0095-1137/07/$08.00⫹0 doi:10.1128/JCM.00234-07
Copyright © 2007, American Society for Microbiology. All Rights Reserved.
Exceptionally High Representation of
Burkholderia cepacia
among
B. cepacia
Complex Isolates Recovered from the Major Portuguese
Cystic Fibrosis Center
䌤
Mo
´nica V. Cunha,
1Ana Pinto-de-Oliveira,
1,2Luı´s Meirinhos-Soares,
3Maria Jose
´ Salgado,
4Jose
´ Melo-Cristino,
4Susana Correia,
5Celeste Barreto,
5and Isabel Sa
´-Correia
1*
IBB—Institute for Biotechnology and Bioengineering, Centre for Biological and Chemical Engineering, Instituto Superior Te´cnico,
Av. Rovisco Pais, 1049-001 Lisboa, Portugal1; Instituto de Microbiologia, Faculdade de Medicina da Universidade de Coimbra,
Rua Larga, 3004-504 Coimbra, Portugal2; Direcc¸a˜o de Comprovac¸a˜o da Qualidade do Infarmed, Parque de Sau´de de Lisboa,
Avenida do Brasil, no. 53, 1749-004 Lisboa, Portugal3; and Laborato´rio de Bacteriologia4e Centro Especializado em
Fibrose Quı´stica,5Hospital de Santa Maria, Av. Prof. Egas Moniz, Lisboa, Portugal
Received 30 January 2007/Returned for modification 2 March 2007/Accepted 7 March 2007
Burkholderia cepacia, a species found infrequently in cystic fibrosis (CF), was isolated from 85% of patients
infected with bacteria of theB. cepaciacomplex that visited the major Portuguese CF center, in Lisbon, during
2003 to 2005. A detailed molecular analysis revealed that this was mainly due to twoB. cepaciaclones. These
clones were indistinguishable from two strains isolated from intrinsically contaminated nonsterile saline solutions for nasal application, detected during routine market surveillance by the Portuguese Medicines and Health Products Authority.
Burkholderia cepaciacomplex (BCC) bacteria are
opportu-nistic pathogens that may colonize and/or infect patients with cystic fibrosis (CF), an inherited disorder that, among other clinical manifestations, predisposes individuals to recurrent respiratory infections and lung damage (15). Epidemiological surveys carried out in several countries indicated that all nine BCC species can be recovered from respiratory secretions of CF patients, but Burkholderia cenocepacia and Burkholderia
multivoransare predominant (17). A remarkable exception to
this observation is the epidemiological analysis carried out by our laboratory at the major Portuguese CF center (6). Al-though the prevalence ofB. cenocepacia(52%) was confirmed, a significant percentage (36%) of the patients at the CF center of Hospital de Santa Maria (HSM) during 1995 to 2002 were colonized or infected withB. cepacia(6). This contrasts with previous studies performed in Canada, the United States, Italy, and France, whereB. cepaciaincidence ranged from 0.2% to 7.7% (1, 3, 4, 13, 21, 23). The reason for the unexpectedly high representation of B. cepacia during this surveillance period could not be determined. By the end of 2003, a routine market surveillance analysis performed by Infarmed, the Portuguese Medicines and Health Products Authority, revealed that sev-eral batches of nonsterile saline solutions from two local man-ufacturers greatly exceeded the microbiological quality limits (ⱕ102CFU/ml) established by European Pharmacopoeia VII
(7). Preliminary identification by gas chromatography analysis of the fatty acid methyl esters (MIDI; Sherlock, Newark, DE) suggested that the isolated bacteria belong to the species B.
cepacia. Following the confirmation of the identification by
molecular methods described below, the contaminated batches were immediately withdrawn from the market. New contami-nated batches were detected later, in March 2006. Since saline is used in inhalant therapy by CF patients, a correlation be-tween this contamination and the unusually high representa-tion ofB. cepacia registered at the CF center under surveil-lance (6) was considered. To test this hypothesis, molecular analysis of 95 BCC isolates recovered from sputum samples from 13 CF patients on selective B. cepacia solid medium (Selectatab; Mast Diagnostics, Merseyside, United Kingdom) from November 2002 to March 2006 was carried out, and their genetic relatedness to the saline isolates was assessed. In gen-eral, sputum samples from CF patients were obtained every 2 to 3 months during periodic consultation to monitor their clinical status. Samples were cultured more often for patients showing clinical deterioration. All serial isolates obtained from chronically infected patients (80% of patients examined) were included in the study. A patient was considered chronically infected when three positive cultures of BCC strains were isolated within an 8-month period.
