ePrints Soton
Copyright © and Moral Rights for this thesis are retained by the author and/or other
copyright owners. A copy can be downloaded for personal non-commercial
research or study, without prior permission or charge. This thesis cannot be
reproduced or quoted extensively from without first obtaining permission in writing
from the copyright holder/s. The content must not be changed in any way or sold
commercially in any format or medium without the formal permission of the
copyright holders.
When referring to this work, full bibliographic details including the author, title,
awarding institution and date of the thesis must be given e.g.
AUTHOR (year of submission) "Full thesis title", University of Southampton, name
of the University School or Department, PhD Thesis, pagination
Centre for Life Sciences
The effect of the aphid sex
pheromone on the aphid
Myzus
persicae
and its parasitoid
Aphidius
colemani
by
G. Mandela Fernández-Grandon
Thesis for the degree of Doctor of Philosophy
October 2012
I
UNIVERSITY OF SOUTHAMPTON CENTRE FOR LIFE SCIENCES Doctor of Philosophy
THE EFFECT OF THE APHID SEX PHEROMONE ON THE APHID MYZUS PERSICAE AND ITS PARASITOID APHIDIUS COLEMANI
Aphids remain an enormous threat to the sustainability of crops in glasshouse and field environments around the world. It is known that the aphid sex pheromone is used as a kairomone by its natural enemies, such as parasitoids. The focus of this research was how the aphid sex pheromone component, (4aS,7S,7aR)-nepetalactone, affects a host, its parasitoid and the host-parasitoid interaction in a tritrophic system. A model system of Chinese cabbage Brassica rapa sp. Pekinensis Cv. Wong bok, the peach-potato aphid Myzus persicae and the generalist parasitoid Aphidius colemani is applied with a particular emphasis on understanding parasitoid foraging and how it may be affected, and potentially manipulated, by nepetalactone.
Firstly, it was demonstrated that asexual M. persicae are capable of detecting the sex pheromone components, despite their components having no previously known ecological function in parthenogenetic populations. Although it was found that they avoid the odour in high concentrations, it was concluded that performance on an individual or population level were unlikely to be affected. The ability of the parasitoid
A. colemani to detect nepetalactone was confirmed at the electrophysiological level. Nepetalactone did not elicit any behavioural response when presented in isolation but was found to increase retention of the parasitoid within a patch if other host cues were also present. It was found that Nepeta cataria oil, from which nepetalactone can be isolated, increased the success of parasitoid oviposition in the host. To enhance parasitoid foraging, it was investigated whether learning was possible with
nepetalactone; an odour already known to elicit an innate response. Learning through emergence conditioning was ineffective in altering parasitoid behaviour; however, ovipositional experience did induce a change in foraging patterns. This change in foraging pattern did not translate to more effective host location when tested in the laboratory, which led the research towards experimentation in a more complex spatial-temporal environment. Nepetalactone, or the learning of nepetalactone, were not found to have an effect on parasitoid success at this scale. It was found that the introduction of parasitoids into a glasshouse environment reduced aphid population growth at a rate disproportionate to the rate of mummification. This highlighted the importance of indirect consequences of parasitoid visitation on aphid population control. In a separate assay it was identified that aphid population size affects plant fitness, such that smaller aphid populations result in greater plant fitness, thus
demonstrating benefits of parasitoids in biological control which are often overlooked. This work provided a greater insight into the role of nepetalactone in a tritrophic system and how odours may be used by parasitoids during foraging. Finally, the key findings of this study are discussed and the possible direction of future work. A new interpretation of parasitoid foraging is discussed, by the integration of information provided by this study and knowledge generated by previous work.
ABSTRACT ... III Contents ... V List of figures ... XI List of tables ... XIII Declaration of Authorship ... XV Acknowledgements ... XVII
1 General Introduction ... 1
1.1 Aphids ... 1
1.1.1 Lifecycle ... 1
1.1.2 Economic and Ecological Impact ... 2
1.1.3 The peach-potato aphid Myzus persicae ... 3
1.2 Parasitoids ... 3
1.2.1 Abundance and Diversity ... 3
1.2.2 Aphid Parasitoid Lifecycle ... 4
1.2.3 Aphid Parasitoid Aphidius colemani ... 6
1.2.4 Parasitoids in Biological Control ... 6
1.3 Semiochemicals ... 7
1.3.1 Discovery ... 8
1.3.2 Identification of an Aphid Sex Pheromone ... 8
1.3.3 Ubiquity of Nepetalactone in Aphid Species ... 9
1.3.4 Commercial production of aphid sex pheromone ... 10
1.4 Parasitoid Host Selection ... 11
1.4.1 Stages of Host Selection ... 11
1.4.2 Host Habitat Location ... 12
1.4.3 Host Location ... 13
1.4.4 Host Acceptance ... 13
1.4.5 Host Suitability ... 14
1.4.6 Host Regulation ... 14
1.5 The Reliability-Detectability Trade-off ... 14
1.5.1 Theory and Hypotheses ... 15
1.5.2 Infochemical Detour ... 15
1.6 Learning ... 17
1.6.1 Maternal influence ... 18
1.6.2 Emergence conditioning ... 18
1.6.3 Associative learning ... 19
1.6.4 Alpha conditioning ... 19
1.6.5 Success motivated retention time... 20
1.7 Parasitoid response to nepetalactone ... 20
1.8 Thesis aims and hypotheses ... 23
1.8.1 Overall aim of research ... 23
1.8.2 Individual chapter key hypotheses ... 23
2 The effect of nepetalactone on Myzus persicae virginoparae host choice and population growth ... 25
2.1 Introduction ... 25
2.2 Materials and methods ... 27
2.2.1 Aphid and plant cultures ... 27
2.2.2 Y-tube olfactometry ... 27
2.2.3 Effect of nepetalactone on aphid performance ... 27
2.2.4 Effect of nepetalactone on aphid population growth ... 28
2.2.5 Electrophysiology ... 29
2.2.6 Cleaning of Perspex and glassware ... 29
2.2.7 Statistical analysis ... 29 2.3 Results ... 30 2.3.1 Aphid preference ... 30 2.3.2 Aphid performance ... 31 2.3.3 Population growth ... 32 2.3.4 Electrophysiological response ... 33 2.4 Discussion... 34
3 Detection and response of Aphidius colemani to the aphid sex pheromone nepetalactone in a laboratory environment ... 39
3.1 Introduction ... 39
3.2.3 Square assay ... 42
3.2.4 On-leaf retention time ... 43
