Metabolic bone disease and associated risk factors in very LBW and high risk babies
131 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
Metabolic bone disease and associated risk factors in very low birth weight and high risk babies
Singh Poonam1, Mehta Nirali2, Ladumor Mahesh3
1,2,3
Department Of Pediatrics, Associate Professor, Surat Municipal Institute Of Medical Education And Research ( SMIMER), Surat, Gujarat.
INTRODUCTION
Premature infants are known to be at risk of developing metabolic bone disease (Rickets or osteopenia), and this risk is inversely rel ated to the infant's birth weight. The pathog enesis of rickets in preterm infants is consid
*Corresponding Author
Dr Nirali J. Mehta
Department of Pediatrics, SMIMER Hospital,
Umarvada,Sahara Darwaja, Surat, Gujarat.- 395010
M. No : 9099024880
Mail id : drniralim@gmail.com
ered multifactorial. In addition to inadequ ate mineralintake includes phosphorous, cal cium and vitamin D deficiency as a major contributing factor2,7chronic co-morbidities like chronic lung disease,short bowel syndr ome, and parenteral nutrition associated ch olestasis (PNAC) could also be related fact ors2,8. Medications used in the neonatal inte nsive care unit (NICU), such as steroids,me thylxanthines, and diuretics could theoretic ally increase the risk of inadequate bone mi neralization2,9,10,11 To prevent rickets of pre maturity - vitamin D, calcium and phosphor ous supplementation is needed.
Osteopenia of prematurity in very low birth
ORIGINAL ARTICLE
ABSTRACT
BACKGROUND: Premature infants are at a risk of developing metabolic bone disease (MBD). Reports of oste openia of prematurity are increasing due to the improved survival of low birth weight infants. There is a felt ne ed to study the risk factors for MBD. This study aimed at finding the incidence and risk factors contributing to MBD in our NICU. MATERIALS & METHODS: We included all newborns of NICU who full-filled the incl usion criteria like birth weight ≤1.5 kg. and/ or gestational age ≤ 32 weeks. Other babies with risk factors, neon atal or maternal were also included. History, clinical examination, anthropometry and investigations (routine wit h serum calcium, phosphorous and alkaline phosphatase, urinary calcium, creatinine, vitamin D) were done. So me comorbid conditions were analysed to see for correlation with MBD. Statistical analysis was done using SPS S software with Univariate analysis by Chi square test & independent t test. RESULTS: The total numbers of b abies analysed were 50.The overall incidence of MBD was 18%. Risk factors that significantly correlated with d evelopment of MBD were less gestational age and birth weight. Maximum incidence was in babies between 28 to 30 weeks - 66.6%(p=0.001) followed by those between 30 to 32 weeks which was 16.66%.100% babies le ss than 1 kg (p value 0.031) & 46.6% those between 1-1.25kg,(p value 0.001) developed MBD. Mean gestation al age of babies with MBD was lower, 29.94±1.184 weeks as against those without MBD 31.585±1.264 week s and was statistically significant (p 0.006). Mean birth weight of babies with MBD was lower, 1137±122 gms a nd those without MBD was1355±106 gm which was statistically significant (p=0.0000001). None of the babie s had any other significant neonatal risk factors contributing to development of MBD. In patients with MBD ave rage level of alkaline phosphatase was 834+/-164 IU against babies without MBD where it was 538=/-189- this had statistical significance of p value 0.0001. Urinary Ca/Cr ratio was found to be significant at 2 weeks 0.97+/ -0.45 with MBD versus 0.53+/_0.24 which had a statistical significance of 0.001. A combination of serum alkal ine phosphatase (>800 IU) and urinary calcium creatinine ratio >0.6 at 2 weeks yields a sensitivity of 66% and specificity of 97%. Positive predictive value of these two together was 85% and negative predictive value was 9 3% for development of MBD. Maternal risk factors which were significantly correlated were PIH (p value 0.0 1), antenatal steroid administration (p value 0.001) and maternal serum calcium levels 8.68 mg versus 9.22 mg ( p value 0.013).Co morbid neonatal conditions like sepsis, hyperbilirubinemia , acidosis, and C-pap support were found to significant fordevelopment of MBD. CONCLUSIONS: We should screen all babies <1250g and gesta tional age ≤32 wks. Babies with birth weight ≥1250 -1500g and gestational age ≥32- 34 weeksshould be screene d if they have additional co morbid conditions because MBD is a preventable and treatable condition.