Distribution of isolates from CF patients and saline among
BCC species. During the 3.5-year surveillance period in this
study, 85% of BCC-positive CF patients under surveillance at the HSM CF center harbored strains of the speciesB. cepacia(recA
HaeIII restriction fragment length polymorphism [RFLP] type D, E, K, Z, or AG). This conclusion was based on polymorphisms of the recA gene with HaeIII and species-specific recA-directed PCR, performed as described before (16).B. cenocepacia(recA
HaeIII RFLP type G, AN, or AU) was present in 23% of the CF patients examined, 15% of them also being colonized or infected
withB. cepacia(Table 1). WhileB. cenocepaciainfections
pre-dominated until 2001 (6), B. cepacia became the most repre-sented species after November 2002, its incidence peaking in 2004 (Table 1). The analysis of saline isolates indicated that they be-longed torecAHaeIII RFLP type D or E (Table 2).
* Corresponding author. Mailing address: IBB. Centre for Biological and Chemical Engineering, Instituto Superior Te´cnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. Phone: 351-218417682. Fax: 351-218419199. E-mail: isacorreia@ist.utl.pt.
䌤Published ahead of print on 14 March 2007.
1628
on May 16, 2020 by guest
http://jcm.asm.org/
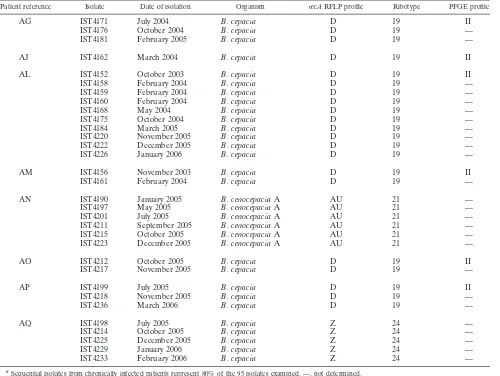
TABLE 1. Results of molecular analysis of BCC isolates from CF patients under surveillance at HSM from November 2002 to March 2006a
Patient reference Isolate Date of isolation Organism recARFLP profile Ribotype PFGE profile
O IST4135 November 2002 B. cepacia AG 12 —
IST4137 January 2003 B. cepacia AG 12 —
IST4139 April 2003 B. cepacia AG 12 —
IST4142 June 2003 B. cepacia AG 12 —
IST4146 August 2003 B. cepacia AG 12 —
IST4150 September 2003 B. cepacia AG 12 —
IST4157 January 2004 B. cepacia AG 12 —
IST4165 March 2004 B. cepacia AG 12 —
IST4167 May 2004 B. cepacia AG 12 —
IST4240 September 2004 B. cepacia AG 12 —
IST4177 November 2004 B. cepacia AG 12 —
IST4180 January 2005 B. cepacia AG 12 —
IST4183 March 2005 B. cepacia AG 12 —
IST4189 May 2005 B. cepacia AG 12 —
IST4204 July 2005 B. cepacia AG 12 —
IST4209 October 2005 B. cepacia AG 12 —
IST4216 November 2005 B. cepacia AG 12 —
IST4227 January 2006 B. cepacia AG 12 —
IST4230 February 2006 B. cepacia AG 12 —
R IST4144 July 2003 B. cenocepaciaB AN 15 —
IST4149 September 2003 B. cenocepaciaB AN 15 —
IST4155 November 2003 B. cenocepaciaB AN 15 —
IST4164 March 2004 B. cenocepaciaB AN 15 —
IST4178 November 2004 B. cenocepaciaB AN 15 —
IST4203 July 2005 B. cenocepaciaB AN 15 —
IST4205 August 2005 B. cenocepaciaB AN 15 —
IST4210 September 2005 B. cenocepaciaB AN 15 —
IST4219 November 2005 B. cenocepaciaB AN 15 —
IST4228 January 2006 B. cenocepaciaB AN 15 —
IST4231 February 2006 B. cenocepaciaB AN 15 —
