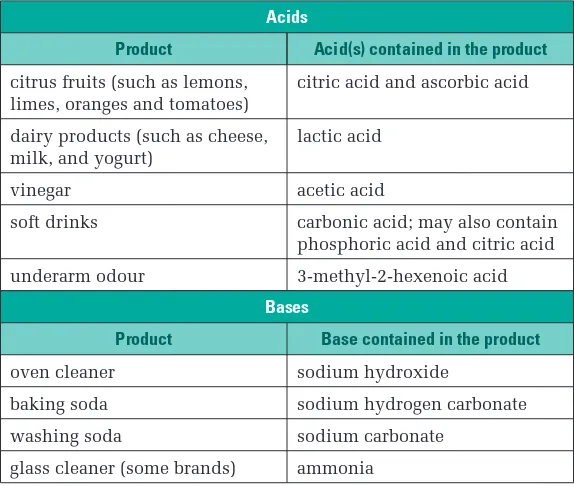
22 Acid Theory
Full text
Figure

Related documents
To assign a deck property or apply a variation to a span, click Item 7.1 Deck Section and the Assign/Show Deck Sections button on the Bridge Wizard, or on the Bridge Object
A spectrum is usually displayed with peak velocity amplitude units on the vertical axis, while the horizontal axis can show frequency in hertz (cycles per second), cycles per
During the last century Africa started to experience the Demographic Transition. In particular most Sub-Saharan African countries have already experienced a substantial decline
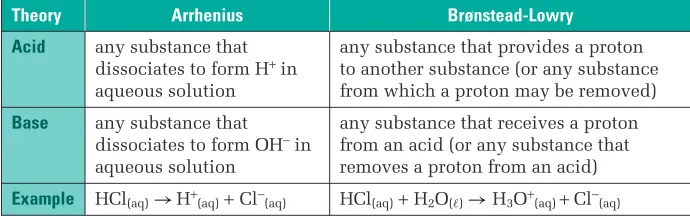
But it does include all of the classic strong acids/strong bases we see in general chemistry, and most of the common acids and bases that function in water.. Arrhenius Bases –
Acid: Hydronium ions (H 3 O + ) in water are
Since NH 4 Cl is produced by the reaction of HCl (a strong acid) with NH 4 OH (a weak base) which is combination to produce acidic solutions. 1) Salt of strong acid and
ν The stronger the acid, the weaker the conjugate base associated with that acid. ν Strong bases accept H + ions
Titration: Analytical method used to determine the concentration of acids and bases in a neutralization reaction.. Measured volume of an acid or base