Metabolic bone disease and associated risk factors in very LBW and high risk babies
132 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
weight infants was first reported in the liter ature in the study of Ylpo et al.3,13 In1943B enjamin et al.,3,14 while carrying out metabo lic balance analysis in preterm newborn inf ants (PNB), concluded that maternal milk a s the only source of nutrition was insufficie nt in terms of minerals, thus favoring alterat ions in bone mineralization. In corroboratio n to these findings, Von Sidow et al.3,15 des cribed osteopenia of prematurity innewborn s (NB) on exclusive breastfeeding and with out phosphorus (P) supplementation. Initiall y, the alterations in mineralization observed in VLBW newborns were called osteopeni a of prematurity. This term was employed i n an attempt to characterize a situation of b one hypomineralization. In other studies, th is condition was called rickets of prematurit y, due to the X-ray findings similar to those of vitamin D deficiency. In 1985, it was de termined that metabolic bone disease (MB D) be the term used.
In the 1980s, the prevalence of rickets in ex tremely low birth weight (ELBW) infants was around 50% and2,17,18 after the introduc tion of a high mineral containing diet in t he late 1980s, the prevalence decreased to 3 0% or lower.2,19,20Thus the aim of our st udy was to determine the incidence of meta bolic bone disease in VLBW babies and hi gh risk babies and to correlate risk factors ( both maternal & neonatal) contributing in metabolic bone disease.
MATERIAL AND METHODS
Study design: This prospective cohort stud y was conducted in admitted babies of NIC U of Pediatric department of a tertiary hosp ital. Study population: The inclusion criter ia34 were birth weight ≤1.5 kg and gestatio nal age ≤ 32 weeks. Other neonates with ris k factors like total or partial parenteral nutri tion >2weeks, cholestasic jaundice >2week s, drug intake history > 2weeks of any drug like furosemide, theophylline, dexamethaso ne or soda bicarbonate, babies with chronic lung disease or bowel ileal disease. Neonat es with maternal risk factors like mothers w ith PIH, eclampsia, prolonged rupture of m embranes,maternal infections like UTI, TO RCH or chorioamnionitis, maternal hypoth yroidism, diabetes, maternal drug intake lik e anticonvulsants, diuretics, corticosteroids
, were also included in the study. Matern al anthropometry after delivery and materna l calcium, phosphorous and alkaline phosph atase were done. We tried to correlate como rbid conditions like neonatal sepsis, hyperb ilirubinemia requiring intensive photothera py, apnea, hypoxia, acidosis,oxygen suppor t, ventilator requirement as correlates in dev elopment of MBD.The patients were enroll ed within 48 hours of birth and followed up at 2, 6 and 10 weeks. The anthropometry, c linical features of rickets like craniotabes, wide AF, rickety rosary, frontal bossing, Ha rrison sulcus, respiratory distress and failur e to wean from ventilator were checked on followup. Blood investigations at enrollme nt were those routinely done in our neonatal unit. In addition to this, investigations like serum calcium, phosphorous, and alkaline phosphatase, were done at 2 days, 2 weeks, 6 weeks and 10 weeks. Urinary calcium, ph osphorous, creatinine, calcium creatinine ra tio, urinary tubular phosphate reabsorption were done at 2 weeks. Vitamin D 3 levels were done at 6 weeks of age in patients w ho could afford it. Radiographs of chest, lef t wrist and humerus were done at 6 weeks a nd evaluated by radiologist. Follow up X-ra y of wrist and humerus were done at 10 we eks in patients who had been diagnosed as having MBD at 6 weeks. All babies were o n breast milk. If this was insufficient, donor milk from milk bank was given to fulfill da ily requirements. Human milk fortification (HMF: 2gm/day) with breast feeding were given to all VLBW babies till weight achi evement of 2 kg and/or gestational age of 3 8 weeks whatever first44(One HMF sachet c ontained 50mg of calcium; 25mg of phosph orus; 40IU vitamin D) as per AAP guidelin es. After that calcium and phosphorus were given according to AAP guidelines (calciu m 140–160 mg/kg/day, phosphate intake va ry from 95 to 108 mg/kg/day and vitamin D for preterm infants is 400 IU/day) as per pr otocol up to 1 year.
Therapeutic treatment was started in infa nts who had been detected with osteopenia on x-ray and/or laboratory parameters in th e form of increase serum alkaline phosphat e more than (> 800 IU/L) at 6 weeks of life.