IST4232 February 2006 B. cenocepaciaB AN 15 —
IST4235 March 2006 B. cenocepaciaB AN 15 —
V IST4148 September 2003 B. cepacia E 17 I
IST4241 June 2004 B. cepacia E 17 I
IST4169 June 2004 B. cepacia E 17 I
IST4200 July 2005 B. cepacia E 17 I
IST4224 December 2005 B. cepacia E 17 I
AB IST4136 January 2003 B. cenocepaciaA G 7 —
IST4140 May 2003 B. cenocepaciaA G 7 —
IST4141 May 2003 B. cenocepaciaA G 7 —
IST4151 October 2003 B. cenocepaciaA G 7 —
IST4153 November 2003 B. cenocepaciaA G 7 —
IST4154 November 2003 B. cenocepaciaA G 7 —
IST4166 April 2004 B. cenocepaciaA G 7 —
IST4170 June 2004 B. cenocepaciaA G 7 —
IST4173 August 2004 B. cepacia D 19 II
IST4179 January 2005 B. cenocepaciaA G 7 —
IST4182 March 2005 B. cenocepaciaA G 7 —
IST4191 June 2005 B. cenocepaciaA G 7 —
IST4187 April 2005 B. cenocepaciaA G 7 —
IST4202 July 2005 B. cenocepaciaA G 7 —
IST4213 October 2005 B. cenocepaciaA G 7 —
IST4234 February 2006 B. cenocepaciaA G 7 —
AF IST4193 December 2004 B. cepacia K 2 —
IST4186 April 2005 B. cepacia K 2 —
IST4188 May 2005 B. cepacia K 2 —
IST4192 June 2005 B. cepacia K 2 —
IST4194 October 2005 B. cepacia K 2 —
IST4206 October 2005 B. cepacia K 2 —
IST4207 October 2005 B. cenocepaciaB AN 15 —
IST4221 November 2005 B. cepacia E 17 I
IST4238 March 2006 B. cepacia K 2 —
IST4237 March 2006 B. cepacia K 2 —
Continued on following page
on May 16, 2020 by guest
http://jcm.asm.org/
Genetic relatedness of clinical and saline isolates of B. ce-pacia. The 60 clinical isolates of B. cepaciatested generated five ribopatterns, designated 2, 12, 17, 19, and 24 (Fig. 1; Table 1), while the 35 B. cenocepacia isolates generated three ri-bopatterns, designated 7, 15, and 21 (Table 1). Ribotyping was
performed as described by Cunha et al. (6), using as a probe fluorescein-labeled 16S and 23S rDNA from B. cenocepacia
J2315 chromosomal DNA, amplified with the primers 16SF (5⬘-GATTGAACGCTGGCGGCATG-3⬘), 16SR (5⬘-GAGGT GATCCAGCCGCACCT-3⬘), 23SF (5⬘-AAGCGATCAAGT GCATGTGGTG-3⬘), and 23SR (5⬘-GATCAAGCCTTACGG GCAATTA-3⬘). The 33 B. cepacia isolates recovered by Infarmed in December 2003 and March 2006 from nine contam-inated lots of saline generated four different ribopatterns (Fig. 2A; Table 2). Isolates with ribopattern 19 were recovered on both occasions from the saline produced by manufacturer B. Remark-ably, ribopatterns 17 and 19, generated by 23B. cepaciasaline isolates, were also generated by 28B. cepaciaisolates obtained from 9 CF patients receiving care at HSM from September 2003 to March 2006 (Table 1). RFLP–pulsed-field gel electrophoresis (PFGE) analysis, carried out according to standard protocols (22), confirmed the clonality of clinical and saline B. cepacia
