A Descriptive Survey of Pediatric Human Immunodeficiency
Virus–infected Long-term Survivors
Karin Nielsen, MD, MPH*; George McSherry, MDi; Ann Petru, MD**; Toni Frederick, PhD§; Diane Wara, MD‡‡; Yvonne Bryson, MD*; Natasha Martin, FMIBS‡; Cecelia Hutto, MD#;
Arthur J. Ammann, MD‡; Samuel Grubman, MD¶; James Oleske, MD, MPHi; and Gwendolyn B. Scott, MD#
ABSTRACT. Objective. To identify the population of human immunodeficiency virus–infected pediatric long-term survivors (LTS) followed in major medical institu-tions in California, Florida and New Jersey.
Methods. A cross-sectional survey was performed with data collection forms sent to all investigators. De-mographic, clinical, and laboratory data were obtained on all living patients 8 years infected in the perinatal period with human immunodeficiency virus.
Results. A total of 143 perinatally infected and 54 children infected by neonatal transfusion were identi-fied. Fifty-four children (27%) had absolute CD4 counts 500 cells/mm3(group 1: mean age 9.8 years), 54 children
(27%) had CD4 counts between 200 and 500 cells/mm3
(group 2: mean age 10.1 years), and 89 children (45%) had CD4 counts <200 cells/mm3 (group 3: mean age 10.4
years). Ninety-five (48%) patients had developed AIDS defining conditions; 14 (26%) in group 1, 26 (48%) in group 2, and 55 (62%) in group 3. Ninety-two percent of patients had received antiretrovirals. Perinatally human immunodeficiency virus-infected children tended to be younger (mean age 9.8 years) than children infected via a blood transfusion (mean age 11 years). Generalized lymphadenopathy was the most prevalent clinical find-ing. Lymphoid interstitial pneumonia and recurrent bac-terial infections were the most prevalent acquired im-mune deficiency syndrome-defining conditions. Twenty percent of LTS had CD4 counts 500 cells/mm3and no
immune deficiency syndrome-defining conditions. Conclusions. Pediatric LTS were in variable stages of disease progression. The proportion of children within each CD4 strata did not differ by mode of acquisition of infection. Increased CD4 counts were inversely propor-tional to age. Only 20% of pediatric LTS had minimal to no disease progression.Pediatrics 1997;99(4). URL: http: //www.pediatrics.org/cgi/content/full/99/4/e4; HIV, pedi-atric long-term survivors, slow disease progression.
ABBREVIATIONS. HIV-1, human immunodeficiency virus type 1; AIDS, acquired immunodeficiency syndrome; LTNS, long-term nonprogressive survivors; LTPS, long-term progressive survivors; LIP, lymphoid interstitial pneumonitis.
Human immunodeficiency virus type 1 (HIV-1) disease in the pediatric population progresses more rapidly than in adults and has a bimodal distribution of disease presentation.1,2The group of children who develop early and severe disease, frequently with the development of acquired immunodeficiency syn-drome (AIDS)– defining illnesses and with a higher likelihood of death in the first years of life, have been identified as rapid progressors.2There is, however, a group of children who have survived for many years after the diagnosis of HIV infection. In this group, two populations have emerged: the long-term non-progressive survivors (LTNS), who have remained asymptomatic or only mildly symptomatic over a period of years, and those who have survived de-spite clinical and laboratory evidence of disease progression, the long-term progressive survivors (LTPS). The availability of antiretroviral therapy and early medical intervention with prophylaxis and treatment of infections has altered to some degree the natural history of HIV infection and increased survival time of infected patients.3Long-term survi-vors provide a unique opportunity to investigate the immunologic, virologic, and genetic characteristics of HIV-infected children to determine what factors may slow progression to disease and death.
In October 1993, the Pediatric AIDS Foundation sponsored a workshop on HIV-infected pediatric long-term survivors with the participation of inves-tigators from seven research institutions. The work-shop focused on a discussion of virological, immu-nological, and genetic factors that potentially could contribute to long-term survival. As a result of this workshop, investigators representing five large uni-versity affiliated medical centers nationwide volun-teered to cooperate in a collaborative study of their patient populations. We summarize the results of an initial survey conducted at these sites of children with perinatal HIV infection or with transfusion-associated HIV-acquired infection describing their demographics, clinical findings, immunological sta-tus, and antiretroviral therapy.
