Ten years of biosimilars in Europe: development and evolution of the regulatory pathways
Full text
Figure
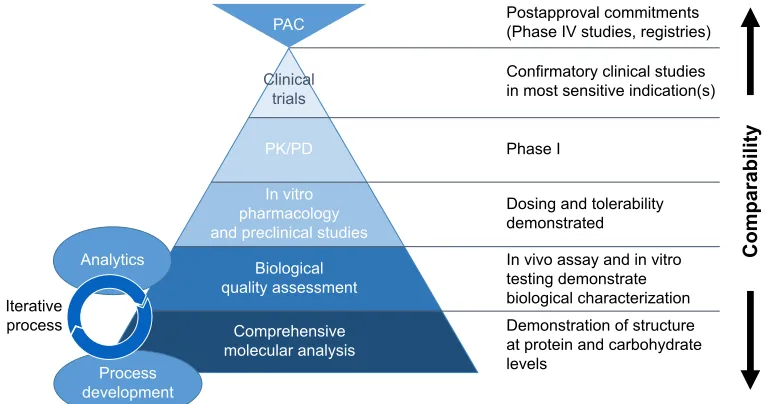
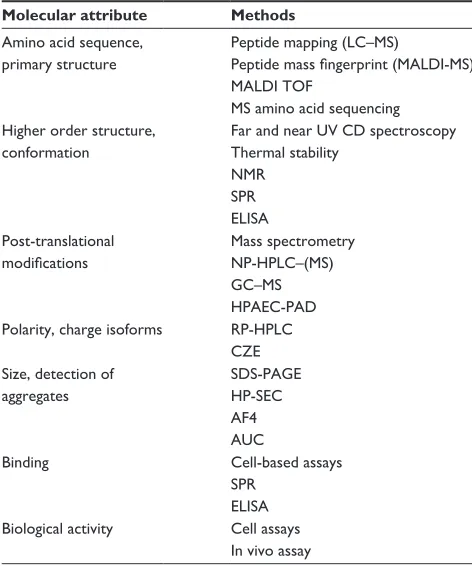
Related documents
To create and assign device models, refer to the procedures for the signal model command in the Allegro PCB and Package Physical Layout Command Reference.
Standardization of herbal raw drugs include passport data of raw plant drugs, botanical authentification, microscopic & molecular examination, identification of
Surgical Site Infection Reduction Through Nasal Decolonization Prior to Surgery..
Abstract: This report discusses recent developments of psychotraumatology mainly related to the recently published ICD-11, but also from a societal point of view.The selected aspects
The proposed Peyton Slough Hydraulic Relief Project consists of removing an existing hydraulic restriction in Peyton Slough to improve water exchange between McNabney Marsh and Suisun
Results obtained in the present study revealed mild alterations in the kidney function, reflected as a significant increase in urea of KT/WR-treated rats and a significant decrease
повышается также скорость входа протонов в матрикс (рис.. Влияние МрТр на трансмембранный обмен ионов в митохондриях. Открывание МРТР, как уже было показано нами