3.2.5 Single-attack assay ... 44
3.3 Results ... 47
3.3.1 Electroantennography ... 47
3.3.2 Square assay ... 47
3.3.3 On-leaf retention time ... 48
3.3.4 Single-attack assay ... 50
3.4 Discussion ... 54
4 Effect of learning on the behaviour of parasitoid Aphidius colemani to an already innate cue; nepetalactone ... 61
4.1 Introduction ... 61
4.1.1 Maternal Influence ... 62
4.1.2 Emergence conditioning ... 62
4.1.3 Ovipositional experience ... 63
4.1.4 Hierarchy of response ... 63
4.1.5 Alpha conditioning and the aphid sex pheromone response ... 63
4.2 Materials and methods ... 64
4.2.1 Parasitoid cultures ... 64 4.2.2 Parasitoid experience ... 65 4.2.3 Four-arm olfactometry ... 65 4.2.4 Attack/success rate ... 67 4.2.5 Square assay ... 69 4.2.6 Motivation assay ... 69
4.2.7 Leaf location assay ... 70
4.3 Results ... 71
4.3.1 Four-arm olfactometry ... 71
4.3.2 Attack/Success rate ... 74
4.3.3 Square assay ... 77
4.3.4 Motivation assay ... 79
5 The value of parasitism and nepetalactone in an applied context ... 89
5.1 Introduction ... 89
5.1.1 Effect of aphid feeding on plant fitness ... 89
5.1.2 Parasitoid application in biological control ... 90
5.1.3 Challenges to successful parasitoid application ... 90
5.1.4 Potential solutions to foraging inefficiency ... 91
5.2 Materials and Methods ... 92
5.2.1 Effect of aphid feeding on vegetative fitness ... 92
5.2.2 Enhancing foraging through learning in a complex spatial-temporal environment ... 93
5.2.3 Manipulating parasitoid numbers in a complex spatial-temporal environment ... 96
5.3 Results ... 97
5.3.1 Vegetative fitness ... 97
5.3.2 Enhancing foraging through learning in a complex spatial-temporal environment ... 101
5.3.3 Manipulating parasitoid numbers in a complex spatial-temporal environment ... 103
5.4 Discussion... 109
5.4.1 Vegetative fitness ... 109
5.4.2 Learning in a complex spatial-temporal environment ... 110
5.4.3 Manipulating release numbers ... 112
6 General Discussion ... 115
6.1 Key findings from each chapter ... 115
6.2 Understanding the role of nepetalactone in a tritrophic context: a new synthesis ... 117
6.3 Potential benefits to biological control ... 120
6.4 Future work ... 121
6.4.1 Nepetalactone to increase consistency of response ... 122
6.4.2 Understanding foraging strategies ... 122
Appendix B ... v Bibliography ... ix
Figure
1.1 Typical lifecycle of a peach-potato aphid. ... 2Figure
1.2 Typical aphid parasitoid lifecycle... 4Figure
1.3 Picture an aphid mummy. ... 5Figure
1.4 The two ubiquitous aphid sex pheromone components. ... 9Figure
2.1Preference of Myzus persicae virginoparae ... 30Figure
2.2 MRGR of M. persicae virginoparae. ... 32Figure
2.3 Natural rate of increase of Myzus persicae virginoparae. ... 33Figure
2.4 Electrophysiological responses of Myzus persicae virginoparae. ... 34Figure
3.1 Layout of the square assay arena ... 43Figure
3.2 Diagram of single- ... 46Figure
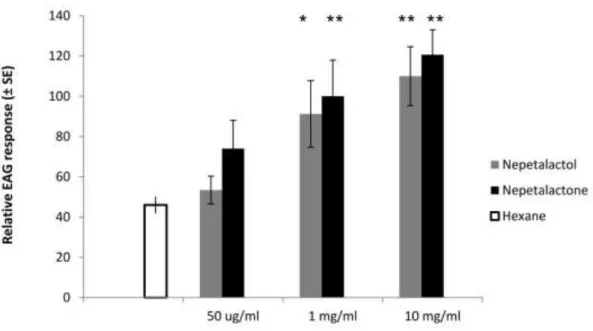
3.3 Aphidius colemani EAG response. ... 47Figure
3.4 Time spent by Aphidius colemani in each area of the square olfactometer. 48 Figure
3.5 On-leaf retention time set 1 ... 49Figure
3.6 On-leaf retention time set 2 ... 50Figure
3.7 Time to first attack ... 51Figure
3.8 Offspring after 14 days ... 51Figure
3.9 Fecundity following parasitism ... 52Figure
3.10 Effect of parasitoid attack on fecundity ... 53Figure
3.11 Success of attack ... 53Figure
4.1 Four-arm olfactometer ... 66Figure
4.2 Layout of the leaf location assay. ... 71Figure
4.3 Mean time spent in a given arm per visit. ... 73Figure
4.4 Attack rate and success rate in parasitoids ... 74Figure
4.5 Time spent grooming within the assay for each treatment. ... 75Figure
4.6 Time of first attack. ... 76Figure
4.7 Success rate of ovipositional attacks. ... 77Figure
4.8 Time spent in square assay treatments. ... 78Figure
4.9 Time spent in square assay quadrants. ... 79Figure
4.10 Effect of treatment on the turning of A. colemani. ... 80Figure
4.11 Parasitoid orthokinesis. ... 81Figure
4.12 Parasitoid leaf and host location. ... 82Figure
5.1 Layout of glasshouse compartment. ... 94Figure
5.2 Parasitoid release chamber. ... 95Figure
5.3 Placement of trap plants. ... 97Figure
5.4 Leaf numbers as an effect of aphid attack. ... 98Figure
5.5 Percentage of leaf growth following aphid attack. ... 99Figure
5.6a)-e) Aphid populations from days 2-10 of the foliar fitness experiments. 100 Figure
5.7 Number of mummies by treatment. ... 101Figure
5.8 Effect of parasitoid attack on aphid population growth. ... 102Figure
5.9 Correlation of mummies to aphid population growth. ... 103Figure
5.10 Number of mummies found for each treatment group. ... 104Figure
5.11 Effect of nepetalactone and parasitoid numbers on day 2 aphid populations. ... 105Figure
5.12 Effect of nepetalactone and parasitoid numbers on final aphid population. ... 105Figure
5.13 The effect of nepetalactone proximity on 2 day aphid counts. ... 106Figure
5.14 Proportion of mummies in trap and experimental plants... 107Figure
5.15 Effect of parasitoid visit on the growth of aphid populations. ... 108Figure
5.16 Correlation between mummies and aphid numbers. ... 108Figure
... 109Figure
6.1 Visual representation of the effect of nepetalactone on parasitoid foraging. ... 119Table
1.1 Ratios of nepetalactol to nepetalactone found in entrainments collected from oviparae of different aphid species (taken from Stewart-Jones et al., 2007). ... 10 Table
1.2 Parasitoids which have exhibited a response to aphid sex pheromones (taken from Powell & Pickett, 2003). * This study came out after the original publication and has been added by the author ... 21 Table
4.1 Retention time of Aphidius colemani in the four-arm olfactometer after exposure to various pre-treatments and treatments. The P value was acquired by comparing the proportion of time spent in treatment arm to the expected proportion (0.25) using a one-sample t-test. NS = Not Significant. ... 72 Table
4.2 Percentage of visits to an arm by Aphidius colemani after exposure to a pre-treatment/treatment set. The P value was acquired by comparing the proportion of visits made to the treatment arm to the expected proportion (0.25) using aone-sample t-test. NS = Not Significant. ... 72 Table
6.1 Findings from this thesis which may offer potential benefits or challenges to the application of aphid sex pheromones in biological control. ... 121I, Gabriel Mandela Fernández-Grandon declare that this thesis and the work presented in it are my own and has been generated by me as the result of my own original research.