From the *Department of Pediatrics, UCLA School of Medicine, Los Ange-les, California; ‡Pediatric AIDS Foundation, Novato, California; §Pediatric AIDS Surveillance Study, Los Angeles Pediatric AIDS Consortium, Los Angeles County Dept of Health Services, Los Angeles, California;i Depart-ment of Pediatrics, UMD–New Jersey Medical School and Children’s Hos-pital of New Jersey, Newark, New Jersey; ¶Department of Pediatrics, Saint Vincents Hospital and Medical Center of New York, New York; #Depart-ment of Pediatrics, University of Miami School of Medicine, Miami, Florida; **Children’s Hospital Oakland, Oakland, California; and ‡‡Department of Pediatrics, University of California, San Francisco School of Medicine, San Francisco, California.
Received for publication Apr 24, 1996; accepted Jul 15, 1996.
Reprint requests to (G.B.S.) University of Miami School of Medicine, De-partment of Pediatrics D4-4, PO Box 016960, Miami, FL 33101.
METHODS
A survey was initiated with patients recruited from the New Jersey College of Medicine and Dentistry, the University of Cali-fornia San Francisco, Children’s Hospital Oakland, the Los Ange-les Pediatric AIDS Consortium, and the University of Miami. Workshop participants conservatively defined pediatric long-term survivors as HIV-infected children who were at least 8 years regardless of absolute CD4 count. The decision to use this age range as a cutoff was based on the need to clearly define and separate slow from rapid progressors within a minimal margin of ambiguity. Information was obtained on all living children 8 years of age or older who were known to have been vertically infected with HIV or who had acquired their infection through transfusion of blood products shortly after birth, being currently followed at these institutions. By means of standardized forms, specific data were extracted from patient medical charts by investigators at any one of the five sites. In addition, perinatally HIV-infected children and children infected via transfusion of blood products within the first month of life who qualified as long-term survivors were compared in relation to their clinical and laboratory findings at the time of the survey.
Design
This was a cross-sectional survey in which data collection stan-dardized forms were sent to the investigators at each one of the five sites during the months of January and February of 1994. Data for the Los Angeles Pediatric AIDS Consortium was provided by the Pediatric AIDS Surveillance Study in Los Angeles County. Demographic and detailed prospective clinical information were obtained including race/ethnicity, the presence ever of specific clinical symptoms, presence of any AIDS-defining conditions ever, use of antiretroviral and prophylactic therapies, as well as specific laboratory studies.
Study Population
Children 8 years or older who acquired HIV vertically or through transfusion of blood products in the first month of life as documented by HIV culture, DNA polymerase chain reaction or enzyme linked immunosorbent assay confirmed by Western blot (after eighteen months of age). The children followed were not necessarily followed from birth. Entrance into the medical system could have occurred at an older age.
Laboratory Evaluation
All laboratory assays were performed routinely during regu-larly scheduled patient visits as warranted by their clinical condi-tion. HIV-1 antibody enzyme linked immunosorbent assay, HIV-1 antibody Western blots, and HIV-1 p24 antigen antibody enzyme linked immunosorbent assay were performed using commercially available assays. HIV-1 DNA polymerase chain reaction and
HIV-1 peripheral blood lymphocyte cocultures were conducted in accordance with standard procedures.4
Statistical Analysis
Thex2test was used to compare the distribution of children
with perinatal and transfusion-associated infections who had an AIDS-defining condition, the number of children within each CD4 strata, the age distribution of perinatally infected children and children infected by neonatal transfusion, and the age distribution within each CD4 strata. Student’s t test and analysis of variance were used to compare mean absolute CD4 counts. Statistical sig-nificance was evaluated at a .05 level.
RESULTS
A total of 143 perinatally HIV-infected children 8 years or older were identified with the following geographic distribution: Newark, NJ: 62 (43%), Mi-ami, FL: 43 (30%), Los Angeles, CA: 22 (15%), Oak-land and San Francisco, CA: 16 (11%). Fifty-four children who acquired HIV infection through a blood transfusion within the first month of life were identified with 40 (74%) being from Los Angeles County and 14 (26%) from Oakland and San Fran-cisco.