40
Metabolic bone disease and associated risk factors in very LBW and high risk babies
133 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
sphorus 20mg/kg/day40weregiven and con tinued until radiographs show healing and/o r decreasing serum alkaline phosphate level (< 500IU/L),40then prophylactic supplemen tation till one year40. Deficiency of vitamin D3(< 15 ng/dl)44 was treated with Vitamin D 1000 IU/day for 21 days then prophylacti c supplementation of calcium, phosphorous and vitamin D was given till one year of ag e 35.Statistical analysis: Univariate analysi s by Chi square test, independentt test. Excl usion criteria: Infants with serious /lethal c ongenital malformations, infants who expir ed or took LAMA, were lost to follow up or did not give consent to be a part of the st udy. The study was approved by ethical co mmittee of our university and informed wri tten consent was taken from the parents of e ach participant. All babies received treatme
nt as per standard NICU protocol RESULTS
A total of 186 babies (both intramural and e xtramural) were eligible out of which 136 b abies were excluded ( 64 babies expired, 68 babies took DAMA and 4 babies could not be followed up).Thus the total numbers of babies included in the study were 50 (10 e xtramural+40 intramural). Out of a total of 50 babies analyzed, male to female ratio wa s 3:2. 9 babiesdeveloped MBD hence incide nce of metabolic bone disease was 18%.Ma ximum incidence wasin babies between 28 to 30 weeks, 66.6%(p=0.001) followed by t hose between 30 to 32 weeks which was 1 6.66%.100% babies less than 1 kg (p value 0.031), 46.6% those between 1-1.25kg,(p v alue 0.001) developed metabolic bone disea se.
Table 1: Incidence based on demographic data
Total no of babies screened (N=50)
Total no of babies with MBD (n=9)
Incidence (percentage %)
p value
Sex
Male 30 07 14 0.229
Female 20 02 02
-Gestational age
<28 weeks 0 0 0
28-30 weeks 06 04 66.66 0.001
30-32weeks 30 5 16.66 0.764
32-34 weeks 14 0 00
-Birth weight
<1kg 1 1 100 0.031
1-1.250kg 15 7 46.6 0.001
1.251-1.5 kg 34 1 2.94
-Weight for gestation
SGA 32 08 25 0.086
AGA 18 1 5.5
-On comparing the groups of babies who de veloped MBD and those who did not the fol lowing risk factors were found to be correla ted. Mean gestational age of babies with M BD was lower, 29.94±1.184 weeks as again st those without MBD 31.585±1.264 week s and was statistically significant (p 0.006). Mean birth weight of babies with MBD wa s lower, 1137±122 grams and those without MBD was1355±106 gm which was statisti cally significant (p=0.0000001).
Among other neonatal risk factors which we analysed like use of drugs like frusemid e, theophylline, steroids for more than 2 we eks, partial parenteral nutrition and neonata l cholestasis, bowel ileal disease we had onl y one patient with prolonged use of frusemi
de which did not show any significance but this cannot be commented on as number is inadequate. Clinical features suggestive of r ickets like poor weight gain(<10gm/day), fr ontal bossing, harrisson’s sulcus, fractures, rickety rosary, craniotabes , respiratory dist ress, difficult weaning were not seen in any patients of MBD till 6 weeks. Wide anterio r fontanel >2x2 cm was seen at 2 wks age i n one patient of MBD.
Metabolic bone disease and associated risk factors in very LBW and high risk babies
134 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734 e it was 538=/-189 this had statistical signif
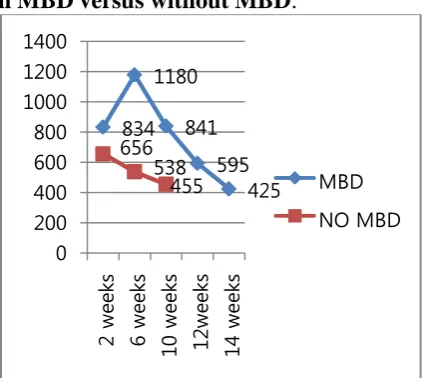
icance of p value 0.0001. Alkaline phosphat ase levels peaked at 6 weeks to1180 IU and then normalized at 14 weeks to 425 IU in p atients with MBD after treatment with calci um and phosphorous supplementation as s hown in graph.
Fig. 1: Serial comparison of serum alkali ne phosphatase (IU) levels in patients wit h MBD versus without MBD.