The effect of the aphid sex pheromone on the aphid Myzus persicae and its parasitoid Aphidius colemani
I confirm that:
1. This work was done wholly or mainly while in candidature for a research degree at this University;
2. Where any part of this thesis has previously been submitted for a degree or any other qualification at this University or any other institution, this has been clearly stated;
3. Where I have consulted the published work of others, this is always clearly attributed;
4. Where I have quoted from the work of others, the source is always given. With the exception of such quotations, this thesis is entirely my own work;
5. I have acknowledged all main sources of help;
6. Where the thesis is based on work done by myself jointly with others, I have made clear exactly what was done by others and what I have contributed myself;
7. Either none of this work has been published before submission:
Signed:
This PhD studentship was supported by a BBSRC CASE Studentship with AgriSense BCS Ltd. I would like to thank Guy Poppy for his supervision throughout the project. Also a great deal of gratitude is owed to Jamie Sutherland and Owen Jones for getting the project started and giving me the opportunity to conduct this research. Throughout the project I was also fortunate enough to have statistical help and general support from Lex Kraaijeveld. I would also like to thank Brian Fenton and Gaynor Malloch for introducing me to the scientific world. Thank you to Christine Woodcock for help on EAG work, Jade Revan for work on the single-attack bioassays and Merry George for isolating the nepetalactone. Thanks also to Karen Girard and Koppert B.V. for
providing me with parasitoids at no cost and allowing me to see IPM in action. It has been a wonderful opportunity to work in a field that fascinates me so much and to feel, in some small part, I may have contributed.
On a personal level I also owe a great deal to a great many. Some of these are the cast of Boldrewood/B85 and the SCR who have provided relief over breakfast, lunch, dinner and nights out. Prammy P., Maaike B., Naimh O. and Yasser A5 Ibrahim for the games nights. All the Lads from back home who ensured I had a warm welcome on the rare chance I had to visit. Specifically I have to mention Michael R. Burns and Ruairidh A. Falconer who have never failed to lift my spirits. Thanks to the basketball teams I have played for/coached: The Ravens, Eagles, Supersonics and Swifts that have kept me fit and happy. My many thanks to Coach Tomasz Sosna for giving so much of his time. Gracias a mis padres, que me han dado tanto. La educación comenzó 100 años antes de que yo naciera. Ustedes han luchado para que nosotros podamos aprender y vivir una vida rica, gracias Modge y Dodge. Gracias a mi familia cerca y lejos. Lalo, Bruni and Lia thank you, you have always looked after me well and expected little in return. Lastly, but far from least, I would like to thank Kirstin J. Williamson for continuing to love and support me. I look forward to the future as we tackle the world together.
1rb > c
1
1
General Introduction
1.1
Aphids
1.1.1
Lifecycle
Aphids are small soft bodied hemipterans. With around 4400 extant species (Resh & Cardé, 2009) aphids are a large and ecologically well distributed group. All but two families of the aphid families are found in the Aphidoidea superfamily (Phylloxeridae and Adelgidae can be found in the Phyllxeroidea superfamily). Aphids use their stylet to pierce plant tissues and enter the phloem where they obtain the sugar-rich sap. Whilst the majority of aphid species are autoecious (completing their life cycle on one or more species of closely related plants) some of those that are hetereocious
(spending different stages of their lifecycle on unrelated hosts) can be highly
polyphagous; feeding on a wide variety of plants. An example is Myzus persicae which is capable of feeding on, and spreading viruses between, over 30 different plant
families (van Emden et al., 1969).
Aphids go through four instars of development before becoming adults. Adults which form wings (the buds of which will be seen in earlier instars) are known as alatae though the majority will remain wingless, known as apterae. Production of alatae provide aphids access to more distant resources and areas subject to less predation, however, the morphological change also displays trade-offs with the reproductive ability of the individual (Dixon et al., 1993), as is generally seen for dimorphic insects (Guerra, 2011).
The majority of aphids studied alternate between sexual and asexual generations (Figure
1.1). Many prominent aphid pests follow a heteroecious feeding pattern: feeding on crop plants during the year and utilising shrubs or trees to overwinter. In holocyclic lifecycles the aphid will produce a sexual form with overwintering eggs. Less common are anholocyclic lifecycles which remain asexual throughout the year. The majority of pest aphid populations are asexual for most of the year but pass through a sexual phase (Powell & Pickett, 2003).Figure
1.1 Typical lifecycle of a peach-potato aphid.Illustration of
M. persicae
lifecyle when peaches are present from Radcliffe
et al.
(1993).
The parthenogenetic reproduction seen during asexual generations produces a
offspring allowing for exceedingly rapid population growth (Blackman & Eastop, 1984). Through this extraordinary reproductive strategy aphid populations are capable of exponential growth (Lin & Ives, 2003), an attribute that adds to difficulty in creating effective pest management strategies.
1.1.2
Economic and Ecological Impact
Aphids are prevalent pests in agricultural systems around the world. This is due to direct damage caused by feeding on the plant phloem, a process which removes many of the plants essential nutrients, but also their efficacy as vectors for a range of plant viruses (Lushai & Loxdale, 2004, Martinez-Torres et al., 1999). Populations of aphids have proven difficult to control because of their rapid rate of reproduction, adaptation to insecticides and polyphagous nature which allows persistence even when the host plant is protected. In the UK aphids are responsible for over half of all plant virus
million per annum (Barker et al., 2003). Traditionally large quantities of pesticides have been applied to reduce aphid populations but this has often proven to be ineffective (Kim et al., 2001) and in some cases has even aided the growth of aphid populations (Chen et al., 1991). Furthermore, the frequent application of pesticides has led to selection for resistant strains (Wang et al., 2007) which has reduced their efficacy (Foster et al., 1998, Foster et al., 2002). Fears raised by the possible effects of pesticide residues (Nasreddine & Parent-Massin, 2002) are reflected in increased consumer distrust of chemical application (Atkinson et al., 2003) and stricter government regulations (Europa, 2008) which have meant that persistent high level use of pesticides is no longer an option for European growers. Among the most devastating pests to agriculture globally is the peach-potato aphid, Myzus persicae.
1.1.3
The peach-potato aphid
Myzus persicae
Myzus persicae are wide spread heteroecious aphids normally found in temperate climates. Asexual generations are produced during the year; as the temperature decreases sexual morphs are produced and, after mating, the oviparous females lay overwintering eggs (Figure
1.1). This typical M. persicae life cycle is mediated by changes in temperature and photoperiod throughout the year (Trionnaire et al., 2008). It is often seen in temperate regions, such as those seen across much of the UK, M. persicae will exclusively reproduce asexually, producing only females in each generation. It was established by Horsfall (1924) that M. persicae go through four instars and live to approximately 23 days, though subsequent studies (MacGillivray & Anderson, 1958) have described five instars and a longevity of around 41 days, the latter of which is supported by personal observations.M. persicae are highly polyphagous, capable of feeding on plants in over 30 different families (van Emden et al., 1969) and able to act as a vector for over 100 plant viruses (Kennedy et al., 1962). As a consequence of the strong selection pressures applied to
M. persicae feeding on crops they exhibit some of the most highly adapted mechanisms of insecticide resistance seen in aphids (Fenton et al., 2004).
1.2
Parasitoids
1.2.1
Abundance and Diversity
There are currently around 68,000 described species of parasitic wasps, or parasitoids, meaning that they alone account for 4% of all known metazoan species (Godfray, 1994). Of this substantial number the majority (around 50,000) are Hymenoptera (Gaston, 1991). They are often considered an intermediate between predators and true parasites, because, like many true parasites they often require only one host to
complete development, however, like predators their feeding also ensures the death of their host. A parasitoid differs from predators and from true parasites in that the death of the host is a necessary part of the lifecycle; this is what defines parasitoids. The word was originally coined by Reuter (1913) to describe an organism which develops as a larva inside other insects and eventually leads to the host death. They have been of great interest to biologists for their curious lifecycles, display of complex learning and the practical application they have in pest population control.
