Symposium on Developmental Genetics: X I I I International Congress of Genetics
PROTEIN SYNTHESIS DIRECTED BY THE RNA FROM A PLANT VIRUS IN A NORMAL ANIMAL CELL
JOHN KNOWLAND
Medical Research Council, Laboratory of Molecular Biology,
Hills Road, Cambridge, England ABSTRACT
RNA from tobacco mosaic virus can be translated inside oocytes of the frog Xenopus laeuis. The main product is a polypeptide with a molecular weight of 140,000. There is no evidence for coat protein synthesis, and it is unlikely that the polypeptide that is made contains either a whole o r a partial coat protein sequence.
The picture of translation of tobacco mosaic virus RNA obtained using oocytes is very much simpler than that found using cell-free protein-synthe- sizing systems, i n which a great many polypeptides are made under the direction of tobacco mosaic virus RNA. The reasons f o r this difference are discussed, and the relative merits of in vivo and in vitro protein-synthesizing systems are compared.
I T
is possible to inject messenger RNA into oocytes of the frog Xenopus laevis and in this way to study the translation of mRNA inside a normal, intact animal cell (LANE, MARBAIX and GURDON 1971). A variety of defined messengers from vertebrates and one from an insect have been translated in the oocyte (reviewed by LANE and KNOWLAND). In all of these cases, the product made in the oocytes proved to be the normal one expected and not a precursor to or modi- fication of it, which greatly facilitated identification and characterization of the material made in the oocyte.This article explores the value of the oocyte as a means of identifying the trans- lational products of messenger RNA in cases where they are not known or pre- dictable, and compares this aspect of the oocyte system with the use of cell-free protein-synthesizing systems. It is illustrated with some results which show that tobacco mosaic virus RNA can be successfully translated in the oocyte, thereby extending the range of the system to include the translation of RNA from plants. It emerges that the translational product of tobacco mosaic virus RNA in the oocyte is not the coat protein that might have been expected, but a polypeptide of
very much higher molecular weight.
MATERIALS A N D METHODS Viruses
The common strain of tobacco mosaic virus (referred to as wild-type or WT), and the nitrous acid mutant 568, i n which the threonine residues at positions 5 and 107 are replaced by isoleucine
384 J. KNOWLAND
and methionine respectively, and the isoleucine residue at position 129 is replaced by threonine (WITTMANN-LIEBOLD and WITTMANN 1965), were a gift from DR. P. J. G. BUTLER of this laboratory.
Extraction of R N A
RNA was isolated from virus according to the procedures of MARCUS, EFRON and WEEKS (1974). It was dissolved i n water and stored in sealed glass capillaries under liquid nitrogen.
Injection and labelling of oocytes
Oocytes were injected with RNA and labelled by incubation in saline containing radioactive amino acids as described by GURDON et al. (1971). Each oocyte typically received about 50 nl of RNA at 3-5 mg/ml. Up to 15 oocytes were incubated in 20 nl of medium in a small hole drilled in a block of Perspex.
Translation of R N A in reticulocyte lysate
LINGREL (1969).
Preparation of labelled oocytes f o r SDS-gel electrophoresis
10 oocytes were homogenized in 100 pl of 0.125 M Tris-HC1, 0.012 M disodium EDTA, pH 6 8; contaming 0.1 mg/ml of pancreatic ribonuclease A (WORTHINGTON). The homogenate was incubated at 37" for 5 minutes and then centrifuged at 2000g for 5-10 minutes at room tem- perature in order to sediment the yolk and to float the lipid. Ten p1 of 2-mercaptoethanol and 40 pl of solution containing 4% SDS, 20% glycerol and 0.002% bromophenol blue were added to the supernatant. The mixture was heated in boiling water for IO minutes, and was used directly for SDS-gel electrophoresis o r SDS-gel filtration. After removing the yolk in this way, it is possible to apply the supernatant from as many as 5 oocytes to a single slot on a slab gel, while
if the yolk is not removed only about 0.5 oocyte can be applied without overloading the gel. Tests showed that very little radioactive protein is lost with the yolk and that no selective losses seem to occur.