[image:3.585.43.543.80.456.2]isolates with ribopattern 19 or 17, since all the isolates with the same ribotype gave rise to identical RFLP-PFGE patterns (Fig. 2B). This result indicates that the majority of the respiratory infections withB. cepaciaregistered in 2003 to 2005 were due to TABLE 1—Continued
Patient reference Isolate Date of isolation Organism recARFLP profile Ribotype PFGE profile
AG IST4171 July 2004 B. cepacia D 19 II
IST4176 October 2004 B. cepacia D 19 —
IST4181 February 2005 B. cepacia D 19 —
AJ IST4162 March 2004 B. cepacia D 19 II
AL IST4152 October 2003 B. cepacia D 19 II
IST4158 February 2004 B. cepacia D 19 —
IST4159 February 2004 B. cepacia D 19 —
IST4160 February 2004 B. cepacia D 19 —
IST4168 May 2004 B. cepacia D 19 —
IST4175 October 2004 B. cepacia D 19 —
IST4184 March 2005 B. cepacia D 19 —
IST4220 November 2005 B. cepacia D 19 —
IST4222 December 2005 B. cepacia D 19 —
IST4226 January 2006 B. cepacia D 19 —
AM IST4156 November 2003 B. cepacia D 19 II
IST4161 February 2004 B. cepacia D 19 —
AN IST4190 January 2005 B. cenocepaciaA AU 21 —
IST4197 May 2005 B. cenocepaciaA AU 21 —
IST4201 July 2005 B. cenocepaciaA AU 21 —
IST4211 September 2005 B. cenocepaciaA AU 21 —
IST4215 October 2005 B. cenocepaciaA AU 21 —
IST4223 December 2005 B. cenocepaciaA AU 21 —
AO IST4212 October 2005 B. cepacia D 19 II
IST4217 November 2005 B. cepacia D 19 —
AP IST4199 July 2005 B. cepacia D 19 II
IST4218 November 2005 B. cepacia D 19 —
IST4236 March 2006 B. cepacia D 19 —
AQ IST4198 July 2005 B. cepacia Z 24 —
IST4214 October 2005 B. cepacia Z 24 —
IST4225 December 2005 B. cepacia Z 24 —
IST4229 January 2006 B. cepacia Z 24 —
IST4233 February 2006 B. cepacia Z 24 —
a
Sequential isolates from chronically infected patients represent 80% of the 95 isolates examined. —, not determined.
TABLE 2. Results of the molecular analysis ofB. cepaciaisolates recovered from contaminated saline solutions by Infarmed at
the end of 2003 and in March 2006
Manufacturer
(brand) Date of isolation
No of isolates
recA HaeIII profile
Ribotype PFGE profilea
A (K) December 2003 3 E 17 I
A (K) November-December 2003
5 E 18 —
A (K) November 2003 2 E 20 —
B (X) November-December 2003
8 D 19 II
B (X) March 2006 6 D 19 II
B (Y) March 2006 5 D 19 II
B (Z) March 2006 4 D 19 II
a—, not determined.
on May 16, 2020 by guest
http://jcm.asm.org/
[image:3.585.42.282.596.715.2]two strains, with ribopatterns 17 and 19 and RFLP-PFGE profiles I and II, respectively, that were indistinguishable by ribotyping and RFLP-PFGE profiling from the twoB. cepaciaclones iso-lated in 2003 and 2006 from the intrinsically contaminated saline solutions. Moreover, prior to the date of the detection of the first lots of contaminated saline solutions (the end of 2003), noB.
