© 2008, INSInet Publication
Health Risks of Electromagnetic Radiation from Mobile Phone on Brain of Rats
Sahar M. Awad and Nahed S. Hassan
1 2
Department of Physics, Faculty of Science, Alazhar University, Cairo Egypt. 1
Department of Biochemistry, National Research Center, Cairo Egypt. 2
Abstract: Tremendous concerns have been raised about the possibility that exposure to the electromagnetic
radiation (EM R) from mobile phones could affect people’s health. This study was carried out in order to investigate the impact of exposure to the (EM R) of mobile phones. Since recent experimental studies suggest a possible link between mobile phone use and reactive oxygen species (ROS) in EM R-induced oxidative damage in tissues. In this study, rats were divided into three groups, The first group was used as control group and the other Two groups were exposed to 900 M Hz EM R from mobile phone for one week (1 h/day) and for two weeks (1 h/day). Control group was prepared by turning off the mobile phone while the animals were in the same exposure conditions (sham exposed to EM R). Subsequently, oxidative stress markers and pathological changes in brain tissue were examined for all groups. The results indicated significant increase in plasma lipid peroxide (PLPO) and malondialdehyde (M DA) levels. Also there was significant decrease in brain superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GSH-Px) activities in brain tissue. These alterations were indicative for oxidative damage and disturbance in antioxidant system. Histopathological studies revealed cellular injury in brain tissue induced by mobile phone EM R exposure. In conclusion reactive oxygen species may play a role in the mechanism that has been proposed to explain the biological side effects of mobile phone (M P) in brain tissue.
Key w ords: Electromagnetic radiation, M obile phones, Brain, Rat
INTRODUCTION
Concern continues about exposure to radiofrequency (RF) fields which are increasingly available in our daily environment, for example, mobile phone (M P) transmitters/ receivers, radars, satellites, radio/TV transmitters, video display terminals, microwave ovens and occupational devices[1 ,2 ]. A particular concern has been raised about the possibility that exposure to the radiofrequency fields emitted by M Ps could affect people’s health and recent alarming reports demand further investigations on this subjects . M Ps operate on[3 ] wireless technology, with communication typically occurring via a 900-1800 M Hz signal that is pulsed at 217 Hz. This signal carries essentially no power when the user is not talking or receiving, but when the user communicates the power of this pulsed electromagnetic field reaches a maximum of 250 mW .[4 ]
The duration period for mobile phone use and its frequency are important factors, but the exact duration differs from individual to individual. Today’s mobile telephones, with a total power output of about 2 W , are estimated to produce insignificant local heating, which is unlikely to produce any deleterious effects[5 ,6 ]. Recent research from many countries suggests, however, that
there are ‘non-thermal’ effects on living tissue, ranging from changes in the permeability of the blood-brain barrier to changes in encephalogram
and ocular symptoms[7 ,8 ].
However, mobile phone antennas give localized RF exposures predominantly to the head. Thus, it is necessary to determine the local SAR and its distribution in the head to properly evaluate health consequences. Calculation of the maximum temperature rise in the head from RF exposure during mobile telephone use suggests that increases of no more than about 0.1°C would be expected[9 ,1 0 ]. Thus if there are health effects from RF exposure, they are unlikely to be due to any temperature increase. So-called non-thermal mechanisms of RF action in tissues have been proposed .[2 ]
There are many studies in the literature about the biological interactions with EM F and the direct biological effects which such exposure could originate[1 1 -13 ]. Recent in vitro and in vivo studies observed the occurrence of DNA damage[1 4 -1 6 ],as well as micronucleus (M N) generation, which is a well-accepted index for genotoxicity evaluation, after the EM F exposure[1 7 -1 9].However, it is not clear how EM F interacts with living systems. Some authors pointed out a possible role of oxidative stress in this process, and proposed mathematical models explaining how weak
Corresponding Author: Nahed S. Hassan, Department of Biochemistry, Division of Genetic Engineering and Biotechnology,
National Research Center, Cairo Egypt.
e l e c t r o m a g n e t i c f i e l d s c o u l d i m p a ir r a d ic a l r e c o m b in a tio n , t h u s in c r e a sin g fr e e r a d ic a ls generation[20 ,2 1 ]. In agreement with this hypothesis some authors suggested that EM F might also increase free radicals formation, based on the assumption that ROS are implicated in several types of tissue injury[2 2 -26 ]. ROS are scavenged by SOD, and also the enzymes glutathione peroxidase (GSH-Px) and catalase (CAT)[2 7 ]. The aim of the current study is to investigate the possible harmful effects of exposure of brain to emitting levels of EM R from mobile phone in the rats, focusing on changes in the antioxidant enzyme activities and various oxidant parameters of the brain.