SDS-gel electrophoresis
Slab gels (1.6 mm thick, 20 cm wide, 20 cm long) made using recrystallized acrylamide and bisacrylamide (LOENING 1967), were prepared according to LAEMMLI (1970), except that the acrylamide to bisacrylamide ratio was changed from 37.5:l to 2M:l resulthg in a great improve- ment in the sharpness of the oocyte protein bands. Gels were run at up to 50 mA at constant current until the tracking dye had moved 10-12 cms. They were stained for 1-2 hours in 0.1% Coomassie Brilliant Blue in 9% acetic acid, 46% methanol; de-stained in 7.5% acetic acid, 45% methanol; dried and autoradiographed. Slices of dried gels were dissolved by heating at 37" overnight in 0.25 ml of a mixture of 30% hydrogen peroxide and 0.88 ammonia (95:5 by volume) under a layer of liquid paraffin. Four ml of Aquasol (New England Nuclear) containing 20 of glacial acetic acid was added for scintillation counting; the liquid paraffin in the sample had little effect on counting efficiency.
SDS-gel filtration
ammonium formate, 0.1 % SDS, 0.1 % 2-mercaptoethanol, containing O.O@% sodium azide.
Recovery of protein fr om solution in SDS
Protein solutions in SDS were made 20% in TCA and kept ice-cold for 20 minutes. After brief warming at 37" to dissolve precipitated SDS, the precipitated protein was collected on
siliconized glass fiber discs by suction filtration. The precipitate was washed with 20% TCA at room temperature, and then 100 ml of chloroform was passed slowly through the filter to remove SDS bound to protein (REYNOLDS and TANFORD 1970). The recovery of pure protein was nearly TMV-RNA was assayed in rabbit reticulocyte lysate prepared as described by LOCKARD and
Columns of Bio-gel A-l5m, 200-44Kl mesh (Bio-Rad) were made up and run in 0.05M
PLANT VIRUS RNA I N ANIMAL CELLS 385
Cyanogen bromide cleavage of proteins
Protein was eluted from filters (above) with 98% fosrmic acid. It was digested at 5-10 mg/ml in 70% formic acid with an equal weight of CNBr fosr 18-24 hours at 25". The digest was lyophilized, incubated a t 5 mg/ml in pH 6.5 pyridine-acetate buffer (pyridine: 100 ml; acetic acid: 3 ml, water to 1000 ml) for 16-24 hours a t 60" to open the homoserine lactone ring and lyophilized again.
Preparation and analysis of tryptic peptides released from the translation product of TMV-RNA The pdypeptide made in oocytes under the directio'n of TMV-RNA and labelled with 14C-valine was prepared by SDS-gel filtration (see text) and mixed with unlabelled tobacco mosaic virus coat protein (FRANKEL-CONRAT 1957). The mixture was precipitated with TCA, and the bound SDS was extracted with chlorosform. After lyophilization to remove residual TCA and chloroform, the protein was oxidized with performic acid f o r 3 hours a t ON", lyophilized twice, and suspended in 0.8 m l of 2% (w/v) ammonium bicarbonate. 0.1 m g of TPCK-trypsin (WORTHINGTON) dissolved in 0.1 ml of 2% ammonium bicarbonate was added, and the mixture was incubated at 37" for 2 hours. Mo're trypsin (0.1 mg) was added, and incubation continued for an additional 18 hours. The digest was lyophilized twice, suspended in 0.1 ml pH 6.5 pyridine- acetate buffer on Whatman 3MM paper at 3000 volts for 1 hour in the first dimension, and i n the seco'nd dimension by electropho'resis at 3000 volts f o r 1.5 hours a t p H 3.5 (the buffer is pyridine, 5 ml; acetic acid, 50 ml; water to 1000 ml) . The paper was dried and autoradiographed, and the TMV-coat protein peptides were located from their fluorescence in ultraviolet light after staining with Fluoram (fluorescamine Roche) dissohed at 2.5 mg per 100 ml of 0.5% (v/v)
pyridine in acetone. Some peptides were then run in a third dimension using descending chroma- tography in BAWP (butan-1-01, 30 ml; acetic acid, 6 ml; water, 24 ml; pyridine, 20 ml).