1.2.2
Aphid Parasitoid Lifecycle
Parasitoids display a vast array of complex lifecycles which are intimately related to those of their hosts. For aphid parasitoids the lifecycle commences with the female locating a viable host and ovipositing (Figure
1.2). Aphid parasitoids are generally found to prefer juveniles, producing their largest offspring if ovipostion occurs in hosts at the 3rd instar of development (Sequeira & Mackauer, 1992a).Figure
1.2 Typical aphid parasitoid lifecycle.The parasitoid illustrated here is
Lysiphlebus testaceipes
(taken from Hoffmann & Frodsham,
1993).
Following oviposition, the parasitoid larva will complete development inside the host utilising it as a source of energy and protection. In many parasitoids superparasitism may occur in which several eggs may develop within the same host, however, aphid
During the early stages of parasitoid larval development the aphid host may employ sufficient to entirely prevent parasitoid development, however, if it fails to prevent parasitoid development the larva will absorb all the nutrients required for its growth and begin form a silk cocoon within the host to complete pupal growth. During this stage of development parasitoids are themselves vulnerable to parasitism. In natural environments, and those produced by glasshouses, this form of secondary parasitism, or hyperparasitism, frequently occurs and can provide difficulty in implementing sustainable parasitoid-based biological control solutions. If the primary parasitism
le becoming hardened displaying the bronze/gold colour characteristic of an aphid
(Figure
1.3).Figure
1.3 Picture an aphid mummy.Image showing the circle from where the parasitoid has emerged from the host. Courtesy of
the Abbot Lab, University of Vanderbilt.
Once the pupa is fully matured it will chew a circle out of the upper rear of the mummy (Figure
1.3) through which it will escape, emerging fully developed and ready to mate or locate a host. Males tend to have a shorter gestation time, most likely due to their smaller body size (Sequeira & Mackauer, 1992a). Once emerged, males will often remain in the patch ready to mate with emerging females. As is typical of manyhymenopterans, aphid parasitoids are haplodiploid meaning that fertilized eggs laid by the female will develop into females and the unfertilised eggs will develop into males (Hickman et al., 2004). This allows mated females control over the sex of the egg that
is deposited in the host. It is normally found that females are laid in higher quality host (Charnov, 1982) though can be related to other factors such as the age of the mother (He & Wang, 2008).
1.2.3
Aphid Parasitoid
Aphidius colemani
Aphidius colemani is a generalist parasitoid (Hymenoptera:Braconidae) that most likely originated from Northern India or Pakistan though is now found Australia, Europe and across North and South America (Starý, 1975). A. colemani has been reared on dozens of different aphid species, however, due to a lack of interbreeding between
populations and slight morphological variations between them it has been suggested that Aphidius colemani is actually a collection of sibling species (Messing & Rabasse, 1995). If this is the case then A. colemani may not be as generalist as previously believed since not all the studies were conducted with the same populations. A. colemani are commercially produced and available as a control agent in the UK, primarily for M. persicae and Aphis gosspyii aphid populations (Grasswitz & Resse, 1998). Amongst insects Hymenoptera display a high aptitude for learning and strong reliance on olfaction, making them ideal model specimens for study. In addition A. colemani are prevalent in biological control programmes and widely commercially available providing a potential application for knowledge gained from this study. The knowledge gained in laboratory assays regarding foraging will be tested in a
glasshouse environment (Ch.
5) and it is, therefore, pragmatic that widely used biological control agent is used.1.2.4
Parasitoids in Biological Control
Biological control, or biocontrol, is the use of living organisms to control pest
populations. The introduction of such methods is believed to have started over 1600 years ago in ancient China where the arboreal ant Oecophylla smaragdina was first used to protect crops (Wei et al., 2005). The first known successful introduction of a natural enemy for biological control in modern times is believed to have occurred in the eighteenth century (DeBach & Rosen, 1991). Parasitoids have been a prominent feature in integrated pest management since the early 19th century and have proven an integral component (Orr & Suh, 1998) with 66% of successful control plans involving a species of hymenopteran parasitoid (Thacker, 2002). Parasitoids are effective in biological control programmes because they are often capable of attacking a large number of hosts each day and many species can be reared on a large scale. In aphid biological control, unsuccessful applications of parasitoids have been ascribed to a lack of synchrony with the host (Powell & Pickett, 2003), poor quality of the parasitoids (Fernandez & Nentwig, 1997) or failure in the initial stages of foraging (Lewis et al.,
1990). A potential solution to problems in synchrony and foraging may be the application of semiochemicals.
1.3
Semiochemicals
A semiochemical is any chemical used by organisms that conveys information concerning the organism itself or the environment (Law & Regnier, 1971). The term comes from the Greek routes of semeion (σημειου) meaning signal and was suggested as a simple term covering both inter- and intra-specific chemical communication (Law & Regnier, 1971)
ted by an animal to the outside and (Karlson & Butenandt, 1959). The word is offered as an alternative to previously used
in meaning to carry and horman to excite (Karlson & Butenandt, 1959). Although a pheromone in its true sense refers to intraspecific communication it is often used to encompass the range of
olfactory signals produced by an organism. Semiochemicals are most commonly divided by the cost/benefit relationship that exists between the signaller and the receiver. A kairomone is a semiochemical received by an individual of another species to their benefit. A synomone is a semiochemical received by a member of another species which benefits both the signaller and the receiver, though this is often at the expense of another species. An allomone is a semiochemical received by a member of another species that will elicit behaviour which benefits the signaller.
It is recognised that all these interpretations of pheromone communication are subjective. To take the example of the aphid sex pheromone: it is produced by a calling female aphid to attract male conspecifics, in this sense it is a classic
pheromone. However, the reception of the signal by a parasitoid using the aphid as a host makes it a kairomone. In addition the odour may be used by a hyperparasitoid, which will attack the parasitoids, benefiting the aphids and could therefore be viewed as a synomone. It is also recognised that in this sense the term signaller can be
however, in many cases the signal is produced passively with negative consequences to the individual e.g.
obtaining a balance of nutrients and energy but when received by a natural enemy can be a kairomone to aid in the location of a host. The terms ascribed to semiochemical subcategories can be useful in describing an olfactory interaction, though it is
important to recognise that the complexity of multitrophic systems means that each odour may be involved in a range of interactions, whether this is the intention of the signaller or not.
1.3.1
Discovery
The first observation of pheromones is credited to Jean-Henri Fabré at the end of the 19th century. The discovery was made when Fabré noted that a virgin female emperor
o (Fabré & Miall, 1911). He went on to find that it was still attracting males so long as the covering was not airtight, and from this deduced that a chemical component must be involved. The first full identification of such a pheromone only
dissected 500,000 silk moths in order to remove the 12mg of fluid from the hormone glands needed to analyse and identify the chemical constituents of a pheromone for the first time (Butenandt et al., 1959). Since then, the techniques for identifying semiochemicals have advanced enough that often only one individual is needed. As a result a wide variety of semiochemicals have been characterised, including those of several aphid species.