M ATERIALS AND M ETHODS
Animal model: The animals involved in this study were
maintained and used in accordance with the Animal W elfare Act and the Guide for the Care and Use of Laboratory Animals prepared by the National Research Center , Animal Ethical Committee. Twenty four M ale W istar Albino rats obtained from the Laboratory Animal Production Unit of National Research Center were used in the study, (each weighing 250-300 g and approximately 3 months old at the time of the experiment). They were housed individually in polycarbonate cages. The housing room was kept in an environment of controlled temperature (24-26 <C), humidity (55-60%), and controlled photoperiod (12h/12h of light and dark) for 1 week before the start of the experiment. A standard balanced diet and tap water were provided ad libitum.
The exposure system: consisted of a plastic tube cage
(length: 15 cm, diameter: 6.5 cm) and a mobile phone antenna. Unanesthetized male W istar rats were confined in the cages. T he heads of the rats were positioned toward the antennas of the mobile phones, and each tube was ventilated to decrease the stress of the rat while in the tube. T his exposure system was based on a previous report of Tsurita et al.[2 8 ]. In the present study, a 900 M Hz electromagnetic near-field signal for GSM (Global System for M obile communication at 900 M Hz, continuous wave, analog phone) system was used. T he peak specific absorption rate (SAR) of the brain was 2 W /kg and the average SAR of the whole body was 0.25 W /kg.
Experimental design: Animals were randomly grouped
as follows: group ² (n=8), sham-operated control group, group Ð (n=8) rats were exposed to EM R in the above referred conditions for 1 h/day for 7 days and group Ø
(n=8) rats were exposed to EM R for 1 h/day for 15 days. M obile phones were activated by calling each other. The rats of control group were also placed in the tube with the same environmental room conditions as the exposure groups but without exposure to EM R (mobile phone off).
Biochemical evaluation: To evaluate the biological
effects of the EM Rexposure from mobile phone to the brain, the antioxidant enzyme activity of the brain tissue was evaluated. Brains were removed immediately after decapitation and sectioned sagittally as right and left hemispheres. Right hemispheres were used for histopathological examination. Left hemispheres were washed twice with cold saline solution, placed into glass bottles, labeled and stored at 30 ºC until processing (maximum 10 h). Tissues were homogenized in a four volumes of ice-cold Tris - HCl buffer (50 mmol/l, pH 7.4) using a glass Teflon homogenizer (Ultra Turrax IKA T18 Basic) after cutting of the brains into small pieces with a scissors (for 2 min at 5000 rpm) M alondialdehyde (M DA) activity was carried out at this stage. The homogenate was then centrifuged at 5000 × g for 60 min to remove debris. Clear upper supernatant fluid was taken, glutathione peroxidase (GSH-Px), Glutathione Reductase (GR) and catalase (CAT) activities were carried out in this stage. The supernatant solution was extracted with an equal volume of an ethanol/ chloroform mixture (5/3, volume per volume [v/v]). After centrifugation at 5000 × g for 30 min, the clear upper layer (the ethanol phase) was taken and used in the superoxide dismutase (SOD) activity. All preparation procedures were performed at 4 ºC.