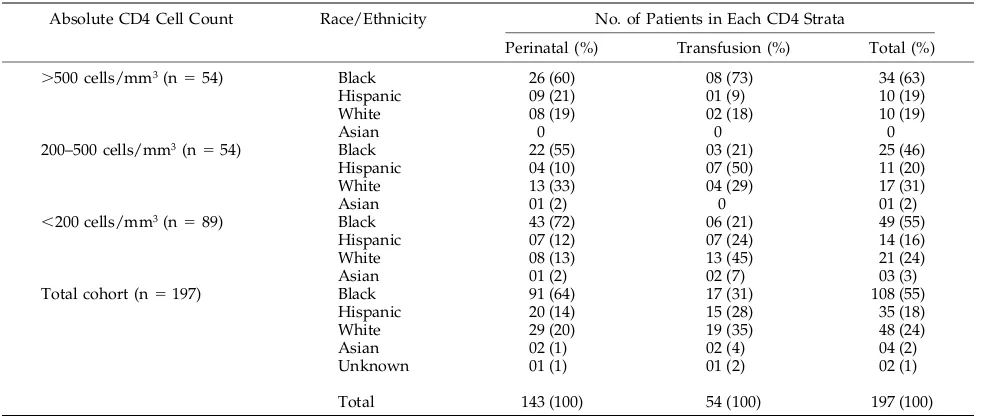
The racial distribution among perinatally HIV-in-fected children and those children with transfusion associated infection differed as seen in Table 1. The majority of perinatally infected children were pre-dominantly black, followed by relatively equal pro-portions of white and Hispanic children. The propor-tion of black patients predominated in all absolute CD4 count groups. Children infected by transfusion had a relatively uniform racial distribution overall although there were small differences in the propor-tion of children within each CD4 strata. Asians were a minority in both groups.
As seen in Table 2, of 197 children enrolled in the survey, 54 (27%) had absolute CD4 counts $500 cells/mm3(group 1), 54 (27%) had between 200 and 500 cells/mm3 (group 2), and 89 (35%) had CD4 counts ,200 cells/mm3 (group 3). Ninety-five chil-dren (48%) had had AIDS-defining conditions (whether one or multiple episodes throughout the course of their disease) according to Centers for
Dis-TABLE 1. Racial Distribution of Long-term Survivors by Mode of Infection Within Each CD4 Strata
Absolute CD4 Cell Count Race/Ethnicity No. of Patients in Each CD4 Strata
Perinatal (%) Transfusion (%) Total (%)
.500 cells/mm3(n554) Black 26 (60) 08 (73) 34 (63)
Hispanic 09 (21) 01 (9) 10 (19)
White 08 (19) 02 (18) 10 (19)
Asian 0 0 0
200–500 cells/mm3(n554) Black 22 (55) 03 (21) 25 (46)
Hispanic 04 (10) 07 (50) 11 (20)
White 13 (33) 04 (29) 17 (31)
Asian 01 (2) 0 01 (2)
,200 cells/mm3(n589) Black 43 (72) 06 (21) 49 (55)
Hispanic 07 (12) 07 (24) 14 (16)
White 08 (13) 13 (45) 21 (24)
Asian 01 (2) 02 (7) 03 (3)
Total cohort (n5197) Black 91 (64) 17 (31) 108 (55)
Hispanic 20 (14) 15 (28) 35 (18)
White 29 (20) 19 (35) 48 (24)
Asian 02 (1) 02 (4) 04 (2)
Unknown 01 (1) 01 (2) 02 (1)
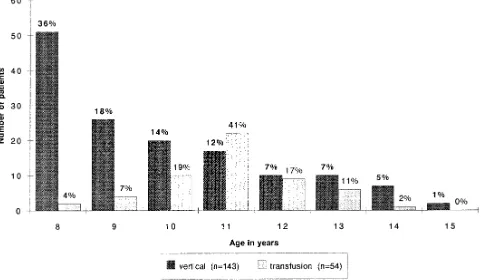
ease Control and Prevention guidelines. Of those, 72 were perinatally infected and 23 were infected via blood products. There were no differences in the proportion of children with AIDS (50% in perinatal group vs 43% in transfusion group, P..05), between mean absolute CD4 counts (379 cells/mm3 vs 269 cells/mm3, P..05) and in the proportion of children within each CD4 strata (30% vs 20% in group 1, 28% vs 26% in group 2, and 42% vs 54% in group 3, P. .05) by mode of acquisition of infection. There was, however, a difference in age distribution between the two groups as seen in Fig 1. Perinatally infected children had a skewed distribution toward the younger years although children infected by blood transfusion had an age distribution clustering around 11 years (P , .001). CD4 counts tended to decrease with increasing age ($500 cells/mm3: mean age 9.8 years, 200 to 500 cells/mm3: mean age 10.1 years,#200 cells/mm3: mean age 10.4 years, P5.03). Most children in the younger age distribution were perinatally HIV-infected.
