i s case, t h e d e f i n i t i o n of a Fermi Level i s questionable and charging e f f e c t s can d i s p l a c e t h e
photoelectron l i n e s . *
If t h e charging domains a r e inhomogeneous, a d i s t o r t i o n of t h e peak shape i s observed. Changes i n t h e f l u x a r e a l s o observed apparently due t o r e t a r d a t i o n caused by t h e s u r f a c e p o t e n t i a l .
In most experimental systems, t h e f l u x l e v e l s of t h e secondary e l e c t r o n s cannot be c o n t r o l l e d . The magnitude of t h e VC i s o f t e n i r r e p r o d u c i b l e because of t h e v a r i a t i o n x-ray f l u x d e n s i t y and s u r f a c e contamination31. The v o l t a i c p o t e n t i a l introduces considerable u n c e r t a i n t y a s regards t h e p o s i t i o n of t h e photoelectron l i n e s i n non-metallic samples.
Siegb&n-type e l e c t r o n spectrometers show charging e f f e c t s of up t o 3 eV, while monochromatic systems can show s h i f t s of up t o 130 eV because of t h e
Back of s t r a y e l e c t r o n s . Measurement of a b s o l u t e binding energies r e q u i r e s a c a l i b r a n t o r a device c o n t r o l l i n g t h e i r r a d i a t i o n of t h e by low-energy e l e c t r o n s o r both.
CaEibrant standards a r e u s u a l l y deposited e x t e r n a l l y on t h e sample,
The Uppsala group used t h e carbon l i n e from adsorbed pump o i l . This l i n e was assigned a binding energy o f 285.0 eV, Since many s p e c i e s have been
observed i n t h i s s u r f a c e ~ o n t a m i n a n t ~ ~ , t h i s r e f e r e n c e i s u n s a t i s f a c t o r y . Seven c a r b o n components have been found i n experiments on Ag HgI c r y s t a l s
2 4
i n t h i s p r e s e n t work.
Other r e f e r e n c e standards a r e obtained by d e p o s i t i o n of g r a p h i t e from a s o o t y flame33, by u t i l i z i n g t h e carbon from double-stick adhesive t a p e 3 4 o r by evaporation of gold o r lead onto t h e sample. The l a s t procedure i s
accepted a s t h e most r e l i a b l e one35,
A d i f f i c u l t y which may a r i s e from t h e d i s p o s i t i o n of t o o t h i n a gold l a y e r i s formation of i s l a n d s of only a few hundred angstroms i n diameter. The v o l t a g e d i f f e r e n c e between gold and t h e sample m a t e r i a l a r i s i n g from t h e d i f f e r e n c e i n t h e photo y i e l d i s given by
where
L E e l e c t r o n d i f f u s i o n length I.--
0 r a d i u s of c a l i b r a n t i s l a n d s
no-nl 5 d i f f e r e n c e i n photo y i e l d
ns z y i e l d of Bow energy e l e c t r o n s i n s i d e t h e sample
9 z p e n e t r a t i o n depth of x-rays,
0
I f t h e e l e c t r o n d i f f u s i o n l e n g t h i s assigned t h e t y p i c a l v a l u e of -500A, ro i s given t h e same v a l u e , and t h e x-ray p e n e t r a t i o n depth i s a few microns, then eq. 17 T ~ ~ U C ~ S t o
. n O - n l
v = 3
ns ev. ' (18)
32. O l g i l v i e , J . L . , and Wolberg, A . , Applied Spectroscopy,26,401
-
(1972) 3 3 . Brundle, C . R . , ~ S p e c t r ~ s c o p ~ ~ 25, 8 (1971)34. Jasgensen, C.K., Chimia, 25, 213 ( n 7 1 )
FIGURE 7 BSCa Line S h i f t versus Flood Gun Current
F ( I s ) Teflon Sample
34
Since n s < l , d e t e c t a b l e s h i f t s should r e s u l t f o r An>O.Ol. S i m i l a r p h o t o v o l t a i c s h i f t s should occur i n o t h e r metal i n s u l a t o r systems, and
AV could be s e v e r a l v o l t s 3 6 .
For i n t e r n a l c a l i b r a t i o n s s e v e r a l schemes have been developed such a s
mixing t h e s m p l e with g r a p h i t e 3 1 , and impregnating t h e sample with a common element32. I n c a s e s , where methyl o r a l i p h a t i c carbons a r e p r e s -
e n t i n t h e sample t h e s e can be used a s a r e f e r e n c e r e p r e s e n t i n g energies o f 285.0 and 285.4 eV r e s p e c t i v e l y 3 7 9 38. These s t a n d a r d s o f f e r c o r r e c t i o n s
f o r Mandelung ~ o t e n t i a l s ~ ~ and v a r i a t i o n s i n t h e work f u n c t i o n . This method was used throughout i n t h i s t h e s i s work.