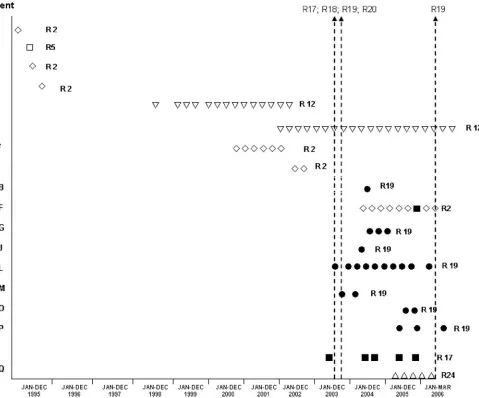
cepaciaisolate with ribopattern 17 or 19 had been recovered from
CF patients (Fig. 1) (6). Furthermore, patients with ribopattern 17 or 19 isolates had never been colonized/infected with BCC bacteria (Fig. 1). Indeed, the very strong increase in the incidence
of B. cepacia in the CF center under surveillance, registered
during 2003 and 2004, coincided with the detection in the market of contaminated saline solutions. ThreeB. cepaciastrains differ-ent from those presdiffer-ent in the contaminated saline also colonized and/or infected the CF patients receiving care at HSM from 2003 through 2006 (Table 1). These strains also contributed to the unusually high representation of B. cepaciaspecies in this CF
center during the surveillance period, but the source of infection remains unclear.
Clinical outcome of CF patients infected withB. cepaciaor
B. cenocepacia.During the surveillance period in this study, no death was registered among the CF patients harboring BCC bacteria. In general, the CF patients chronically infected with
B. cenocepaciaorB. cepaciastrains remained clinically stable,
in particular those harboring strains indistinguishable from the saline clones. The only exception was patient AF, who had already presented with moderate lung disease before testing positive forB. cepaciabut whose clinical condition suffered a rapid deterioration (as indicated by lung function and number of hospitalizations) following colonization for almost 18 months with aB. cepaciastrain of ribopattern 2. Although this clinical strain was unrelated to the saline clones, aB. cepacia
[image:4.585.60.539.69.467.2]strain with ribopattern 17 was sporadically isolated from this patient, as well as aB. cenocepaciastrain (Table 1).
FIG. 1. Ribopatterns (〫, R2;䊐, R5;ƒ, R12;■, R17;F, R19;‚, R24) of theB. cepaciaisolates obtained from CF patients visiting the HSM CF center between January 1995 and March 2006. ClinicalB. cepaciastrains identical to strains isolated from the contaminated saline solutions, with ribopatterns 17 and 19, are represented by solid symbols. The arrows indicate the date of detection of contaminated saline solutions. Patients G, O, AB, and AF also harboredB. cenocepaciaisolates. This representation is based on results in Table 1 and on previous results (6).
on May 16, 2020 by guest
http://jcm.asm.org/
Concluding remarks. Bacteria of the BCC are resistant to multiple antimicrobials and to diverse growth inhibitors, which they can even use as carbon sources (5). These bacteria also have minimal nutritional requirements, which enables them to grow in aqueous products, including disinfectants (10, 19). Contamination of albuterol and sulbutamol nebulization solutions (2, 9), nebu-lizers (11), mouthwash (18), nasal sprays (8), and ultrasound gel (12) has resulted in outbreaks of nosocomially acquired infection by BCC bacteria. Although results from this study appear to suggest an epidemiological relationship between the intrinsically contaminated saline solutions and CF patients colonized/infected with the less commonly isolated speciesB. cepacia, it was not possible to establish a definitive link between the use of contam-inated saline solutions and patient contamination. Furthermore, other B. cepacia strains with no direct relation to the clones detected in the contaminated saline solutions also contributed to the unusually high representation ofB. cepaciaregistered in this CF center during the period under analysis, suggesting other sources of infection. A significant proportion of the CF patients that were not colonized with the two clones under discussion harbored unique strains of B. cepacia or B. cenocepacia. This observation indicates that although transmission of these bacteria is significant in the colonization of CF patients, in a CF center that follows the recommended control measures, as is the case at the Lisbon CF center, other primary sources of infection must ac-count for many of the cases. It is likely that the environment may act as reservoir for novel BCC infections (14, 20, 24). This study supports the recommendation for the exclusive use of sterile sa-line solutions by CF patients. It also highlights the importance of the continuous monitoring of medications for microbial contam-ination and the surveillance of unexplained outbreaks involving less common pathogens. In particular, attention should be given to the usually poorly represented species, likeB. cepacia,
espe-cially when patients with underlying lung disease and increased risk, such as CF patients, are involved.