Determination of M DA: M DA levels were estimated
by the double heating method of Draper and Hadley[2 9 ]. The principle of the method is the spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid (TBA) with M DA and is expressed as nanomoles/ gram (nM g ) wet tissue. -1
Determination of SOD activity: Total (Cu-Zn and M n)
SOD activity was determined according to the method of Durak et al.[3 0]. The principle of the method is based briefly on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine/ xanthine oxidase system as a superoxide generator. Activity was expressed as units per gram (Ug ) protein.-1
Determination of GSH-Px activity:. Glutathione
peroxidase (GSH-Px) activity was measured by the method of Paglia and Valentine[3 1 ]. The enzymatic reaction in the tube that contained reduced nicotinamide adenine dinucleotide phosphate, reduced glutathione, sodium azide and glutathione reductase was initiated by
2 2
the addition of hydrogen peroxide (H O ) and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was given in units per gram (Ug ) protein.-1
Determination of CAT activity: Catalase (CAT)
activity was measured according to the method of Aebi[3 2 ]. The principle of the assay is based on the determination of the rate constant k (dimension: S , -1 k) of hydrogen peroxide decomposition. By measuring the
absorbance change per minute, the rate constant of the enzyme was determined. Activities were expressed as k
(rate constant) per gram (k g ) protein. -1
Determination of GR activity: Glutathione reductase
(GR) was assayed by the method of Stall et al.[3 3 ] and expressed as nmol g protein.-1
Determination of PLPO activity: Blood was collected
from the rats in all previous mentioned groups. Plasma was separated and kept in 80 °C until analyzed for plasma lipid peroxide activity (PLPO) using the method of Yagi[3 4 ] and expressed as nmol ml-¹s.
Histopathological examination: After removing the
brain from skull, brains weresectioned sagittally. Right hemispheres were removed and fixed with a buffered 10% formalin solution for 24 h and embedded in paraffin. Tissues were then sectioned at 5 Am, stained with hematoxylin and eosin (H&E) and examined for histopathological changes using light microscope. The occurrence of ‘‘dark neurons’’ was judged semi-quantitatively by the pathologist as A (non occasional dark neurons), B (moderate occurrence of dark neurons) and C (abundant occurrence of dark neurons).
Statistical analysis: D ata were presented as means
± SE. All analyses were made using the SPSS statistical software package. A one-way ANOVA test was applied to data to detect significant differences initially. At the second step, T ukey’s post-hoc test was used to compare the groups. Differences were considered significant at P<0.05
RESULTS AND DISCUSSION
Results: To estimate the role of reactive oxygen species
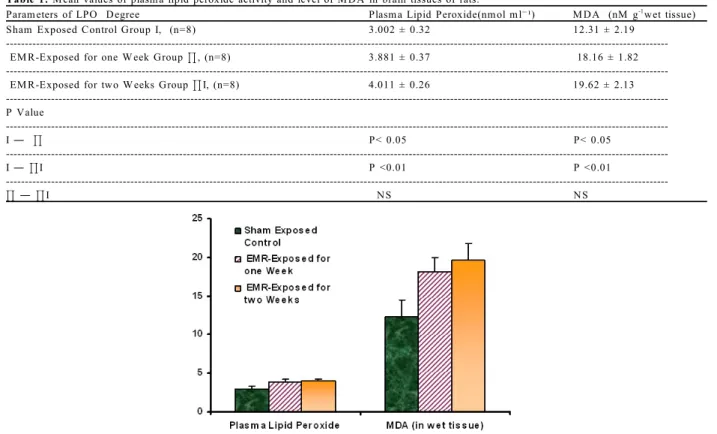
induced by exposure to EM R from mobile phone for one and two weeks, LPO degree and antioxidant status were determined in sham exposed (control) and EM R-exposed groups. Exposure to electromagnetic radiation 'EM R' from mobile phone device produced a significant increase in tissue level of M DA and plasma lipid peroxides (indicator of LPO degree) in group Ð
(EM R-exposed for one week, p <0.05) and group Ø
(EM R- exposed for two weeks, p <0.01) as compared with control sham exposed group ², (Tale 1, Figure1). W hile activities of antioxidant enzymes (indicator of antioxidant status) were found to be decreased in EM R-exposed groups when compared with control sham exposed group ² (Table 1, Figure 1).
The level of SOD activity was decreased in group
Ð (p < 0. 01) and group Ø (p < 0. 001) and values of GSH-Px activity was found to be decreased in EM R group (p < 0. 05). Also EM R exposure revealed a non significant decrease in CAT activity in group Ð and
significant decrease in group Ø (p < 0. 01). EM R induced significant decrease in GR activity in both group Ð (p < 0. 05) and group Ø (p < 0. 001) when compared to those of control
Values as shown in (Table 2, Figure 2).