RESULTS
Stimulation of protein synthesis in oocytes following injection of T M V - R N A I n the first experiments performed, oocytes were injected with TMV-RNA and labelled with a 14C-amino acid mixture. I n general, injection of TMV-RNA stimu- lated incorporation of amino acids by a factor of two o r three. The proteins were then separated by SDS-gel electrophoresis, and 1 mm sections of the gels were counted. Despite the increase in protein synthesis, no TMV-stimulated products were found in this way. I n particular, no coat protein was found, although it would easily have been detectable (SINGER 1971 ; ZAITLIN and HARIHARASUBRA- MANIAN 1972). I t therefore seemed possible that some other protein was made in response to injection of TMV-RNA, but that it could not be clearly detected simply by cutting gels into 1 m m sections. I n general, this practice may not give enough resolution to reveal narrow bands, which may well occupy less than a single slice; this possibility was pursued by using the much more discriminating technique of autoradiography to reveal the radioactive bands on the gels.
When oocytes are labelled with 35S-methionine and the radioactive proteins in an SDS-gel are located autoradiographically, a great many bands can be seen (LASKEY, GURDON and CRAWFORD 1972), often separated by only a very small distance, emphasizing the value of this method of analysis if additional transla- tion of RNA is to be reliably detected against the background of protein synthesis by the oocyte.
Synthesis of new protein following injection of T M V - R N A into oocytes
386 J. K N O W I A N D
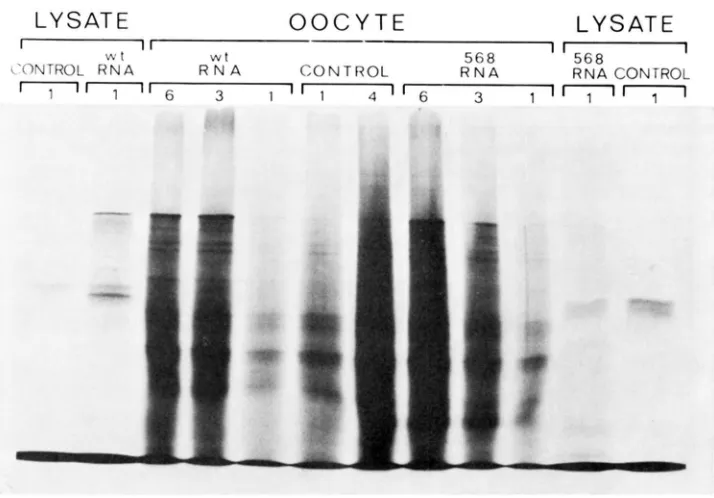
a label, any coat protein synthesis will not be revealed because the wild-type coat protein contains no methionine. However, if RNA from the mutant Ni-568 is used, synthesis of coat protein will be detectable because the coat protein of Ni- 568 does contain methionine. In fact. neither RNA supports production of coat protein (molecular weight 17,500), but both cause a new protein, with a very much higher molecular weight (approximately 140,000). to be synthesized (Figure 1 )
.
The new protein is clearly visible as a new band on the gel when the yolk is removed from the oocytes before electrophoresis, but cannot be seen at all clearly if the yolk is present. Apparently the new protein can somehow be ob- scured by the yolk protein which, although it does not incorporate radioactivity, has a very similar molecular weight and is present in such large amounts that it intcrferes seriously with the separation of proteins in this molecular weight range.A. protein with a molecular weight of 140,000 is also made in a reticulocyte lysate protein-synthesizing system primed by either variety of TMV-RNA
(Figure I), and this strongly suggests that the response in the oocyte is the result of translation of the injected RNA and is not due to increased synthesis of an
PLANT VIRUS R N A I N A N I M A L CELLS 387
oocyte protein. It is exceedingly unlikely that the 140,000-dalton polypeptide is
an aggregate of smaller polypeptides because the extraction involves pro1 onged boiling in SDS and mercaptoethanol. Various other proteins are also made in the lysate after adding TMV-RNA. They are all smaller than 140,000 daltons, and, as discussed later, may represent intermediate stages in the synthesis of the largest polypeptide produced.