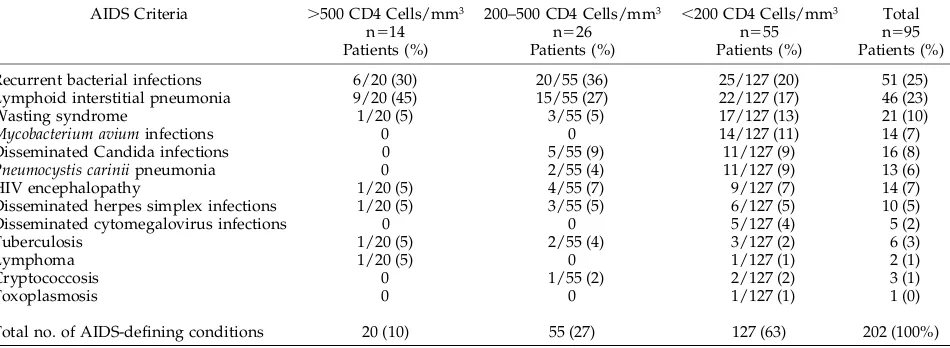
As seen in Table 3, among the 95 long-term
survi-vors who had developed AIDS-defining conditions, 14 (15%) had absolute CD4 counts$500 cells/mm3 26 (27%) had values between 200 and 500 cells/mm3, and 55 (58%) had,200 cells/mm3(26% of group 1, 48% of group 2, and 62% of group 3 children). Among children with CD4 counts $500 cells/mm3, lymphoid interstitial pneumonitis was the most com-mon AIDS-defining condition followed by recurrent bacterial infections. Other AIDS-defining conditions present in very small numbers throughout all ranges of CD4 counts were HIV encephalopathy, dissemi-nated herpes simplex infection, lymphoma, and tu-berculosis. Children with intermediate and lower CD4 counts had a higher prevalence of frequent re-current bacterial infections followed by lymphoid interstitial pneumonitis. Only children in the lower CD4 count range developed disseminated cytomeg-alovirus infection, Mycobacterium avium intracellulare infections and toxoplasmosis. Therefore, among chil-dren in the cohort who had had an AIDS-defining condition, lymphoid interstitial pneumonitis (LIP) was the most common finding in the $500 cells/
TABLE 2. Pediatric Cohort of Long-term Survivors
Absolute CD4 Cell Count
Mode of Transmission
No. Patients (%)
Mean Age (yr)
Mean CD4 (Cells/mm3)
No. Patients w/AIDS in Each CD4 Strata (%)
No. Patients Using Antiretrovirals* (%)
.500 cells/mm3 Perinatal: 43 (30) 9.5 858 12/43 (28) 39 (91)
Transfusion: 11 (20) 11.1 751 02/11 (18) 08 (73)
200–500 cells/mm3 Perinatal: 40 (28) 9.7 356 21/40 53) 35 (88)
Transfusion: 14 (26) 11.2 368 05/14 (36) 13 (93)
,200 cells/mm3 Perinatal: 60 (42) 10.2 51 39/60 (65) 59 (98)
Transfusion: 29 (54) 10.9 38 16/29 (55) 25 (86)
Total perinatal 143 (73) 9.8 379 72/143 (50) 133 (93)
Total transfusion 54 (27) 11.0 269 23/54 (43) 46 (85)
* Current or previous use of one or more of the following antiretrovirals: zidovudine, didanosine, and zalcitabine.
mm3 strata with recurrent bacterial infections being the most common cause of AIDS in the other CD4 groups. There were no differences in the prevalence of AIDS-defining conditions between perinatally in-fected children and children inin-fected by transfusion of blood products (data not shown).
Because children with a history of lymphoid inter-estitial pneumonitis are known to have a better prog-nosis than those with other AIDS-defining condi-tions, the prevalence of an AIDS diagnosis within each cohort was evaluated excluding patients with only LIP as their AIDS-defining condition (as seen in Table 4). The proportion of children with AIDS de-creased from 26% to 19% in the .500 CD4 group, from 48% to 44% in the 200 to 500 CD4 cohort and from 62% to 56% in the,200 CD4 group. Therefore, by excluding children who had LIP as their only AIDS-defining condition from the AIDS cohort the number of children with significant clinical immu-nosuppression within each CD4 group did not change substantially. However, few children had only LIP as an AIDS-defining condition. When pa-tients were further stratified according to presence or absence of LIP and absolute CD4 count range as seen in Table 5, children with lower absolute CD4 counts and a positive history for this disease had a much lower prevalence of other AIDS-defining conditions (29%) than the group of patients who had never had LIP (71%) but also had very low CD4 counts. This finding, however, was not observed within the
co-horts of children with higher and intermediate abso-lute CD4 counts; among these patients the propor-tion of other AIDS-defining condipropor-tions was equally distributed regardless of history of LIP.