The contributions of L. Lito (HSM) and M. Miranda, A. Galva˜o, and E. Be´rtolo (Infarmed) to this study are gratefully acknowledged. M. V. Cunha is the recipient of a fellowship (SFRH/BPD/14911/ 2004) from Fundac¸a˜o para a Cieˆncia e a Tecnologia (FCT).
REFERENCES
1.Agodi, A., E. Mahenthiralingam, M. Barchitta, V. Giannino, A. Sciacca, and S. Stefani.2001.Burkholderia cepaciacomplex infection in Italian patients with cystic fibrosis: prevalence, epidemiology, and genomovar status. J. Clin. Microbiol.39:2891–2896.
2.Balkhy, H. H., G. Cunningham, C. Francis, M. A. Almuneef, G. Stevens, N. Akkad, A. Elgammal, A. Alassiri, E. Furukawa, F. K. Chew, M. Sobh, D. Daniel, G. Poff, and Z. A. Memish.2005. A National Guard outbreak ofBurkholderia cepaciainfection and colonization secondary to intrinsic contamination of albu-terol nebulization solution. Am. J. Infect. Control33:182–188.
3.Brisse, S., C. Cordevant, P. Vandamme, P. Bidet, C. Loukil, G. Chabanon, M. Lange, and E. Bingen.2004. Species distribution and ribotype diversity of Burkholderia cepaciacomplex isolates from French patients with cystic fibro-sis. J. Clin. Microbiol.42:4824–4827.
4.Campana, S., G. Taccetti, N. Ravenni, F. Favari, L. Cariani, A. Sciacca, D. Savoia, A. Collura, E. Fiscarelli, G. De Intinis, M. Busetti, A. Cipolloni, A. d’Aprile, E. Provenzano, I. Collebrusco, P. Frontini, G. Stassi, M. Tran-cassini, D. Tovagliari, A. Lavitola, C. J. Doherty, T. Coenye, J. R. W. Govan, and P. Vandamme.2005. Transmission ofBurkholderia cepacia complex: evidence for new epidemic clones infecting cystic fibrosis patients in Italy. J. Clin. Microbiol.43:5136–5142.
5.Coenye, T., and P. Vandamme.2003. Diversity and significance ofBurkholderia cepaciaoccupying diverse ecological niches. Environ. Microbiol.5:719–729. 6.Cunha, M. V., J. H. Leita˜o, E. Mahenthiralingam, P. Vandamme, L. Lito, C.
Barreto, M. J. Salgado, and I. Sa´-Correia. 2003. Molecular analysis of Burkholderia cepaciacomplex isolates from a Portuguese cystic fibrosis cen-ter: a seven-year study. J. Clin. Microbiol.41:4113–4120.
7.Directorate for the Quality of Medicines of the Council of Europe.European Pharmacopoeia, 4th ed. Council of Europe, Strasbourg, France.
8.Dolan, S., E. Dowell, S. Valdez, J. J. LiPuma, and J. James.2005. An outbreak ofBurkholderia cepaciacomplex associated with an intrinsically contaminated nasal spray product. Am. J. Infect. Control33:e110–e111. 9.Ghazal, S. S., K. Al-Mudaimeegh, and E. M. A. Fakihi.2006. Outbreak of
Burkholderia cepaciabacteremia in immunocompetent children caused by contaminated nebulized sulbutamol in Saudi Arabia. Am. J. Infect. Control
34:394–398.
10.Holmes, B.1986. The identification ofPseudomonas cepaciaand its occur-rence in clinical material. J. Appl. Bacteriol.61:299–314.