Although TMV coat protein is not produced in oocytes injected with TMV- RNA, it is possible that the entire coat protein sequence is present within the 140.000-dalton polypeptide that is synthesized. I n principle, this could be tested by examining tryptic peptides released from the large polypeptide. If the 140,000-
daltm product does contain coat protein as an integral part, the variety synthe- sized under the direction of 568-RNA would release a characteristic "S-methio- nine coat protein peptide that would be absent in the variety coded for by WT-
RNA. Using this approach, ROBERTS and PATERSON (1973) and ROBERTS, MATHEWS and BRUTON (1973) were able to show that when a cell-free protein- synthesizing system derived from wheat germ is primed with TMV-RNA, the products jnclude a small amount of this characteristic peptide. I n practice, how- ever, the large background incorporation of methionine in the same molecular weight region in the oocyte (Figure 1) is likely to make unequivocal detection of a particular "S-methionine tryptic peptide rather difficult. It might be simpler to test for the coat protein sequence within the large translation product after label- ling with an amino acid that is present in the coat protein in large amounts, when the background incorporation by the oocyte might be less serious. One suitable amino acid for this purpose is valine, which is present in large amounts in TMV coat protein and is unlikely to be lost in the oocyte by conversion to other com- pounds.
Incorporation of valine into proteins after injection of T M V - R N A into oocytes
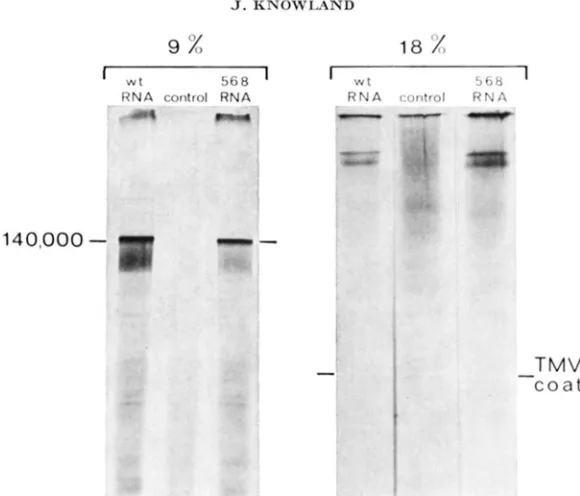
When oocytes are injected with TMV-RNA and labelled with 14C-valine, the 140,000-dalton polypeptide that is labelled by 35S-methionine can be seen very much more clearly, because the background incorporation by the oocyte is enor- mously reduced (Figure 2; 9% gel). The reduction in background also makes it possible to see some radioactive bands that are not detectable when 35S-methionine
is used as the label. Although the main product is clearly the 140,000-dalton polypeptide, another band can now be seen on the 9% gel a little above the main one; it has a molecular weight of approximately 160,000.
Electrophoresis on the 18% gel (Figure 2), combined with the clarity of the results obtained with *4C-valine, strongly suggests that coat protein is not synthe- sized. It also suggests that the large polypeptide may in fact consist of two others with similar molecular weights, although the significance of the apparent split- ting of the large polypeptide into two bands is not entirely clear.
An important practical consequence of the low background incorporation obtained with valine is that the 140,000-dalton polypeptide can be prepared for
388
140,000
J. KNOWIAND
9 X 18 '%,
'
w l 5 6 8'
'
W l 5 6 8 1R N A control R N A R N A control R N A
-
TMV c o a tFIGURE 2.-TransIation of T M V - R N A in oocytes: elrctrophoresis of IC-valinr proteins on a q% and 18% SDS-gel. Ratches of t i oocytes wer'r injected with saline or T M V - R N A and iiiru- hatcd in I C v a l i n e (280 mCi/mMole) at 1 niCi/ml for 21. hours. The low-speecl suprrriatiint from 2 oocytes was r e s o l ~ ~ d by SIX-gel elrctrophorr~is. The p s i t i o n of TMV coat proteiri on the 18% gel is shown.
much lower resolving power of gel filtration. This circumvents the difficulty of cxtracting the band in good yield from a dry gel, and makes measurement of the amount of radioactivity that enters the band vcrv much easier. Using gel filtra- tion in SDS, it is found that about 30% of the total valine incorporated enters the polypeptidc synthesized under the direction of TMV-RNA (Figure 3). Syn- thesis of this polypeptide corresponds to translation of about 65% of the RNA. It is possible that the translation product includes part or all of the coat protein sequence. or that it is a n entirely independent product. These possibilities are examined in the experiments described below.
Tests for coat protein sequmces in the 140.000-dnlton polyppptidcp synthcriwd in oocytes under the direction of TMV-RNA
PLANT VIRUS R N A IN A N I M A L CELLS 389
4 0
30
20
10 Y
0
3
.cr 0
-
mL
30
?