Lymphadenopathy was the most common clinical finding in all children (n5160/197), and within each strata of CD4 count (present in 71% to 86% of pa-tients in each category). Hepatomegaly was the sec-ond most frequent finding (n 5 117) overall. Oral thrush and recurrent episodes of otitis media were the third most frequent clinical finding (n 5 86). Splenomegaly and herpes zoster infection were present in 44% of patients; HIV dermatitis was present in 25%, parotid enlargement in 21%, throm-bocytopenia in 17%, intermittent diarrhea in 16%, HIV cardiomyopathy in 10%, and HIV nephropathy in 5%. The prevalence of clinical findings overall increased as CD4 counts decreased with the excep-tion of generalized lymphadenopathy, recurrent oti-tis media, parotid enlargement, and HIV nephropa-thy that were found less often in the lower CD4 strata than in the intermediate or higher CD4 count range. There were no differences noted by mode of acqui-sition of infection.
Overall, 179/197 children (92%) had received an-tiretroviral therapy (zidovudine, didanosine, and/or zalcitabine). As seen in Table 2, the use of antiretro-viral therapy was highly prevalent in this cohort of children regardless of CD4 count or the presence of an AIDS-defining condition.
When subjects were categorized by presence of an AIDS-defining condition and CD4 count, as shown in Fig 2, the use of antiretrovirals, oral candidiasis, recurrent diarrhea, HIV cardiomyopathy, hepato-megaly, herpes zoster infections, HIV nephropathy, parotid enlargement, splenomegaly, and recurrent otitis media were findings more prevalent among children who had had AIDS-defining conditions. Lymphadenopathy and thrombocytopenia had an equal prevalence in both AIDS and non-AIDS groups although HIV dermatitis was a more frequent find-ing among children without AIDS although in both groups the prevalence increased as CD4 counts de-creased.
Forty long-term survivors (20%) who had never
TABLE 3. AIDS-defining Conditions Among Pediatric Long-term Survivors
AIDS Criteria .500 CD4 Cells/mm3
n514 Patients (%)
200–500 CD4 Cells/mm3
n526 Patients (%)
,200 CD4 Cells/mm3
n555 Patients (%)
Total n595 Patients (%)
Recurrent bacterial infections 6/20 (30) 20/55 (36) 25/127 (20) 51 (25)
Lymphoid interstitial pneumonia 9/20 (45) 15/55 (27) 22/127 (17) 46 (23)
Wasting syndrome 1/20 (5) 3/55 (5) 17/127 (13) 21 (10)
Mycobacterium avium infections 0 0 14/127 (11) 14 (7)
Disseminated Candida infections 0 5/55 (9) 11/127 (9) 16 (8)
Pneumocystis carinii pneumonia 0 2/55 (4) 11/127 (9) 13 (6)
HIV encephalopathy 1/20 (5) 4/55 (7) 9/127 (7) 14 (7)
Disseminated herpes simplex infections 1/20 (5) 3/55 (5) 6/127 (5) 10 (5)
Disseminated cytomegalovirus infections 0 0 5/127 (4) 5 (2)
Tuberculosis 1/20 (5) 2/55 (4) 3/127 (2) 6 (3)
Lymphoma 1/20 (5) 0 1/127 (1) 2 (1)
Cryptococcosis 0 1/55 (2) 2/127 (2) 3 (1)
Toxoplasmosis 0 0 1/127 (1) 1 (0)
Total no. of AIDS-defining conditions 20 (10) 55 (27) 127 (63) 202 (100%)
TABLE 4. Proportion of Patients With AIDS-defining Condi-tions
Absolute CD4 Cell Count
All Patients
Excluding Patients With LIP Only*
.500 cells/mm3 14/54 10/54
26% 19%
200 to 500 cells/mm3 26/54 24/54
48% 44%
,200 cells/mm3 55/89 50/89
62% 56%
Total 95/197 84/197
48% 43%
had an AIDS-defining condition and had an absolute CD4 count$500 cells/mm3were identified as LTNS. This group was comprised of 31 perinatally infected children and 9 children infected by neonatal transfu-sion. Their mean age was 10.0 years (range 8 to 14 years), mean absolute CD4 count was 823 cells/mm3 (range 504 to 3030 cells/mm3); 26 children (65%) were black, 8 (20%) were white, and 6 (15%) were Hispanic. Eighty-two percent of these children had received antiretroviral therapy, and as with the other children in the study, lymphadenopathy was present in a large number of patients (85%). Hepatomegaly was present in 37% of patients, splenomegaly in 25%, 35% had had recurrent episodes of otitis media, 20% had had herpes zoster infections and/or oral thrush, 15% had had thrombocytopenia, with diarrhea and HIV cardiomyopathy present in 10% and 7% of pa-tients, respectively. In the LTNS children, the find-ings of parotid enlargement and HIV dermatitis were found more frequently than in the LTPS children (25% vs 21% and 22% vs 7%, respectively). These differences, however, were not statistically signifi-cant (P..05).