Urinary investigations done were urinary calcium, phosphate, creatinine and urinary t ubular phosphate reabsorbtion%. Among th ee Ca/Cr ratio was found to be significant a t 2 weeks 0.97+/-0.45with MBD versus 0.5 3+/_0.24 which had a statistical significanc
e of 0.001.A combination of serum alkaline phosphatase(>800 IU) and urinary calcium creatinine ratio >0.6 at 2 weeks yields a se nsitivity of 66% and specificity of 97%. Po sitive predictive value of these two together was 85% and negative predictive value wa s 93%. The levels of serum calcium and ph osphate were not statistically different amo ng both groups at any stage of evaluation. Earliest X Ray changes of rickets were seen at 6 weeks only in one patient in wrist with MBD which had a significant p value of 0. 031.
Among the neonatal comorbid conditions, b abies with sepsis(proven/probable) (p>0.00 8), hyperbilirubinemia requiring photothera py(0.01), acidosis(0.043), CPAP (p 0.001) were showing statistical significance as the y had associated MBD. Mean duration of st ay in NICU and feedingof donor milk did n ot show any statistical significance among t he two groups. Vitamin D3 levels were don e in all 9 patients of MBD at 6 weeks and in 35 out of 41 patients in those without MB D. Average levels were 29.32ng versus 37. 12ng in babies without MBD this was not st atistically significant. .
Table 2:- Univariate analysis of risk factors for metabolic bone disease Total no of babies
with MBD (n=9) (%)
Total no of babies without MBD (n=41) (%)
p value
Epidemiological data
Gestation age 28-30 weeks 4 (44.44) 2 (4.87) 0.001
Birth weight (<1 kg ) 1 (11.11) 0 0.031
Birth weight (1-1.250 kg) 7 (77.77) 8 (19.51) 0.001 Clinical characteristic
Wide open fontanels more than 2×2 cm. at 2 weeks 1(11.11) 00 0.013 Co – morbid condition
Sepsis 6 (66.66) 9 (21.95) 0.008
Probable 4(44.44) 4 (9.7) 0.010
Hyper bilirubinemia requiring intensive phototherapy (3)(33.33) (2)(4.87) 0.01
Acidosis 4(44.44) 6(14.63) 0.043
C - PAP support (3)(33.33) 00 0.0001
Maternal risk factors
PIH 3 (33.33) 2 (4.4) 0.010
Antenatal Steroids 7(77.77) 5(12.19) 0.0001
Investigations at 2 weeks of age
Serum alkaline phosphatase (IU/L) 834 ± 164 538 ± 189 0.0001 Urinary calcium creatinine ratio 0.97 ± 0.45 0.53 ± 0.24 0.001 Investigations at 6 weeks of age
Serum alkaline phosphatase (IU/L) 1180 ± 301 455 ± 140 0.0001 Changes of rickets in wrist X –Ray 01(11.11) 00 0.031 Maternal blood investigations
Serum calcium(mg/dl) 8.68 ± 0.59 9.22 ± 0.55 0.013
834 1180
841 595
425 656
538 455
0 200 400 600 800 1000 1200 1400
2
w
ee
ks
6
w
ee
ks
10
we
ek
s
12
we
ek
s
14
we
ek
s
Metabolic bone disease and associated risk factors in very LBW and high risk babies
135 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
Maternal risk factors analysed were PIH, ec lampsia, administration of antenatal steroid s, antenatal infections, maternal diabetes, h ypothyroidism and drugs intake.
The ones which were statistically significa nt for development of MBD were PIH (p va lue 0.01), antenatal administration of steroi ds to mother (p value0.001) and maternal se rum calcium levels at delivery 8.68 versus 9.22 (p value 0.013). Though univariate ana lysis showed significance of many risk fact ors multivariate logistic regression analysis did not show any factor to be independently associate with development of MBD.DISC USSION: The reported incidence of rickets in low birth weight may be as high as 32 %.
(47, 48.)