The pathological examination revealed a significant positive relation between EM R exposure duration time and number of dark neurons appeared as scattered and grouped neuron cells, which were often interspersed, shrunken nerve cells and dark staining so called dark neurons.
Dark neurons were seen in all locations, but especially in the cortex, hippocampus and basal ganglia, mixed in among normal neurons (Figure3). The number of dark neurons increased as the duration time of EM R exposure increased in group Ð and Ø, on comparing with the sham exposed group ².
D iscussion: The use of mobile phones is currently one
of the fastest growing technological developments. The close proximity of the antenna of such a device to the head and consequently to the brain has raised concerns about the biological interactions between EM R and the brain tissues. The direct biological effects of exposure to 900-M Hz EM R have not been studied extensively.
The present study has shown that exposure to EM R with a frequency of 900 M Hz had a significant effect on rat brain, suggesting that RO S were generated under the experimental conditions employed. A significant increase was observed in M D A and PLPO levels in the exposed group. The change in activities of antioxidant enzymes with M DA and PLPO levels may be regarded as an indicator of increased ROS production occurring during the exposure period and may reflect the pathophysiological process of the exposure. Thus, impaired oxidant /antioxidant balance in brain might be partially responsible for the adverse effects of mobile phone use. It was reported that free radical mediated LPO is involved in EM R-induced tissue injury[3 5 ]. M oustafa et al.[3 6 ] indicated that acute exposure to EM R may modulate the oxidative stress of free radicals by enhancing lipid peroxidation and reducing the activation of SOD and GSH-Px, which are free radical scavengers. Irmak et al. have shown that EM R with frequency of[5 ] 900 M Hz has no significant effect on rabbit brain, suggesting that oxygen free radicals were not generated; but, they observed a significant increase in serum SOD activity in the exposed group.
However, our results have revealed that there is an oxidative stress-induced LPO in brain after mobile phone exposure, Likewise, M DA and PLPO levels may be an important marker showing the degree of impairment in oxidant/antioxidant balance in brain. Comparative evaluation of our study with previous studies mentioned above has revealed an agreement in results obtained.
T able 1: M ean values of plasm a lipid peroxide activity and level of M D A in brain tissues of rats.
Param eters of LPO D egree Plasm a Lipid Peroxide(nm ol m lG¹) M D A (nM g wet tissue)-1
Sham Exposed C ontrol Group I, (n=8) 3.002 ± 0.32 12.31 ± 2.19 EM R -Exposed for one W eek Group J, (n=8) 3.881 ± 0.37 18.16 ± 1.82 EM R -Exposed for two W eeks Group JI, (n=8) 4.011 ± 0.26 19.62 ± 2.13 ---P Value ---I ¯ J P< 0.05 P< 0.05 ---I ¯ JI P <0.01 P <0.01 ---J ¯ JI N S N S
Fig. 1: Levels of plasma lipid peroxide and M DA in brain tissues of rats in shame exposed control, EM R-exposed
for one week and EM R-exposed for two weeks groups. T able 2: M ean values of antioxidant enzym es activities in brain tissues of rats.
param eters of Antioxidant Status SO D (U g-1 protein) GSH -PX (U g-1 protein) C AT (K g-1 protein) GR (nM g-1 protein) Sham Exposed C ontrol
Group I, (n=8) 0.64±0.041 1.861±0.231 4.06 ±0.21 1.29±0.098 ---EM R -Exposed for one W eek
Group J, (n=8) 0.49±0.037 1.326±0.162 3.67±0.19 0.96±0.121 ---EM R -Exposed for two W eeks 0.41±0.052 1.21± 0.201 3.12±0.24 0.72±0.113 Group JI, (n=8) P Value ---I ¯ J P <0.01 P< 0.05 N S P< 0.05 ---I ¯ JI P <0.001 P< 0.05 P <0.01 P <0.001 ---J ¯ JI N S N S P< 0.05 N S
Kula et al.[3 7 ] demonstrated that The degree of oxidative damage on the biomolecular level can be determined via the assessment of antioxidant enzyme activities and the densities of mid- and end products of lipid peroxidation. M DA exists among the end products of lipid peroxidation. At the end of the reaction, M DA is produced alongside many compounds such as alkenes, 2-alkenals, and 4-hydroxyalkenals. An increase of M DA level indicates the severity of oxidative damage. In this study, M DA levels increased on days 7 and 14 on
comparison with controls, It has been reported by various researchers that exposure to EM F of 50 Hz and at different magnitudes caused lipid peroxidation in different living species[3 8 ,3 9 ].