1.3.2
Identification of an Aphid Sex Pheromone
During sexual phases of the aphid lifecycle female aphids will produce sex pheromone demonstrated by the attraction of male aphids in an olfactometer (Pettersson, 1970), and later through chemical analysis (Dawson et al., 1987). Oviparous females emit the pheromone from glands on the metathoracic tibia (Marsh, 1975). When calling, the female typically raises her hind legs and waves them in the air allowing for a greater exposure of the odour emitting glands (Pettersson, 1970). The odour is then received by male aphids via the second rhinaria of the antennae (Pickett et al., 1992).
Using gas chromatography techniques Dawson et al (1987) were able to analyse extracts from excised legs of the vetch aphid (Megoura viciae) to find two active ingredients: (+)-(4aS,7S,7aR)-nepetalactone and (-)-(1R,4aS,7S,7aR)-nepetalactol. When tested individually neither of these isomers were found to elicit a strong response in males. The two combined did show a response equal to that of the
original extract, demonstrating that the blend of the two components was important to aphid response.
It has been found that oviparous females do produce trace amounts of sex pheromone when not calling (Dawson et al., 1990) but it is not known whether the amount
produced would be sufficient to attract males. The sex pheromone production of the (Goldansaz & McNeil, 2003). It has been shown for the greenbug aphid Schizaphis graminum productivity is greatest between 6-8 days of the adult stadium (Eisenbach &
Mittler, 1980) and for the rosy apple aphid, Dysaphis plantaginea, pheromone
production is greatest on the 8th day of the adult stadium (Stewart-Jones et al., 2007). The quantity of pheromone that aphids emit varies widely by species with the vetch aphid, Megoura viciae, producing around 100-150 ng per ovipara per day (Hardie et al., 1990) to the rosy apple aphid, Dysaphis plantaginea, producing only 60-90 ng per ovipara per day (Stewart-Jones et al., 2007).
An understanding of the parasitoid response to semiochemicals may assist biological control programmes in overcoming some of the challenges faced by foraging
parasitoids. A promising application of semiochemical technology may be through genetically modified plants (Poppy & Powell, 2004), which have already shown capable of expressing the aphid alarm pheromone and had success in attracting parasitoids (Beale et al., 2006). The promising results of such work has encouraged further study in the form of full-scale field trials (Pickett et al., 2012). However, the use of
genetically modified plants is also facing strong public opposition or unease (Grant, 2003) and it is likely any introduction of semiochemical technology in pest control in the near future will rely on pre-existing methods of application.
1.3.3
Ubiquity of Nepetalactone in Aphid Species
The two active components of the aphid sex pheromone were found to be the monoterpenoid isomers (4aS, 7S, 7aR)-nepetalactone and (1R, 4aS, 7S, 7aR
)-nepetalactol (Figure
1.4) (Dawson et al., 1987). Neither component was found to be attractive to male vetch aphids, Megoura viciae, when presented alone; however, a combination of the two compounds resulted in a behavioural response equal to that elicited by the leg extract. Since this discovery it has been noted that nepetalactone and nepetalactone are ubiquitous components of aphid sex pheromones (Dawson et al., 1990). They appear as primary constituents in the pheromones of all aphids, with the only exceptions observed to date being the damson-hop aphid (Phorodon humuli) which utilises two different nepetalactol diastereoisomers (Campbell et al., 1990) and possibly the additional compound (1S, 2R, 3S)-dolichodial produced by the rosy apple aphid, Dysaphis plantaginea (Dewhirst et al., 2008).Figure
1.4 The two ubiquitous aphid sex pheromone components.Left: (4aS, 7S, 7aR)-nepetalactone, Right: (1R, 4aS, 7S, 7aR)-nepetalactol (taken from Birkett &
Pickett, 2003).
With two active compounds comprising the majority of aphid sex pheromones it is understood that the males differentiate between species by the ratio of lactol to -lactone. The blend of many aphid sex pheromones have been described (Table
1.1), although it is observed that the ratio of compounds that the female produces can vary during her adult phase (Hardie, 1990).Table
1.1 Ratios of nepetalactol to nepetalactone found in entrainments collected from oviparae of different aphid species (taken from Stewart-Jones et al., 2007).Common name Species name Ratio (ol:one) Reference
Cabbage aphid Brevicoryne brassicae 0:1 (Gabryś et al., 1997)
Black-berry cereal aphid
Sitobion fragariae 0:1 (Hardie et al., 1992)
Grain aphid Sitobion avenae (trace) 0:1 (Lilley et al., 1994a)
Spiraea aphid Aphis spiraecola 1:2a (Jeon et al., 2003)
Peach aphid Tuberocephalus
momonis
1:4 (Boo et al., 2000)
Vetch aphid Megoura viciae 1:5–1:12 (age effect) 1:6 (Hardie, 1990)
(Dawson et al., 1990)
Spiraea aphid Aphis spiraecola 1:6–1:8 (age effect)b (Jeon et al., 2003)
Black bean aphid Aphis fabae 1:29 (Dawson et al., 1990)
Pea aphid Acyrthosiphon pisum 1:1 (Dawson et al., 1990)
Peach-potato aphid Myzus persicae 1.5:1 (Dawson et al., 1990)
Rosy apple aphid Dysaphis plantaginea 3.7:1 3.7:1–3.3:1 (age
effect)
(Stewart-Jones et al.,
2007)
Potato aphid Macrosiphum
euphorbiae
4:1–2:1 (age effect) (Goldansaz & McNeil,
2003)
Greenbug Schizaphis graminum 8:1 (Dawson et al., 1988)
Currant aphids Cryptomyzus spp. 30:1 (Guldemond et al., 1993)
Bird cherry-oat aphid Rhopalosiphum padi 1:0 (Hardie et al., 1994c)
Damson hop aphid Phorodon humuli 1*:0 (Campbell et al., 1990)
a Field-collected oviparae. b Laboratory-reared oviparae
Using M. viciae it has been shown that the males are responsive to sex pheromone blends associated with other aphid species but had the strongest response to the ratio represented by their own species (Hardie, 1990).
1.3.4
Commercial production of aphid sex pheromone
Behavioural bioassays conducted by Hardie et al (1997) confirmed that the enantiomeric purity of the compounds needed to be high in order for them to be biologically active. This discovery made the previous methods of nepetalactone/lactol
from citronellol extraction unfeasible (Dawson et al., 1996). The relatively small quantity of pheromone produced by the aphids themselves meant that any production on a commercial scale would require an alternative source. Male aphids of M. viciae,
Aphis fabae and Acyrthosiphon pisum respond to synthetic sex pheromone in the laboratory (Hardie, 1990) and Sitobion fragariae males have shown attraction to synthetic sex pheromone in the field (Hardie et al., 1992) along with a range of parasitoid species (Hardie et al., 1991). While synthesis of the compounds from raw materials is possible, and ecologically applicable, it is too costly to be performed on large scale (Birkett & Pickett, 2003). A steam distillation system was developed using the catmint plant, Nepeta cataria, which has allowed low cost and high yield
production of the sex pheromone components. The essential oil is extracted from the fresh plant via steam distillation with the addition of cyclohexane to collect volatile oil. The steam and cyclohexane are entered into a condenser where the cyclohexane fraction and the water are separated. Vacuum distillation is performed on the cyclohexane fraction to remove the volatile oil (Birkett & Pickett, 2003). To slow the rate of release, and improve commercial potential, the extract can be infused into a polymer strip of ethylene vinyl acetate, polyvinyl acetate or polyvinyl chloride. The inexpensive mass production of nepetalactone has opened-up opportunities for commercial application of the pheromone in biological control programmes. If parasitoid foraging is to be enhanced through the use of nepetalactone it is first necessary that the processes involved in parasitoid host selection and the role that odours play is understood.