In the 1980s, fortified mineral formu las were introduced to reduce the rates of ri ckets and fractures in very low birth weight (VLBW < 1,500 g) infants. Reports at that time indicated that up to 30% of VLBWs h ad rickets with fractures. With advances in preterm nutrition, especially with the introd uction of mineral fortified formulas, theoret ically the incidence of rickets of prematurit y should have dropped. However, for increa sing the survival of ELBW infants, signific ant co-morbid conditions could affect actua l mineral intake which might mitigate the ef fect of high mineral fortification.49
In our study the incidence of metabolic bon e disease in very low birth weight babies (babies< 1.5kg) was 18% and in ELBW ba bies(<1kg) was 100%. Incidence of MBD i n babies with gestational age 28-30 weeks was 66.66%, gestational age of 30-32 week s was 16.66% in our study. Osteopenia has been reported in 23% of VLBW infants an d in 55% to 60% of ELBW (< 1,000g)47. It is well recognized that incidence of MB D is inversely related to birth weight and ge stational age.1,40,44
We studied various co – morbidities like NEC, sepsis, hypoglycemia, hyper -bilirubi nemia requiring phototherapy, birth asphyxi a, apnea, oxygen support, c-pap support, ve ntilatory support, mean NICU duration and use of donor milk. Out of these clinical feat ures - sepsis, hyperbilirubinemia requiring phototherapy, acidosis, and C-pap support were found to be statistically significantly c
orrelating with metabolic bone disease in p reterm very low birth weight babies. In stud y of MinLee and Ran Namgung 49 ELBW i nfants with rickets had a significantly highe r occurrence of PDA (P = 0.025), parenteral nutrition associated cholestasis (PNAC) (P = 0.041), severe PNAC (P = 0.013), BPD ( P = 0.019), and moderate/severe BPD (P = 0.012). Thus there is a need to consider Tes co morbidities in prevention of occurrence of MBD. Since these have not been listed in studies as risk factors and are showing corr elation more studies taking these into consi deration are required to ascertain if they are risk factors for developing MBD. Serum ca lcium values may remain low, normal or sli ghtly elevated, so they are not a good indica tor of presence or severity of MBD42. Simil arly in our study serum calcium at 2 days, 2 weeks and 6 weeks did not show any statist ical significance. Low serum phosphate (<3 .5-4mg/dl) had low sensitivity but high spec ificity40. Low concentration of inorganic ph osphate(<1.3mmol/L) with elevated alkalin e phosphatase(>800)IU/L)40 may be more s ensitive (100%) and specific(70%) for diag nosing inadequate intake and low bone min eral density.40 In our study a combination of the criteria serum total alkaline phosphatas e activity above 800IU/L and urinary calciu m creatinine ratio>0.6 at 2 weeks yields a s ensitivity of 66% and specificity of 97%. P ositive predictive value of these parameters was 85% and negative predictive value of t hese two together was 93%. We could not d o serum inorganic phosphate levels so we c annot comment of its specificity or sensitivi ty. We had done Vitamin D level in 44 pati ents; out of which 11 patients had vitamin D deficiency but it was not statistically sign ificant to developed MBD. The study by K oo et al also suggest that level of vitamin D are not significantly low in patients with M BD53
Metabolic bone disease and associated risk factors in very LBW and high risk babies
136 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
mg/dl), serum phosphorus(mg/dl), serum al kaline phosphatase(IU/L). Out of these risk factors, antenatal use of steroids (p=0.001), PIH (p=0.010) and maternal serum calcium level (p=0.013) were found to be significant for development of MBD in preterm baby. In study by Yokoyama in 2010 prolonged ( >5days) of iv magnesium sulphate showed radiographic bone changes in neonates but was not statistically significant51. No other studies were found on literature search for maternal risk factors influencing developm ent of MBD so these could not be compare d. The present study clearly highlights the magnitude of the problem due to MBD. Th e incidence is likely to increase as smaller b abies survive, unless a parallel reduction in other risk factors occurs. By preventing pre maturity, controlling or minimizing risk fac tors and meticulous screening of at risk pret erm, it may be possible to reduce the incide nce of MBD.
Since MBD is essentially asymptomatic in t he early stages, standards of practice now d emand carefully timed screening, prophylax is and treatment of at risk infants for MBD t o minimize the risk of bony weakness in th ese infant.
Recommendations
1. We should screen all preterm babies wit hbirth weight <1250g and gestational ag e ≤32 weeks, irrespective of risk factors or co – morbid conditions.
2. Babies with birth weight ≥1250 -1500g
and having gestational age 32- 34 week s should be screened if they have additi onal co – morbid conditions because M BD is a preventable and treatable condit ion.
3. Serum alkaline phosphatase and urinary calcium creatinine ratio at 6 weeks can be used as screening test for early detect ion of MBD and alkaline phosphatase t o guide treatment.