It was documented by Albers and Beal,[4 0 ] that the mitochondrial respiratory chain is the major site for the
2 2 2 generation of superoxide radicals (O , H O ). It is -possible that EM R may affect the mitochondrial membranes to produce large amounts of oxygen radicals ROS under our experimental conditions. These
Fig. 2: Activities of antioxidant enzymes in brain tissues of rats in shame exposed control, EM R-exposed for one week and EM R-exposed for two weeks groups.
Fig. 3: Histopathological appearance of hippocampus in brain tissues of rats in (A) shame exposed control, (B)
EM R-exposed for one week and (C) EM R-exposed for two weeks groups. continuously produced ROS are scavenged by SOD,
glutathione peroxidase (GSH-Px), catalase (CAT) and glutathione reductase (GR). Under some circumstances, these endogenous antioxidative defenses are likely to be perturbed as a result of overproduction of oxygen radicals, inactivation of detoxification systems, consumption of antioxidants, and failure to adequately replenish antioxidants in tissue. It has been demonstrated in numerous studies that ROS are directly involved in oxidative damage of cellular macromolecules such as lipids, proteins, and nucleic acids in tissues[2 4 ].
Salford et al.,[41 ] first reported the evidence for neuronal damage caused by nonthermal microwave exposure. The cortex as well as the hippocampus and the basal ganglia in the brains of exposed rats contain dark neurons. Ilhan et al.,[2 2 ] demonstrated that mobile phones caused oxidative damage biochemically and produced histopathological changes in brain tissue in a rat model exposed to EM R. T hey suggested that there was significant relationship between EM R dose and number of dark neurons, which have been causally linked to the generation of ROS and oxidative stress. This was concomitant with results observed in this study (Figure 3).
Scientists have warned children for the possible hazardous effect of mobile phones, since young person are growing up. Indeed, in young individuals, cellular defenses against free radical-induced protein oxidation by antioxidant enzymes are likely in prime shape and the proteases and protein synthesis machinery are fully functional. Thus, there is little or no change in the level of protein carbonyls during the first 45 years. However, when normal individuals reach 45 years, enzymes, including proteases and antioxidant proteins, and smaller antioxidant molecules in the individual become progressively inactivated due to the failure of the antioxidant systems to overcome the constant influx of ROS. Consequently, the accumulation of free radical-induced carbonylated proteins accelerates, indicating the age when cells in the individual become increasingly more susceptible to ROS-mediated damage[4 2 ]. Since cumulated oxidative stress in brain cells can lead to neurodegenerative diseases and an excess of free radicals in cells has been suggested to be the cause of vario us hum an d iseases (P arkinso n’s disease, Alzheimer’s disease and amyotrophic lateral sclerosis, etc.). On the basis of Ilhan et al., findings[2 2 ] elder people must be more cautious for EM R-induced
oxidative damage. A lthough p resent scientific information does not indicate the need for any special precautions for use of mobile phones, if individuals are concerned, they might choose to limit their own EM R exposure by limiting the length of calls, or using ‘‘hands-free’’ devices to keep mobile phones away from the head and body.
REFERENCES
1. Nakamura, H., I. M atsuzaki, K. Hatta, Y. Nobukuni, Y. Kambayashi and K. Ogino, 2003. Nonthermal effects of mobile-phone frequency microwaves on utero p lacental functio ns in p regnant rats. Reproductive Toxicology, 17: 321-6.
2. Repacholi, M .H., 2001. Health risks from the use of mobile phones. Toxicology Letters., 120: 323-31. 3. M aes, A., M . Collier, L. Verschaeve, 2001. Cytogenetic effects of 900 MHz (GSM ) microwaves on human lymphocytes. B ioelectromagnetics, 22: 91- 6.
4. Croft, R.J., J.S. Chandler, A.P. Burgess, R.J. Barry, J.D. W illiams, A.R. Clark, 2002. Acute mobile phone operation affects neural function in humans. Clinical Neurophysiology, 113: 1623-32.