1.4
Parasitoid Host Selection
1.4.1
Stages of Host Selection
Divisions of the stages of host selection by a hierarchy of cues, or the order in which they are encountered, are common in the literature (van Alphen & Vet, 1986, Wellings, 1991). One of the most widely adopted categorisations, and that most applicable to this study, is that defined by Vinson (1976). Vinson describes five distinct stages to the host selection process. Although chemicals play a vital role in all stages of host selection it is speculated that odour is most prominent in the initial three stages, which will be the focus of this study. Experiments in this study are designed to address foraging issues associated with these initial three stages although a review is given below of each stage to provide understanding of the complete process. It is also noted that visual and vibrational cues can be important in host location (Fischer et al., 2001 respectively, Michaud & Mackauer, 1995), though it is odour that is most extensively covered in the literature and will be discussed here. It is recognised that these stimuli may be used by the parasitoid in conjunction (Wäckers & Lewis, 1994) but appears that
odour is the most important cue in long range foraging. All statements made regarding host selection in the following sections are supported directly by Vinson (1976) unless an alternative reference is provided. Division of host selection into five stages is applicable to all foraging parasitoids; however the focus of this text is the application of the theory to aphid parasitoids. Therefore, unless otherwise stated, the context of explaining host selection is provided with relation to aphid parasitoids.
1.4.2
Host Habitat Location
To initiate the host selection process it is necessary for the parasitoid to locate the habitat of its host. Whilst the ideal chemical signal for a parasitoid is one that relates directly to the presence of a suitable host, these are not always readily available. Any cue released by the host that is easily detectable to a parasitoid from a distance will be selected against. For this reason, few signals of this nature exist or are broadcast over a large distance and instead parasitoids resort to utilising other cues from the
environment which may relate to the presence of the host. Plants that are preferred by hosts tend also to be preferred by parasitoids. Due to the greater volume of odour produced, plant volatiles are much more readily available than pheromones produced by the host and are, therefore, frequently the main cue used by a foraging parasitoid at long distance. Plants subjected to damage by the host will often produce a greater volatile output (Kessler & Baldwin, 2001) with an altered ratio of compounds (Paré & Tumlinson, 1999). Plants releasing volatiles indicating a level of stress are selected by the parasitoid over a healthy one because it is more likely to have feeding hosts (Du et al., 1998), and preference for a plant will be greater still if the preferred host is present (De Moraes et al., 1998). The cause of the volatile output differences are not fully understood, though it is believed that in aphids components of the saliva elicit the distinct volatile complex. The role of these salivary proteins in aphid feeding is not completely understood either but it is hypothesised that that they play an important role or would have been selected against considering the disadvantage of being more easily located by parasitoids (Powell & Pickett, 2003). Since aphids are commonly found in sporadically located but densely populated patches it would be expected that the parasitoids would be adapted to respond to signals released upon aggregation of aphids to increase the chance of encountering a patch rich in hosts. Indeed Aphidius ervi have been seen to have a threshold of 40 aphids before significant levels of response are seen (Powell et al., 1998). Similarly Cotesia vestalis, a parasitoid of
Plutella xylostella was shown to display a density dependent response to the odour of feeding caterpillars (Girling et al., 2011).
1.4.3
Host Location
When a parasitoid locates a patch containing the host, it encounters an additional range of odours, some of which are produced directly by the host. At this stage in location, aphid-associated cues such as exuviae and honeydew can be utilised by the parasitoid or any of the semiochemicals produced by the host e.g. alarm pheromones that are often employed by aphids to signal the presence of an enemy. The alarm pheromone, (E)-β-farnesene, is secreted by the aphids through the cornicles causing conspecifics to scatter or seek other parts of the plant. This is a highly informative signal because it demonstrates the presence of an aphid colony, although only one that is already making attempts to escape the area. By utilising host waste produce or their pheromones as kairomones parasitoids are able to gain more reliable information as to the presence of the host.
1.4.4
Host Acceptance
When a host is successfully encountered and available, the parasitoid may still select not to oviposit in the individual, this stage of selection is the host acceptance. The basis for host acceptance includes shape, size, movement and sound of the host, but again odour is recognised as having an essential role. The presence of the host odour is sufficient to elicit the parasitoid piercing with its ovipositor (Picard, 1922) but it appears other factors are necessary to induce the eggs to be released. These factors could be olfactory stimuli or tactile chemical communication but this remains
unknown, and is likely to be a combination of these factors (Battaglia et al., 1993). Acceptance levels by the parasitoid to certain hosts vary based on size of the host or age, which is often correlated. It has been shown that aphids of different ages
produce differing levels of pheromones, and it is therefore likely that certain chemical cues have diminished in adults that would otherwise induce oviposition. It is also possible that the preferences of the parasitoid could be learnt relative to previous success and may therefore vary depending on their environment or life history. Another factor in host acceptance is the pheromones left by previous parasitisation. Upon contacting the host a parasitoid may leave a chemical marker, intentially or not, indicating that the host has already been parasitized, or visited. This avoids the parasitoid wasting more eggs in the same host and creating competition between its offspring but it also reduces possible resource competition with other parasitoids. It is speculated here that if the host marker odour is strong enough it may play a role in the previous stages in host selection as it signals that viable hosts were available and therefore the environment is conducive to the feeding of the host species and the presence of a host colony.
1.4.5
Host Suitability
The initial three stages of host selection contribute to aspects of the foraging process. The location of an appropriate host is vital, but alone it does not guarantee
reproductive success for the parasitoid. Host suitability relates to the ability of the parasitoid larva to develop inside the host. If superparasitism has occurred then competition may take place within the host for resources or more direct conflict may arise between the larvae. Toxins may also be released to eliminate a competitor or alter the physiological state of the host, some of which will be induced by the mother during oviposition. Another barrier to successful development is toxins which may already be carried within the host or diseases that it may carry which the parasitoid is susceptible to. Due to disease or predation the host may die before the parasitoid larva is able to complete the necessary stages of development. This is one of the reasons that the use of pesticides and parasitoids in pest control would have to be carefully controlled; ensuring that hosts are not made unviable due to the levels of pesticides in their body which would also eliminate the parasitoid population.
1.4.6
Host Regulation
to permit its own survival. Host regulation is very closely linked to host suitability in many aspects. This stage can be viewed as the processes carried out by the parasitoid to make the host an adequate source of food and shelter. One of the first and most significant steps in host regulation for many species of parasitoid is the paralysation of the host. This can be permanent or transient and allows control over the host body. The dose of venom must be carefully controlled to ensure the beneficial effect for the parasitoid without killing the host. A parasitoid may also regulate growth rate, food consumption, development, morphology, behaviour, respiration, biochemical and physiological activities within the host (Vinson & Iwantsch, 1980). The regulation of the host may be necessary to ensure the survival of the host until the parasitoid has reached the required stage of development. It follows through kin selection theory that the premature death of a parasitized aphid will enhance the survival of its genes and therefore be selected for. It is recognised that regulation is caused by the larva whilst within the host but also much of it is initiated by the parasitoid mother during oviposition.