:
20X I a '10
0
0
30
20
10
0
I I I I I I
Control
2'0
F r a c t i o n number
FIGURE 3.-SDS-gel filtration of 14C-valine proteins made i n oocytes after injection of TMV- RNA. The SDS-supernatant from 100 oocytes treated as described in the legend to Fig. 2 was passed at 15 ml/hour down a column of Bio-gel A-15m (82 x 2.5 cm) as described in MATERIALS
AND METHODS. Five ml fractions were collected, and 0.25 ml from each was counted. The elution
positions of molecular weight standards are indicated: BSA: bovine serum albumin (69,OCO); CT: a-chymotrypsinogen (25,7001) ; RNase: ribonuclease A (13,7oI) ; BPB: bromophenol blue
(included volume marker). Fractions 48-52 were pooled as indicated for further analysis.
product of WT-RNA with cyanogen bromide, which cleaves peptides specifically at methionine residues, will produce one fragment that encompasses the entire coat protein sequence, while the corresponding fragment released from the translation product of 568-RNA4 will be split into two pieces. Consequently, it might be possible to detect a coat protein sequence within the 140,000-dalton polypeptide by comparing the molecular weights of the fragments in one cyano-
390 J. KNOWLAND
0
I I I
control
0
*U
80 100 1 2 0m m from origin
FIGURE 4.-Electrophoresis of cyanogen bromide fragments released from l 4 C - v a h e products specified by TMV-RNA. The cyanogen bromide digest of the pooled fractions obtained in Figure 3
was treated with 2-mercaptoethanol and SDS and part of it was analyzed by electrophoresis on an 18% SDS-gel. The markers are: TMV: TMV coat protein (1 7,500) ; CT: a-chymotrypsinogen (25,700) ; RNase: ribonuclease A (13,7001). Note that in this molecular weight range the order of migratim of SDS-proteins does not necessarily correspond to their molecular weights (ROBERTS and PATERSON, 1973).
RNA may indeed contain a whole coat protein sequence. While this result suggests that the 140.000-dalton product of TMV-RNA may contain a whole coat protein sequence, it is only a weak suggestion. A more stringent test involves comparing the behavior of tryptic peptides released from the 140,000-dalton polypeptide with those released from authentic coat protein.
PIANT VIRUS R N A IN A N I M A I . CEI.1.S 391
I:ic,unc ,j.--l.'iiig(.ri)riiit of Iwptitlrs rrlriisrd TI-OIII ii niixturr of wih!-typr T\I\' rniit prntviii
i i : d thr 1) >lyp:ytidv synthrsizrtl on wild-typr T Y V - R S A in o x y t r s . Thr 14!).(~W)-tIii1ton p l y -
prptidr pui-ifir*l from onrytrs iriirctrcl with IVT TU\'-RSA and lnhrllrcl with 1 'C:-valinr \viis
niixrtl with airthrntic IVT cont protrin. Thr mixturr \viis cligcstrcl with trypsin. and the protlucts wrrr scy~irnlrtl by r~rctrophorrsis i i n d chroniiitogriiphy os clrsrrilmt it8 MATERI.AIS A N D >II5TIIODS. R:itlioiictivity wiiq clrtrrtetl hy aiitoracliogriil)tiy. iintl the authentic coat protrin prptitlrs (oirtlinrd) w * w Inratccl usjiig Flirorilni.
to rcwiovc SIX, oxidized with performic acid and digested with tr!.psin as tlrscribetl in n m T I m A 1 . s A N D METHODS. T h e tryptic peptides were seplrated by high-voltage electrophoresis on paper. first a t pH 6.5 and then at pH 3.5. T h e result (Figure 5 ) shows that a f t e r these two steps, the majority of the authentic coat protein peptides contained no radioactivity. Some peptides did. h o w w r . appear to contain some radioactivity, but they could bc entirely separated from radioactivity by chromatography in RAWP. This expcriment strongly suggests. therefore. that the 140,000-dalton polypeptide spccified by TMV-RNA contains w i t h e r a whole nor a partial coat protein scquence. It also indicates that the difference between the products of WT-RNA and 568-RNA suggested by the cyanogen bromide cleavage experiment described earlier is ii consequence of a
392 J. KNOWLAND
chains to such an extent that the peptides released no longer migrate with authen- tic coat protein peptides in the separation methods used. However, no abnormal
or unexpected secondary modifications of polypeptides synthesized on messengers injected into the oocyte have yet been observed, so this possibility seems a remote one.