DISCUSSION
A survey of HIV-infected children with long-term survival was conducted to better define the popula-tion of children who live for prolonged periods of time with HIV infection. The population surveyed had significant clinical findings of immunodeficiency with 50% of vertically infected children and 43% of children infected by a blood transfusion within the first month of life having had an AIDS-defining con-dition by 8 years or older at their last contact date. The majority of children (92%) regardless of the mode of acquisition of infection, had received anti-retroviral therapy. Many of the patients who had not had an AIDS-defining condition had had symptoms (ie, at least 70% of all patients had experienced lymphadenopathy) although clinical findings varied with CD4 strata as expected. A considerable propor-tion of patients (42% in the perinatal group and 54%
in the transfusion group) had absolute CD4 counts less than 200 cells/mm3.
The age distribution of long-term survivors was skewed toward the younger age group (8 years) among perinatally HIV-infected children which is in accordance with reports on the natural history of HIV infection1,5,6and also the timing of the pediatric epidemic (historically HIV acquired via blood trans-fusion occurred earlier in the AIDS epidemic whereas the perinatally acquired HIV epidemic peaked years later). The cohort surveyed only in-cluded living children and not children who might have died before or after 8 years because this survey was conducted in anticipation of future long-term survivor studies. Children infected by transfusion of blood products who were long-term survivors tended to be 10 years or older primarily due to the mode of acquisition of infection that became unlikely after 1985.7 Because there were fewer children in-fected via transfusion of blood products after this year, older children were more prevalent within this cohort. The mean age of long-term survivors tended to increase although moving from a higher CD4 count to a lower CD4 count category (9.8 years vs 10.1 years vs 10.4 years). These findings are similar to those reported by the Italian Register in which 21% of perinatally HIV infected children 5 years or older had less than 200 CD4 cells/mm3at 61 to 72 months with the proportion rising to 41% at more than 96 months.8Grubman et al9also observed that 50% of children older than 9 years of age had CD4 counts ,200 with 57% having an AIDS-defining condition. The population of long-term survivors did not seem to differ significantly when categorized by mode of infection (perinatal vs transfusion). Racial distribution was an exception, with patients of Afri-can descent constituting the majority (64%) of verti-cally HIV-infected children and one-third of the chil-dren infected via transfusion of blood products. In both groups a similar proportion of patients had developed AIDS. In addition, when patients were categorized by CD4 strata, there were similar
pro-TABLE 5. Prevalence of Other AIDS-defining Conditions Among Long-term Survivors Stratified According to History of Lymphoid Interstitial Pneumonitis (LIP)
AIDS-defining Conditions .500 CD4 cells/mm3 200–500 CD4 cells/mm3 ,200 CD4 cells/mm3
LIP (n59 Patients)
No LIP (n55 Patients)
LIP (n515 Patients)
No LIP (n511 Patients)
LIP (n522 Patients)
No LIP (n533 Patients)
Recurrent bacterial infections 4 2 12 8 10 15
Wasting syndrome 0 1 3 0 6 11
Mycobacterium avium infections 0 0 0 0 3 11
Disseminated Candida infections 0 0 2 3 2 9
Pneumocystis carinii pneumonia 0 0 0 2 2 9
HIV encephalopathy 0 1 3 1 3 6
Disseminated herpes simplex infections
0 1 1 2 1 5
Disseminated cytomegalovirus infections
0 0 0 0 1 4
Tuberculosis 1 0 2 0 0 3
Lymphoma 0 1 0 0 1 0
Cryptococcosis 0 0 1 0 1 1
Toxoplasmosis 0 0 0 0 0 1
Total no. of non-LIP AIDS-defining conditions
portions of patients within each group. There were also no differences in clinical findings between peri-natally infected children and those infected via blood products. The age distribution between groups dif-fered and this finding was statistically significant (P, .001). The age of long-term survivors infected via blood products, however, was affected by the implementation of HIV blood screening programs as mentioned.
The study design precludes any comparisons re-garding the length of survival time after initial expo-sure to HIV among vertically infected children and neonatal blood transfusion recipients. Recent studies
have indicated that the median symptom-free sur-vival time from birth to symptomatic infection may be longer for neonatal transfusion acquired HIV than for perinatally infected.10Jones et al7also estimated a longer incubation period from time of infection to diagnosis of AIDS among pediatric transfusion ac-quired cases when compared to perinatally acac-quired cases using a nonparametric procedure for truncated data. In adults, studies have attempted to find an association between the route of exposure to HIV-1 and clinical prognosis11with the rate of progression to AIDS among transfusion recipients being report-edly associated with the disease status of the donors at the time.12In perinatally infected infants, the rate of maternal transmission and disease progression appears to correlate with maternal virus burden as well as the timing of infection.13–15In this study, both groups of HIV-infected children who survived until at least 8 years of age had relatively similar clinical profiles.