1.5
The Reliability-Detectability Trade-off
The stages of host selection provide a framework to interpret the process undertaken by a parasitoid. However, it does not fully address some of the challenges faced at each stage or why host selection does occur in these stages. For the purpose of this
during foraging and how they utilise the odours available. The dependency on habitat and host associated cues in the early stages of host selection requires that the
parasitoid evaluate the accuracy of the signal in conveying information regarding the host location. A notable challenge that arises in signal detection is that the value of such a signal is often indirectly related to its availability. This phenomenon is known as the reliability-detectability problem.
1.5.1
Theory and Hypotheses
To optimise foraging potential within a multitrophic context it is necessary that parasitoids evaluate the validity of the chemical information available, and respond accordingly. It is recognised that the most reliable cues for a parasitoid to respond to are those directly emitted by a viable host. However, selection pressures exist to ensure that cues emitted from the hosts remain discrete to avoid such detection by predators or parasitoids. Selection pressure on the parasitoid to find a host is also strong because failing to find a suitable host will mean a failure to produce offspring. Within a multitrophic system there may also exist organisms which suffer no
deleterious effect as a consequence of detection, or may even benefit from it, such as the plants being attacked by herbivores (van Loon et al., 2000). From this arises the reliability-detectability trade-off (Vet & Dicke, 1992) which represents the challenge faced by a parasitoid in an environment where cues of greater detectability are less reliable, and those highest in reliability remain more discrete. Reliability here is defined as odours more accurately indicating the presence of a viable host, while detectability is a measure of how available the odour is to detection.
It is hypothesised that three strategies may be employed by the parasitoid to overcome the reliability-detectability problem:
1. The use of more conspicuous chemical cues from individuals of the host species from a different stage of development (infochemical detour).
2. Responding more strongly to stimuli rele plant.
3. Learning to associate easily detected stimuli to less readily detected but more reliable signals.
1.5.2
Infochemical Detour
An infochemical detour is the use of more conspicuous chemicals released by the host species though not necessarily at a stage viable for parasitisation. In aphidiine
parasitoids the use of the aphid sex pheromone to locate viable hosts (Hardie et al., 1991) could be an infochemical detour: using the pheromone produced by adult females (Hardie, 1990) to locate the juveniles which are used as hosts, a phenomenon
that can also be seen in other host-parasitoid models (Wiskerke et al., 1993).
Infochemicals can also be in the form of waste material produced by the host such as; aphid exuviae for Aphidius ervi (Battaglia et al., 2000), aphid honeydew for Aphidius rhopalosiphi (Budenberg, 1990) or host faecal matter for the lepidopteron parasitoid
Microplitis croceipes (Lewis & Tumlinson, 1988).
As a result of low mobility and a high reproductive rate, juvenile aphids are often found alongside adults. This increases the consistency with which parasitoids find viable hosts using an infochemical detour.
1.5.3
Host/Food Source Interaction
In 1937 Laing noted that a braconid wasp could respond to cue
source (Laing, 1937). In this early case it was uninfested meat that elicited a response from the parasitoid Alysia manducator whose host, the Calliphora maggots, normally feed on meat. This represents the initial stage of host selection, habitat location. In the case of Alysia Manducator exposed meat is reliable as the abundance of Calliphora
species provide a high rate of infestation for any meat exposed to the environment. In a similar fashion aphid parasitoids show a response to plant volatiles (Elzen et al., 1983), however, due to the abundance of such volatiles within the environment the odours do not infer the same reliability. To increase the reliability of response the parasitoids respond more strongly to damaged plants (Guerrieri et al., 1993). It is common for parasitoids to utilise plant volatiles and, if the theory that modern
entomophagous parasitoids evolved from wasps parasitizing plants (Malyshev, 1968) is correct, it would imply that the use of plant volatiles in foraging predates even the use of an insect host. A response is seen most strongly to infested plants, less so to artificially damaged plants and rarely to completely healthy plants (Dicke et al., 1999, Girling et al., 2006, Turlings et al., 1995). The reason appears to be the increased release of volatiles emitted by the plant when damaged (Rose et al., 1996, Turlings et al., 1990) but also the qualitative differences in volatile emission between naturally damaged, artificially damaged and intact plants (De Moraes et al., 1998, Rose et al., 1996). These volatiles are increased enormously following herbivore attack (Elzen et al., 1986)
they can attract more parasitoids to attack the herbivores grazing on them (Kessler & Baldwin, 2001, van Loon et al., 2000).
The relationship of herbivore damage and parasitoid response is in concurrence with the reliability-detectability model in that parasitoids are seen to respond to cues that more closely represent the presence of a suitable host. A further refinement of host habitat detection is the ability of the parasitoid to associate the feeding of suitable
greatly increase the reliability of the cue and decrease foraging time. Indeed this has been observed (De Moraes et al., 1998) and it is believed to be achieved by the use of cues from salivary proteins (Powell & Pickett, 2003 the former suggesting aphid saliva as an elicitor; the latter demonstrating a response from caterpillar saliva, Turlings et al., 1990). For this reason response to a plant previously infested with the host aphid remains more attractive (Du et al., 1998).
It is also implied by the greater detectability and quantity of volatile emissions that the parasitoid would use them at a longer range and focus more on semiochemical
releases from the host itself during closer-range foraging; evidence for this has been seen in trials with A. ervi (Du et al., 1997).
1.5.4
Learning
To overcome the challenge of foraging for a discrete host, a parasitoid may learn cues associated with successful host location. Several modes of learning may be employed in foraging and any other challenges faced in the parasitoid life. To understand the different forms of learning, and the benefits they infer to the parasitoid, all the aspects of learning relevant to parasitoid foraging are reviewed below. Using the reliability-detectability framework also provides insights as to how various chemical cues may be learnt, and how susceptible they will be to learning. Odours which are highly reliable and subject to little variation are more likely to be innate in the parasitoid, conversely, odours which will vary with the environment may retain a more plastic response. This is demonstrated by the innate response of Aphidiine parasitoids to the aphid sex pheromone (Poppy et al., 1997) and the particular plants of their host (Storeck et al., 2000). The latter of which can be subject to some variability (as host will frequently be associated with other plants), and will be readily replaced by learning of chemical signals relevant to the existing enrivonment (Storeck et al., 2000).
1.6
Learning
Learning is defined by Dukas (2008)
information over time. More is now being realised about the rapidity with which parasitoids can learn and the prominence of learning in adapting their foraging behaviour. It was previously believed that insects were largely innate creatures and would not be capable of learning and retaining the new information. Recent studies have shown that hymenopterans are capable of complex levels of learning to entirely novel environments (Giurfa, 2003, Laska et al., 1999, Olson et al., 2003) often using
an olfactory sense considerably greater than electronic olfactometers (Rains et al., 2004).
1.6.1
Maternal influence
Prior to the emergence of the wasp it is likely it will already have acquired some information about its environment, or at least that of its mother . It has been demonstrated in Aphidius colemani that a maternal cue is passed onto the offspring during oviposition (Douloumpaka & van Emden, 2003). This cue is viewed as relatively weak as it is often overwritten by experiences during emergence or experience gained by the parasitoid as an adult.