5. Irmak, M .K., E. Fadillioglu, M .Gulec, H . Erdogan, M . Yagmurca, O. Akyol, 2002. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem Funct., 20: 279-283.
6. Irmak, M .K., E. O ztas, M . Yagmurca, E. Fadillioglu, B. Baki, 2003. Effects of electromagnetic radiation from a cellular telephone on epidermal M erkel cells. J. Cutan Pathol, 30: 135-138.
7. Curcio, G ., M . Ferrara, F. M oroni, G.D. Inzeo, M . Bertini, L. De Gennaro, 2005. Is the brain influenced by a phone call? An EEG study of resting wakefulness. Neuroscience Research, 53: 265-270
8. Balik, H.H., D.T. Balik, K. Balikci, I.C. Özcan, 2005. Some ocular symptoms and sensations experienced by long term users of mobile phones. Pathologie Biologie., 53: 88-91.
9. Van Leeuwen, G.M .J., J.J.W . Lagendijk, B.J.A.M . Van Leersum, A.P.M . Zwamborn, S.N. Hornsleuth, A.N.T. Kotte, 1999. Calculation of brain temperature due to exposure to a mobile phone. Phys. M ed. Biol., 44: 2367.
10. Tahvanainen, K., J. Nino, P. Halonen, T. Kuusela, T. Alanko, T. Laitinen, E. Lansimies, M . Hietanen, H. lindholm, 2007. Effects of cellular phone use on ear canal temperature measured by N T C thermistors. Clin Physiol Funt Imaging, 27: 162-72.
11. M ild, K.H., L. Hardell, M . Kundi, M . M attsson, 2003. M obile telephones and cancer: is there really no evidence of an association? (Review). International J. M olecular M edicine, 12: 67-72. 12. Lönn, S., A. Ahlbom, P. Hall, M . Feychting,
2004. M obile phone use and the risk of acoustic neuroma. Epidemiology, 15: 653-659.
13. Crumpton, M .J. and A.R. Collins, 2004. Are environmental electromagnetic fields genotoxic? DNA Repair, 3: 1385-1387.
14. Zmyœlony, M ., J. Palus, J. Jajte, E. Dziubaltowska, E. Rajkowska, 2000. DNA damage in rat lymphocytes treated in vitro with iron cations and exposed to 7 mT magnetic fields (static or 50 Hz). M utation Research, 453: 89-96.
15. Lai, H. and N.P. Singh, 2004. M agnetic-field-induced DNA strand breaks in brain cells of the rat. Environmental Health Perspectives, 112: 687-694. 16. W olf, F.I., A.Torsello, B. Tedesco, S. Fasanella,
A. Boninsegna, M . D 'Ascenzo, 2005. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: possible involvement of a redox mechanism. Biochimica et Biophysica Acta., 1743: 120-129. 17. Tice, R.R., G.G. Hook, M . Donner, D .I. M cRee,
A.W . Guy, 2002. G enotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics, 23: 113-126.
18. Cho, Y.H.and H.W . Chung, 2003. The effect of extremely low frequency electromagnetic fields (ELF-EM F) on the frequency of micronuclei and sister chromatid exchange in human lymphocytes induced by benzo(a)pyrene. Toxicology Letters, 143: 37-44.
19. Trosic, I., I. Busljeta, B. M odlic, 2004. Investigation of the genotoxic effect of microwave irradiation in rat bone marrow cells: in vivo exposure. M utagenesis, 19: 361-364.
20. Grissom, C.B., 1995. M agnetic field effects in biology: a survey of possible mechanisms with emphasis on radical-pair recombination. Chemical Review, 95: 3-24.
21. T immel, C.R., F. Cintolesi, B. Brocklehurst, P.J. Hore, 2001. M odel calculations of magnetic fields effects on the recombination reactions of radicals with anisotropic hyperfine interactions. Chemical Physics Letters, 334: 387-395.
22. Ilhan, A., A.Gurel, F. Armutcu, S. Kamisli, M . Iraz, O. Akyol, S. Ozen, 2004. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clinica Chimica Acta., 340: 153-162.