DISCUSSION
I n this section certain aspects of the oocyte translation system that are rele- vant to the experiments described here are discussed and compared with
in
vitro translation systems, and the results obtained when TMV-RNA is translated in oocytes are compared with those obtained using in vitro systems, and also with the pattern of protein synthesis in plant cells infected with tobacco mosaic virus itself.Aduantages of the oocyte as an assay system for the proteins specified b y a messenger R N A
When searching for the translational product of a messenger RNA, it is im- portant to use a system with a low background of protein synthesis. One of the main disadvantages of the oocyte system is that it has a high level of endogenous protein synthesis. However, it is shown here that by appropriate choice of labelled amino acid, the background incorporation of radioactivity in oocytes can be re- duced almost to the low level that is one of the great attractions of cell-free sys- tems. It is also important that the translation of the messenger should be efficient and continue for some time, so that the main product is not obscured by the pres- ence of shorter intermediates in the synthesis of the longest polypeptide produced. Translation in oocytes is known to be highly efficient (GURDON et al. 1971) and to continue f o r a very long time (GURDON, LINGREL and MARBAIX 1973), and these particular features, when combined with the reduction in background in- corporation achieved here, make it possible to obtain a clear picture of the trans- lation of TMV-RNA in the oocyte system (Figures 1 and 2). Results obtained with cell-free systems, in which translation is generally less efficient and lasts f o r only an hour or so, are more complex (Figure 1 and ROBERTS and PATERSON
1973) ; there being, in addition to the 140,000-dalton polypeptide, many other products of lower molecular weight. The extra products may represent intermedi-
ate stages in the growth of the longest polypeptide, although other explanations, such as incorrect initiation of protein synthesis, o r cleavage of the largest product, are not excluded.
Protein synthesis in plant cells infected with tobacco mosaic virus
The number of new proteins made in plant cells after infection with TMV is not yet known. Using infected leaves, SINGER (1971 ) Pound no new proteins apart from coat protein or polypeptides apparently related to it, while ZAITLIN and
P L A N T V I R U S R N A I N A N I M A L C E L L S 393 suggested may be related to replicase. It seems then, that protein synthesis dur- ing the normal process of infection with TMV may include the production of high molecular weight polypeptides as well as the coat protein itself.
The chief difficulty in characterizing protein synthesis in plant cells infected with TMV is to find a system in which a large number of cells can be infected simultaneously. A possible approach is to use plant cell protoplasts in culture. Using this system, SAKAI and TAKEBE (1972) found, in addition to coat protein, a polypeptide with a molecular weight of 140,000. Because this product appeared to label normally with histidine, which is absent in coat protein, the authors con- cluded that it is unlikely to contain a coat protein sequence. If the 140,000-
dalton polypeptide found in infected protoplasts corresponds to that made in
injected oocytes, then the experiments described here support their conclusion, and suggest that synthesis of the large polypeptide may proceed independently of coat protein synthesis. The results also imply that, in the oocyte system, part of a n RNA molecule can be efficiently translated while another is not translated at all. The reasons for the restriction on translation of tobacco mosaic virus RNA in oocytes are not at all clear.
C O N C L U S I O N S
The work described above shows that the RNA of a plant virus can be success- fully translated in a normal, intact, animal cell. No factors specific to plant cells are required for the synthesis of a large polypeptide, and the product of trans- lation is stable inside the animal cell.
The main product of translation of tobacco mosaic virus RNA in Xenopus oocytes is a polypeptide with a molecular weight of approximately 140,000, which corresponds to translation of about 65% of the RNA. This contrasts with the results found using cell-free systems, in which only a small amount of the 140,000-dalton polypeptide is made, while a great many smaller polypeptides of uncertain origin are produced. Mature coat protein is not made in the oocyte, and .the available evidence suggests that the large polypeptide does not contain either
a whole o r a partial coat protein sequence.
It is not clear whether the translation of tobacco mosaic virus RNA in oocytes reflects the process as it normally occurs in plant cells because the course of pro- tein synthesis in plant cells following infection with tobacco mosaic virus is not yet fully characterized.