Overall, the majority of AIDS-defining conditions within the cohort were either recurrent bacterial in-fections (n 551) and/or lymphoid interstital pneu-monitis (n 5 46). The prevalence of AIDS defining conditions traditionally associated with a poor prog-nosis such as Pneumocystis carinii pneumonia (n 5 13) and disseminated atypical mycobacterial (n514) infections was very low with the majority of cases occurring in the group with lower CD4 counts. Among the group of children with higher CD4 counts, the majority of AIDS diagnosis was attribut-able to lymphoid interstital pneumonitis (n 5 9) followed by recurrent bacterial infections (n 5 6). The proportion of patients with AIDS in each CD4 cohort did not change significantly when children with LIP as the only AIDS-defining condition were excluded from the AIDS category. Interestingly, however, when children were stratified as to pres-ence or abspres-ence of an LIP diagnosis within each CD4 count range, children who had had LIP had fewer additional AIDS-defining conditions than those with a negative history for this disease within the lower CD4 count strata. This finding is in accordance with reports associating LIP with a better disease progno-sis.
The majority of clinical findings described oc-curred more frequently in the group of children with AIDS, with the exception of generalized lymphade-nopathy that was highly prevalent in the entire co-hort, thrombocytopenia that had a similar prevalence in both groups, and HIV dermatitis that was found more frequently in the children without an AIDS-defining diagnosis. As CD4 counts decreased within the cohort, the prevalence of most clinical findings increased in accordance with the natural history of HIV infection. Exceptions were the prevalence of lymphadenopathy that again did not change as well as recurrent otitis media, parotid enlargement, and HIV nephropathy that were not present as frequently in children with lower CD4 cell counts in our survey. Among this cohort of pediatric long-term survi-vors, 20% of infected children (n540) had an abso-lute CD4 count.500 cells/mm3and no AIDS-defin-ing conditions. The demographics of this population
Fig 2. Prevalence of most frequent clinical findings among pedi-atric long-term survivors stratified by absolute CD4 count and presence or absence of previous AIDS-defining conditions. Diarrh, diarrhea; Dermat, HIV dermatitis; Cardio, HIV cardiomyopathy; Hepatmg, hepatomegaly; Lymph, lymphadenopathy; Nephrp, HIV nephropathy; Parotid inc, parotid increase; Spleen, spleno-megaly; Thromb, thrombocytopenia; Rec otitis, recurrent otitis media;.500,.500 CD4 cells/mm3; 200 to 500, 200 to 500 CD4
was not different from the overall profile of the co-hort. The most prevalent clinical finding was gener-alized lymphadenopathy (85%) followed by hepato-megaly (37%). In addition, 35% of these children had recurrent episodes of otitis media in the past. This population of children probably included a highly selected group of patients who had minimal to no progression of HIV disease such as the cohort of adult nonprogressors described in recent studies.16,17 All patients in the survey were long-term survivors based exclusively on their age. However, it is appar-ent that long-term survivors constitute a diverse pa-tient population within the dynamic process of dis-ease progression. Studies have demonstrated that the median survival time of perinatally HIV-infected children is approximately 8 to 9 years.8,10 Therefore, children who have lived until 8 years of age have survived longer than the median length of time in 50% of cases. As time passes, it is likely that median survival will be longer than 8 years particularly if intervention therapies are effective. In this context, true nonprogressors constitute a very unique group of patients and must be selected for the possibility of future investigational studies. Children who main-tain a relatively normal CD4 count and remain free of significant clinical symptoms are long-term survi-vors who have managed to maintain their immuno-logical status. A detailed investigation of this sub-population will potentially add to the understanding and management of HIV disease.
ACKNOWLEDGMENTS
This investigation was supported by the Pediatric AIDS Foun-dation through the research scholar award 77229 –15-PFD to K.N. Information contained in this manuscript was presented at the Third National Conference on Retroviruses and Opportunistic Infections in January 1996, Washington, DC.
The authors acknowledge the collaboration of the Los Angeles County Pediatric AIDS Consortium (LAPAC) and the Los Angeles County Health Department as well as the assistance of Mrs. Judy Jansen and the exceptional support provided by the Pediatric AIDS Foundation in the preparation of this manuscript. In addi-tion, we thank the families and their HIV-infected children at all sites for their cooperation and fortitude. The clinical and support staff at each site gave their best efforts in finding all their notes and results so important in completing such a study. The authors recognize and thank everyone for their kindness and persistence.