11.Hutchinson, G. R., S. Parker, J. A. Pryor, F. Duncan-Skingle, P. N. Hoffman, M. E. Hodson, M. E. Kaufmann, and T. L. Pitt.1996. Home-use nebulizers: a potential source ofBurkholderia cepaciaand other colistin-resistant, gram-neg-ative bacteria in patients with cystic fibrosis. J. Clin. Microbiol.34:584–587. 12.Jacobson, M., R. Wray, D. Kovach, D. Henry, D. Speert, and A. Matlow.
2006. Sustained endemicity ofBurkholderia cepaciacomplex in a pediatric institution, associated with contaminated ultrasound gel. Infect. Control Hosp. Epidemiol.27:362–366.
13.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam.2001. Disproportionate distribution ofBurkholderia ce-paciacomplex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med.164:92–96.
14.LiPuma, J. J., T. Spilker, T. Coenye, and C. F. Gonzalez.2002. An epidemic Burkholderia cepaciacomplex strain identified in soil. Lancet359:2002–2003. 15.Lyczak, J. B., C. L. Cannon, and G. B. Pier.2002. Lung infections associated
with cystic fibrosis. Clin. Microbiol. Rev.15:194–222.
16.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme.2000. DNA-based diagnostic approaches for identification ofBurkholderia cepacia complex,Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol.38:3165–3173.
17.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg.2005. The multi-farious, multirepliconBurkholderia cepaciacomplex. Nat. Rev. Microbiol.
3:144–156.
18.Matrician, L., G. Ange, S. Burns, W. L. Fanning, C. Kioski, G. C. Cage, and K. K. Komatsu.2000. Outbreak of nosocomialBurkholderia cepacia infec-tion and colonizainfec-tion associated with intrinsically contaminated mouthwash. Infect. Control Hosp. Epidemiol.21:739–741.
19.Oie, S., and A. Kamiya.1996. Microbial contamination of antiseptics and disinfectants. Am. J. Infect. Control.24:389–395.
20.Pallud, C., V. Viallard, J. Balandreau, P. Normand, and G. Grundmann.
2001. Combined use of a specific probe and PCAT medium to study Burk-holderiain soil. J. Microbiol. Methods47:25–34.
21.Reik, R., T. Spilker, and J. J. LiPuma.2005. Distribution ofBurkholderia FIG. 2. (A) Ribopatterns generated by B. cepacia isolates from
contaminated saline solutions or from CF patients. Lanes: 1,/HindIII molecular size standard; 2, ribotype 17; 3, ribotype 20; 4, ribotype 18; 5, ribotype 19. Ribotypes 19 and 17 are common to isolates from saline solutions and CF patients. (B) RFLP-PFGE profile II, generated by sevenB. cepaciaisolates that gave rise to ribopattern 19, isolated from contaminated saline solutions or from CF patients. Lanes: 1, bacterio-phage lambda concatemer molecular size standards; 2, saline solution isolate; 3, IST4173 (CF); 4, IST4162 (CF); 5, IST4152 (CF); 6, IST4171 (CF); 7, IST4199 (CF); 8, IST4202 (CF).
on May 16, 2020 by guest
http://jcm.asm.org/
[image:5.585.68.259.69.229.2]cepaciacomplex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol.43:2926–2928.
22.Richau, J. A., J. H. Leita˜o, M. Correia, L. Lito, M. J. Salgado, C. Barreto, P. Cescutti, and I. Sa´-Correia.2000. Molecular typing and exopolysaccharide biosynthesis ofBurkholderia cepaciaisolates from a Portuguese cystic fibrosis center. J. Clin. Microbiol.38:1651–1655.
23.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam.
2002. Epidemiology ofBurkholderia cepaciacomplex in patients with cystic fibrosis in Canada: geographical distribution and clustering of strains. Emerg. Infect. Dis.8:181–187.
24.Vanlaere, E., T. Coenye, E. Samyn, C. Van den Plas, J. Govan, F. De Baets, K. De Boeck, C. Knoop, and P. Vandamme.2005. A novel strategy for the isolation and identification of environmentalBurkholderia cepaciacomplex bacteria. FEMS Microbiol. Lett.249:303–307.