I am grateful to P. J. G. BUTLER for supplies of virus, to J. B. LINCW for advice on the use of reticulocyte lysates, to C. J. BRUTON for advice on techniques of protein chemistry, to J. B. GURDON and C. D. LANE for helpful criticism of the manuscript, and to the Medical Research Council and the Master and Fellows of St. John's College, Cambridge, for financial support. I a m particularly grateful to RUTH LONCTHORNE for invaluable assistance during these experiments.
L I T E R A T U R E C I T E D
394 J. KNOWLAND
FRAENKEL-CONRAT, H., 1957
GURDON, J. B., C. D. LANE, H. R. WGODLAND and G. MARBAIX, 1971
GURWN, J. B., J. B. LINCREL and G. MARRAIX, 1973
LAEMMLI, V. K., 1770
Degradation of tobacco mosaic virus with acetic acid. Virology
Use of frog eggs and oocytes for the stufiy of messxg-r RNA m d its translation in living cells. Nature 233: 117-182.
Message stability in injected frog oocytes: long life of mFmmalian a and ,8 globin messages. J. Mol. Bioml. 80: 539-551.
ClewPge of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 68oL685.
The injection of RNA into living cells: the use of frog oocytes f o r the assay of messenger RNA and the study of the control of gene expression. In: The Biochemistry of Animal Deuelopment. Vol. 3. Edited by R. A. WEBER. Academic Press, New York. (In press.)
Rabbit haemoglobin synthesis in frog cells; the translatioil or reticulo'cyte 9s RNA in frog oxytes. J. Mol. Biol. 61: 73-91.
Translation of Encephalomyocarditis viral RNA in oocytes in Xenopus laeuis. Proc. Natl. Acad. Sci. U.S. 69: 3665-3669.
The synthesis of mouse hemoglobin p-chains in a rabbit reticulocyte cell free system promgrammed with mouse reticulocyte 9s RNA. Biochemical and Biophysical Reseerch Communications 37: 204-21 1.
The fractionetion of high mdecular weight ribonucleic acid by poly- acrylamide gel electrophoresis. Biochem. J. 102: 251-257.
The wheat embryo cell-free system. In: Methods in Enzymology. Vol. 30. Edited by S. P. COLOWICK and N. 0. KAPLAN. Academic Press, New York.
REYNOLDS, J. A. and C. TANFXD, 1970 Blnd'ng cf dodecyl sulphate to proteins at high binding ratios. Posssible implications for the state of proteins in biological membranes. Proc. Natl. Acad. Sci. U S. 686: 100"7--1007.
The efficient translation of TMV RNA and rabbit globin 9s RNA in a cell-free system from commercial wheat germ. Proc. Natl. Acad. Sci. U.S. 70: 2330-2334.
Tobacco mosaic virus RNA directs the synthesis of coat protein pc?tide in a cell-free systcm from wheat. J. Mol. Biol. 80: 733-742.
A non-coat protein synthesized in tobacco mesophyll protoplasts infected by tobacco mxaic virus. Molec. Gen. Genet. 118: 93-96.
Protein synthesis in virus infected plants. I. The number and nature of TMV directed proteins detected in polyacrylamide gels. Virology 46 : 274-255.
Lokalisierung von Aminosaureaustaust- auschen bei Nitritmutanten des Tabakmosaikvirus. Z. Verebungsl., 97 : 305-326.
A gel electrophoresis analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology 47:
29G-305.
4: 1-4.
LANE, C. D. and J. KNOWLAND, 1973
LANE, C. D., G. MARBAIX and J. B. GURDON, 1971
LASKEY, R. A., J. B. G U R D ~ N and L. V. CRAWFORD, 1972 LOCKARD, R. E. and J. B. LINGREL, 1969
LOENINC, V. E., 1967
MARCUS, A., D. EFRON and D. P. WEEKS, 1974
ROBERTS, B. E. and B. M. PATERSON, 1973
ROBERTS, B. E., M. B. MATHEWS and C. J. BRUTCN, 1973
SAKAI, F. and I. TAKABE, 1972
SINGER, B., 1971
WITTMANN-LIEBOLD, B. and €1. G. WITTMANN, 1965