Further studies are required to correlate neo natal and maternal risk factors and comorbd conditions with development of metabolicb one disease to ensure early detection and tre atment of these conditions in our setup. REFERENCES
1. Monique Catache,1 Cléa Rodrigues Leone2;Critical analysis of pathophysiological, diagnostic and therapeutic aspects of metabolic bone disease in very low birth weight infants; review article; 0021-7557/01/77-Supl.1/S53;Jornal de Pediatria.
2. Minerva Pediatr 1989; Weber G, Guarneri MP, Corbella E, Gallia P, Chiumello : Osteopenia in premature children: an emerging problem. G. 41: 347-52
3. Ferrone M, Geraci M. : A review of the relationship between parenteral nutrition and metabolic bone disease. NutrClinPract 2007; 22: 329-39.
4. Venkataraman PS, Han BK, Tsang RC, Daugherty CC. Secondary hyper-parathyroidism and bone disease in infants receiving long-term furosemide therapy. Am J Dis Child 1983; 137: 1157-61.
5. Weiler HA, Wang Z, Atkinson SA. Dexamethasone treatment impairs calcium regulation and reduces bone mineralization in infant pigs. Am J ClinNutr 1995; 61: 805-11.
6. Zanardo V, Dani C, Trevisanuto D, Meneghetti S, Guglielmi A, Zacchello G, Cantarutti F Methylxanthines increase renal calcium excretion in preterm infants. Biol Neonate 1995;. 68: 169-74.
7. Monique Catache,1 Cléa Rodrigues Leone2;Critical analysis of pathophysiological, diagnostic and therapeutic aspects of metabolic bone disease in very low birth weight infants; review article; 0021-7557/01/77-Supl.1/S53;Jornal de Pediatria.
8. Ylppo AZ. Kinderhielkd, apud Rowe JC, Carey DE. Phosphorus deficiency syndrome in very low birth weight infants. PedClin North Am 1987;,24:111, 1919 34:997-1017. 9. Benjamin HR, Gordon HH, Marples
Metabolic bone disease and associated risk factors in very LBW and high risk babies
137 Int J Res Med. 2014; 3(3)131-137 e ISSN:2320-2742 p ISSN: 2320-2734
10. Von Sidow CA ,apud Rowe,. A study of the development of rickets in premature infants. ActaPaediatr 1946, suppl 33:1, 11. Lyon AJ, McIntosh N, Wheeler K,
Williams JE. Radiological rickets in ex-tremely low birthweight infants. PediatrRadiol 1987; 17: 56-8.
12. McIntosh N, Livesey A, Brooke OG. Plasma 25-hydroxyvitamin D and rickets in infants of extremely low birthweight. Arch Dis Child 1982; 57: 848-50.
13. Annu Rev; Greer FR Osteopenia of prematurity. Nutr 1994. 14: 169-85. 14. Lippincott Williams & Wilkins,
MacDonald MG, Seshia MM, Mullett Avery’s neonatology: pathophysiology & management of the newborn. 6th ed. Philadelphia:, 2005
15. Dipak K Guha Guha text book of neonatology;Guha's Neonatology Principles and Practice (2 Vols), 3rd edition ,chapter 62 metabolic disorder, Rickets/osteopenia of prematurity page no 864.
16. Vitamin D Deficiency in Childhood – A Review of Current Guidelines on Diagnosis and Management; Indian
pediatrics; volume 50__july 15, 2013.
17. John p cloherty, ericc.eichenwald, anner.hansen, annr.stark;manual of neonatal care; seventh edition;
chapter59; osteopenia(metabolic bone disease of new born); page no:762 - 763. 18. manual of neonatal care; John p
cloherty, ericc.eichenwald, anner.hansen, annr.stark; seventh edition; chapter25; abnormality of serum calcium and magnesium; page no:300.
19. Kulkaini B, Hall R T, Rhodes P G, et al. Rickets in very low-birth-weight infants. JPediatr 1980; 96: 249-52.
20. Callenbach 3 C, Sheehan M B, Abramson S J, Hall R T. Etiological factors in rickets of very low-birth-weight infants. JPediatr1981 ; 98: 8 21. High Incidence of Rickets in Extremely
Low Birth Weight Infants with Severe Parenteral Nutrition-Associated Cholestasis and Bronchopulmonary Dysplasia; J Korean Med Sci 2012; 27: 1552-155500-5.
22. Akshaya J. Vachharajani, Amit M. Mathur and Rakesh Rao;Metabolic Bone Disease of Prematurity; NeoReviews2009;10;e402-e411; DOI: 10.1542/neo.10-8-e402.