1.6.2
Emergence conditioning
Emergence conditioning is the learning of the chemical environment that occurs in a parasitoid as it emerges from its pupal cocoon. Many parasitoid behaviours previously thought to be innate are now understood to be learnt upon emergence (van Emden et al., 2008, van Emden et al., 1996). The emergence conditioning theory suggests that the parasitoid obtains experience of the volatiles present in the environment by making contact with those that have adhered to the mummy surface. The contact - and learning - is made as the parasitoid cuts its way out of the mummy. Emergence conditioning was first conclusively shown by the removal of the lepidopteron parasitoid
Microplitis demolitor from the cocoon it had created (Hérard et al., 1988). Once
removed from the cocoon it was shown that M. demolitor had lost the host plant odour preferences which are normally exhibited. In 1996 (van Emden et al.) this was shown to be true of the aphid parasitoid Aphidius rhopalosiphi when excised from the
mummies of Metopolophium dirhodum. It has since been shown, using the same aphid parasitoid/host system, that the parasitoid does not only learn the host plant odour but is able to pick-up subtle cues about the presence of other plants within the environment (van Emden et al., 2002). Further evidence for this is that Aphidius colemani raised on Myzus persicae on an artificial diet show no preference for a host plant and will show preference to the host plant of mummy exuviae they are exposed to if removed from their own mummy case before emergence (van Emden et al., 2008). Emergence conditioning is likely to be selected for in an environment where more than one host is used by the parasitoid and/or the host feeds on more than one species of plant. The emergence of the parasitoid is evidence of the success of its mother, by following the same odours the newly emerged parasitoid is, therefore, more likely to achieve success finding a host in this environment.
1.6.3
Associative learning
Associative learning is the association of different stimuli through experience. Associative learning takes place when a parasitoid encounters a reward or aversive stimuli alongside an odour. Following this encounter, or repeats of the same
experience, the parasitoid will learn to associate that odour with reward or the aversive experience. The ability to associate odours in this fashion can be greatly
advantageous to an individual as it allows it to adapt to an unpredictable environment. It is hypothesised that individuals living in a more variable environment will have a greater ability in associative learning and a reduced reliance on innate responses (Vet & Dicke, 1992). Following this theory a generalist parasitoid will show a greater reliance on associative learning as the host present in the environment is subject to change, in
In aphid parasitoids one of the strongest rewards that can be provided is access to a viable host. It has been shown that an odour learnt following ovipositional experience can take precedence over previously learnt cues (Storeck et al., 2000). Although encountering a host may be the optimal reward for a parasitoid, related stimuli are also sufficient in eliciting a learning experience, as has been shown with aphid honeydew and exuviae (Bouchard & Cloutier, 1984, Du et al., 1997), or with the lepidopteran parasitoid Microplitis croceipes (Lewis & Tumlinson, 1988). The relative strength of the learning experience is related to the value of the reward with which it is associated (Battaglia et al., 2000), therefore, cues that are more directly related to a food or host source will be more effective in
reinforcing learnt behaviours. The associative learning effectively collates information consistently dependable cue.
It has been shown that parasitoids have an increased responsiveness to odours which are likely to occur in their environment, odours which are likely to already exist in the
(Turlings et al., 1993). This makes the learning of (Takasu & Lewis, 1993) easier than truly novel odours such as octanal (Olson et al., 2003), as the former are derived from plant sources. Associative learning is essential to parasitoid success as it allows plasticity and adaptability to conditions that may vary between generations.
1.6.4
Alpha conditioning
Alpha conditioning is the process by which the response observed in a parasitoid to a responses, those that relate to odours which vary within the environment, will be
subject to greater levels of enhancement. This is notable with odours such as plant volatiles; it is beneficial to the wasp to respond to plant volatiles innately, however, the volatiles that it responds to may not be available in a given environment. Therefore a rewarded encounter with the volatile will provide alpha conditioning to the odour and may mean this odour takes precedence over other plant volatiles. At present it is not known if alpha conditioning can operate on already strong innate cues. It has been hypothesised that those odours which offer highly reliable information regarding the host may already elicit an optimal response and will not be subject to alpha
conditioning (Glinwood et al., 1999a).
1.6.5
Success motivated retention time
Success motivated retention time is the theory that if an organism has success in the environment (finding a suitable host or food source) it will remain there for a longer encounters an odour associated strongly with a host it may extend the length of time it
- -up time was
originally described by Croze (1970), for carrion crows as the time from finding the circumstance where an organism is foraging a resource patch but is capable of leaving to search for another resource patch. In the lepidopteron parasitoid Venturia
canescens it has been demonstrated that the greater ovipositional success it has in an area the longer it will stay foraging (Bernstein & Driessen, 1996), the patch will also be revisited more frequently which is probably due to host markers which are left at the site. It is hypothesised that aphidiine parasitoids will commit more time foraging a patch if they have already had success in this area, though, in a natural environment other factors such as the possible markers of other parasitoids, health of the plant and proximity of other aphid colonies might be a factor. At present it is still not
established if the reward of energy or a host is required for the parasitoid to learn the environment is favourable or if the encounter of semiochemicals relating to the host is sufficient.
1.7
Parasitoid response to nepetalactone
In 1991 Praon species were used to show that aphid parasitoids could be attracted to the aphid sex pheromone in field trials (Hardie et al., 1991). The synthetic form of the pheromone was used in this initial study but it has since been shown in Aphidius ervi
and Praon volucre that the level of attraction did not vary significantly between synthetic and naturally extracted nepetalactone (Glinwood et al., 1999b) which is the sex pheromone component shown to elicit the greatest response (Hardie et al., 1991).
Various studies since have confirmed that aphid parasitoids are capable of detecting the pheromone components and may utilise it in foraging as a kairomone (Table
1.2). Table
1.2 Parasitoids which have exhibited a response to aphid sex pheromones (taken from Powell & Pickett, 2003). * This study came out after the original publication and has been added by the authorAphidius ervi, which had no previous encounter of aphid sex pheromone and reared on asexual aphid populations, demonstrated a positive response upon encountering the sex pheromone, confirming a genetic basis for the behavioural response (Poppy et al., 1997). Despite being an innate response, if no reward is given during learning there is evidence that the parasitoid may habituate to the pheromone (Glinwood et al., 1999a) showing the flexibility in their learning. While the adaptability of learnt response opens
arasitoid to a specific host/plant complex it also emphasises the complexity of introducing a semiochemical into the ecosystem.
Parasitoid Pest aphid hosts Evidence of response
Aphidius rhopalosiphi Cereal aphids Field experiments
Laboratory bioassays
Electrophysiology
Aphidius ervi Pea aphid
Cereal aphids
Glasshouse aphids
Field experiments
Laboratory bioassays
Electrophysiology
Aphidius eadyi Pea aphid Field experiments
Laboratory bioassays
Aphidius matricariae Glasshouse aphids Electrophysiology
Diaeretiella rapae Brassica aphids
Russian wheat aphid
Field experiments
Laboratory bioassays
Electrophysiology
Praon volucre Wide range of hosts Field experiments
Laboratory bioassays
Electrophysiology
Ephedrus plagiator Wide range of hosts Laboratory bioassays
Aphidius gifuensis Wide range of hosts Electrophysiology*