23. Zmyœlony, M ., P. Politanski, E. Rajkowska, W . Szymczak, J. Jajte, 2004. Acute exposure to 930 M Hz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics. 25: 324-328.
24. Oktem, F., F. Ozguner, H. M ollaoglu, A. Koyu, E. Uz, 2005. Oxidative damage in the kidney induced by 900-M Hz-emitted mobile phone: protection by melatonin. Archives of M edical Research, 36: 350-355.
25. Regoli, F., S. Gorbi, N. M achella, S. Tedesco, M . Benedetti, R. Bocchetti, A. Notti, D. Fattorini, F. Piva, G. Principato, 2005. Pro-oxidant effects of extremely low frequency electromagnetic fields in the land snail Helix aspersa. Free Radical Biology & M edicine, 39: 1620-1628.
26. Yokus, B., D.U. Cakir, M .Z. Akdag, C. Sert, N. M ete, 2005. Oxidative DNA damage in rats exposed to extremely low frequency electro magnetic fields. Free Radical Res., 39: 317-323. 27. Halliwell, B. and J.M .C. Gutteridge, 1999. Free
Radicals in Biology and M edicine, 3rd ed. Oxford University Press, New York.
28. Tsurita G,H. Nagawa, S. Ueno, S. W atanabe, M . Taki, 2000. Biological and morphological effects on the brain after exposure of ats to a 1439 M Hz TDM A field. Bioelectromagnetics, 21: 364-71. 29. D r a p e r , H .H . a n d M . H a d le y, 1 9 9 0 .
M alondialdehyde determination as index of lipid peroxidation. M ethods Enzymol., 186: 421-431. 30. Durak I., Z. Yurtarslani, O. Canbolat, O. Akyol,
1993. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta., 214: 103-104.
31. Paglia, D.E. and W .N. Valentine, 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. M ed., 70: 158-169.
32. Aebi, H., 1974. Catalase. In: Bergmeyer HU, ed. M ethods of Enzymatic Analysis. New Y ork and London: Academic Press. pp. 673-677.
33. Stall, G.E.J., J.C. Van Berkel, J. F. Koster, and J.K. K ruyta, 1973. On the molecular basis pyruvate kinase deficiency : I. Primary defect or consequence of increased glutathione disulfide concentration Biochimica et Biophysica Acta (BBA), 321: 496-502 .
34. Yagi, K., 1984. Lipid peroxidation, Assay for blood plasma or serum. M ethods in Enzymology, 105: 328-331.
35. Stopczyk, D., W . Gnitecki, A. Buczynski, L. M arkuszewski, J. Buczynski, 2002. Effect of electromagnetic field produced by mobile phones on the activity of superoxide dismutase (SOD-1) and the level of malonyldialdehyde (M DA)-in vitro study. M ed Pr., 53: 311-4.
36. M oustafa, Y.M ., R.M . M oustafa, A. Belacy, S.H. Abou-El-Ela, F.M . Ali, 2001. Effects of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. J. Pharm. Biomed Anal., 26: 605-8.
37. Kula, B., A. Sobczak, R. Kuska, 2000. Effects of static and ELF magnetic fields on free-radical processes in rat liver and kidney. Electro M agnetobiol., 19: 99-105.
38. Boland, A., D. Delapierre, D. M ossay, A. Dresse, V. Seutin, 2002. Effect of intermittent and continuous exposure to electromagnetic fields on cultured hippocampal cells. Bioelectromagnetics., 23: 97-105.
39. Eraslana, G., A. Bilgilib, M . Akdoganc, E. Yarsanb, D. Essizd, L. Altintasb, 2007. Studies on antioxidant enzymes in mice exposed to pulsed electro m a g n e tic fie ld s . E co to xico lo gy and Environmental Safety, 66: 287-289
40. Albers, D.S. and M .F. Beal, 2000. M itochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural. Transm., Suppl., 59: 133-54.
41. Salford, L.G., A.E. Brun, J.L. Eberhardt, L. M almgren, B.R.R. Persson, 2003. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ. Health Perspect, 7: 881-3.
42. M oskovitz, J., M .B. Yim, P.B. Chock, 2002. Free radicals and disease. Arch. Biochem .B iophys., 397: 354-9.