REFERENCES
1. Scott GB, Hutto C, Makuch RW, et al. Survival in children with peri-natally acquired human immunodeficiency virus type 1 infection.
N Engl J Med. 1989;321:1791–1796
2. Blanche S, Tardieu M, Duliege A, et al. Longitudinal study of 94 symptomatic infants with perinatally acquired human immunodefi-ciency virus infection. Evidence for a bimodal expression of clinical and biological symptoms. Am J Dis Child. 1990;144:1210 –1215
3. Moore R, Keruly J, Richman D, et al. Natural history of advanced HIV disease in patients treated with zidovudine. AIDS. 1992;6:671– 676 4. AIDS Clinical Trials Group. ACTG Virology Manual for HIV Laboratories.
Bethesda, MD: National Institutes of Health; 1994:1–5
5. The European Collaborative Study. Natural history of vertically ac-quired human immunodeficiency virus type-1 infection. Pediatrics. 1994; 94:815– 819
6. Tovo PA, de Martino M, Gabiano C, et al. Prognostic factors and survival in children with perinatal HIV-1 infection. The Italian Register for HIV Infections in Children. Lancet. 1992;339:1249 –1253
7. Jones DS, Byers RH, Bush TJ, Oxtoby MJ, Rogers MF. Epidemiology of transfusion-associated acquired immunodeficiency syndrome in chil-dren in the United States, 1981 through 1989. Pediatrics. 1992;89:123–127 8. Italian Register for HIV Infection in Children. Features of children perinatally infected with HIV-1 surviving longer than 5 years. Lancet. 1994;343:191–195
9. Grubman S, Gross E, Lerner-Weiss N, et al. Older children and adoles-cents living with perinatally acquired human immunodeficiency virus infection. Pediatrics. 1995;95:657– 663
10. Frederick T, Mascola L, Eller A, et al. Progression of human immuno-deficiency virus disease among infants and children infected perinatally with human immunodeficiency virus or through neonatal blood trans-fusion. Pediatr Infect Dis J. 1994;13:1091–1097
11. Volkow P, Ponce de Leon S, Calva J, Ruiz-Palacios G, Mohar A. Trans-fusion associated AIDS in Mexico. Clinical spectrum, conditional la-tency distribution, and survival. Rev Invest Clin. 1993;45:133–138 12. Ashton LJ, Learmont J, Luo K, Wylie B, Stewart G, Kaldor JM. HIV
infection in recipients of blood products from donors with known duration of infection. Lancet. 1994;344:718 –720
13. Blanche S, Mayaux M, Rouzioux C, et al. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N Engl J Med. 1994;330:308 –312
14. Boyer PJ, Dillon M, Navaie M, Deveikis A, Keller M, O’Rourke S, Bryson Y. Factors predictive of maternal-fetal transmission of HIV-1. Prelimi-nary analysis of zidovudine given during pregnancy and/or delivery.
JAMA. 1994;271:1925–1930
15. Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246 –1247
16. Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characteristics of long-term survivors of human immunodeficiency vi-rus type 1 infection. N Engl J Med. 1995;332:201–208
17. Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term progressive human immunodeficiency virus infection. N Engl
DOI: 10.1542/peds.99.4.e4
1997;99;e4
Pediatrics
Oleske and Gwendolyn B. Scott
Bryson, Natasha Martin, Cecelia Hutto, Arthur J. Ammann, Samuel Grubman, James
Karin Nielsen, George McSherry, Ann Petru, Toni Frederick, Diane Wara, Yvonne
Long-term Survivors
infected
−
A Descriptive Survey of Pediatric Human Immunodeficiency Virus
Services
Updated Information &
http://pediatrics.aappublications.org/content/99/4/e4
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/99/4/e4#BIBL
This article cites 16 articles, 3 of which you can access for free at:
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or in its
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.99.4.e4
1997;99;e4
Pediatrics
Oleske and Gwendolyn B. Scott
Bryson, Natasha Martin, Cecelia Hutto, Arthur J. Ammann, Samuel Grubman, James
Karin Nielsen, George McSherry, Ann Petru, Toni Frederick, Diane Wara, Yvonne
Long-term Survivors
infected
−
A Descriptive Survey of Pediatric Human Immunodeficiency Virus
http://pediatrics.aappublications.org/content/99/4/e4
on the World Wide Web at:
The online version of this article, along with updated information and services, is located
American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.