The method o f i l l u m i n a t i n g t h e sample with e l e c t r o n s o f zero k i n e t i c energy t o n e u t r a l i z e t h e charge can a l s o be used t o o b t a i n a r e f e r e n c e . This technique was f i r s t d e s c r i b e d by ~ u c h i t a ~ ~ ~ a d i s r o u t i n e l y u t i l i z e d i n t h e HP5950A spectrometer, s u p p l i e d with a f l o o d gun o f v a r i a b l e f l u x
(from 0 t o 48 eV).
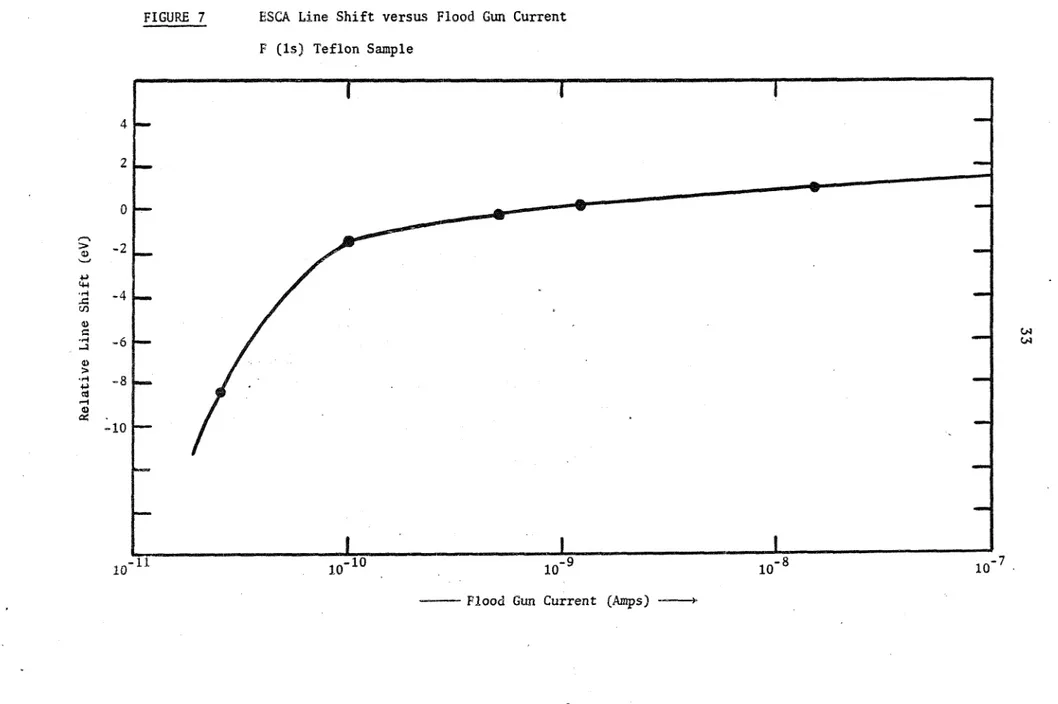
The observed s h i f t o f t h e F l s peak i n experiments w i t h ~ e f l o n ~ ~ has been p l o t t e d versus t h e f l u x from t h e e l e c t r o n gun; s e e Fig. 7. Peak p o s i t i o n s
can be s h i f t e d p o s i t i v e o r n e g a t i v e w i t h a p p r p p r i a t e f l o o d gun c u r r e n t s . Replenishment c u r r e n t s below
lo-''
amps a r e i n s u f f i c i e n t t o avoid charging s h i f t s . The l i n e a r ( l i n e a r AeV, log c u r r e n t ) r e l a t i o n s h i p between cur- refit and peak s h i f t g r e a t l y f a c i l i t a t e s t h e a p p l i c a t i o n of c o r r e c t i o n f a c t o r s .In t h e experiments r e p o r t e d i n Chapter V , t h e photoelectron spectrum of
3 6 . Uebbing, J . J . , F r i v a t e Communication
37, Lindberg* R . J . , Hamrin, K . , Johansson,
G.,
Gelius, U., Fahlman, A . , Nordling, C . , Siegbahn, K . , Physica S c r i p t a , I , 286 (1970)38. Gelius, U . , Hedin, P.F., Hedman, J . , ~ i n d b e r ~ ; B . J . , Manne, Pi.,