Evaluation of
Aspergillus
-Specific Lateral-Flow Device Test
Using Serum and Bronchoalveolar Lavage Fluid for Diagnosis
of Chronic Pulmonary Aspergillosis
Takahiro Takazono,a,bYuya Ito,bMasato Tashiro,aKeitaro Nishimura,aTomomi Saijo,bKazuko Yamamoto,b Yoshifumi Imamura,bTaiga Miyazaki,a,bKatsunori Yanagihara,cHiroshi Mukae,bKoichi Izumikawaa
aDepartment of Infectious Diseases, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
bDepartment of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
cDepartment of Laboratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
ABSTRACT The Aspergillus-specific lateral-flow device (AspLFD) test is a newly devel-oped point-of-care diagnostic method for invasive pulmonary aspergillosis. However, evi-dence of the diagnostic performance of the AspLFD for chronic pulmonary aspergillosis (CPA) is limited. Therefore, we conducted a retrospective study to investigate this in comparison with the galactomannan (GM) -D-glucan (BDG) test. Fifty patients with chronic pulmonary aspergillosis and 65 patients with respiratory disease, as a control, were enrolled in this study. The majority of the CPA disease entities were chronic pul-monary aspergillosis (64.0%,n⫽32), followed by subacute invasive pulmonary aspergil-losis (IPA) (20.0%,n⫽10) and simple pulmonary aspergilloma (SPA) (16.0%,n⫽8). The sensitivity and specificity of the AspLFD test in serum samples were 62.0% and 67.7%, respectively. The GM test (cutoff index, 1.54) showed a sensitivity of 22% and a specific-ity of 92.3%, while the sensitivspecific-ity and specificspecific-ity of the BDG test (cutoff, 19.3 pg/ml) were 48% and 90.8%, respectively. In bronchoalveolar lavage fluid samples, the AspLFD test showed a sensitivity of 66.7% and a specificity of 69.2%, while those of the GM test (cut-off index, 0.6) were 72.7% and 83.1%, respectively. TheAspergillusprecipitating antibody test had 70% sensitivity. Unlike theAspergillusprecipitating antibody test, the AspLFD on serum samples showed similar sensitivity to non-fumigatus Aspergillus species. Patients with false-positive results for the AspLFD on serum samples were of a significantly higher age and had a higher prevalence of cavitary lesions in chest computed tomogra-phy than patients with negative results in the control group. Given the results in this study, the performance of the AspLFD using serum was acceptable as a point-of-care test for the diagnosis of CPA.
KEYWORDS -D-glucan, AspLFD,Aspergillus,AspergillusIgG antibody, chronic pulmonary aspergillosis, diagnosis, galactomannan, lateral flow device
C
hronic pulmonary aspergillosis (CPA) is a slowly progressive disease that presents a variety of clinical courses and radiological findings. The definitive diagnosis of CPA is not easy because of the low sensitivity of mycological culture (1–4) and the difficulty of performing histopathological examination due to patients’ severe general condition. Therefore, serodiagnosis is indispensable for the diagnosis of CPA. However, the galactomannan (GM) assay does not possess high sensitivity or specificity for the diagnosis of CPA (1, 3), unlike the Aspergillus IgG precipitating antibody test (1). AspergillusIgG antibody enzyme-linked immunosorbent assays (ELISAs) are available in several countries, and some of them are reported to show a high sensitivity and specificity for the diagnosis of CPA, though evidence for diagnosing CPA caused by non-fumigatus Aspergillusstrains is lacking (5–7).CitationTakazono T, Ito Y, Tashiro M,
Nishimura K, Saijo T, Yamamoto K, Imamura Y, Miyazaki T, Yanagihara K, Mukae H, Izumikawa K. 2019. Evaluation ofAspergillus-specific lateral-flow device test using serum and bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol 57:e00095-19.https://doi.org/10 .1128/JCM.00095-19.
EditorDavid W. Warnock
Copyright© 2019 American Society for
Microbiology.All Rights Reserved.
Address correspondence to Takahiro Takazono, takahiro-takazono@nagasaki-u.ac.jp.
Received19 January 2019
Returned for modification11 February 2019
Accepted27 February 2019
Accepted manuscript posted online6
March 2019
Published
crossm
26 April 2019
on May 17, 2020 by guest
http://jcm.asm.org/
The number of CPA patients is estimated to be up to 3,000,000 per year worldwide (8); however, some CPA cases are considered to be undiagnosed in resource-limited countries, and therefore, point-of-care (POC) diagnostic methods are required (9). The diagnostic tests described above need at least half a day of turnaround time. The Aspergillus-specific lateral-flow device (AspLFD) is a newly developed POC diagnostic method for IPA. It uses the mouse monoclonal antibody JF5, which binds to a protein epitope present on an extracellular glycoprotein antigen secreted constitutively during the active growth ofAspergillus fumigatus. This test can detectAspergillusantigens in human serum within 15 min (10). An early clinical trial showed that the AspLFD test is comparable to the GM test in serum in terms of diagnosing IPA, with sensitivity and specificity of 81.8% and 98%, respectively (11). However, evidence of the diagnostic performance of the AspLFD for CPA is limited. Therefore, we conducted a retrospective study to investigate the diagnostic performance of the AspLFD in serum and bron-choalveolar lavage (BAL) fluid in comparison with the GM assay in serum/BAL fluid and the-D-glucan (BDG) assay in serum.
MATERIALS AND METHODS
Patients.CPA patients who were diagnosed between 2008 and 2017 and had available stored serum or bronchoalveolar lavage (BAL) fluid samples taken before antifungal treatments were enrolled in this study. Patients with respiratory diseases and without Aspergillus infection who underwent all the following tests for the diagnosis of respiratory infectious diseases in the same period were enrolled as control group patients: the required tests were bronchoscopy and serum/BAL fluid GM, BDG, and Aspergillusprecipitating antibody assays. BAL was performed, using 20 to 40 ml of normal saline, at the site of the pulmonary lesion. Clinical information, including sex, age, underlying diseases, medication, laboratory test results, culture results, and chest computed tomography (CT) findings, was retrospectively collected from medical records. This study was approved by the Nagasaki University School of Medicine Research Ethics Committee (approval number 15032356-2).
Case definition.Criteria for CPA were based on the diagnostic criteria of the European Society for Clinical Microbiology and Infectious Diseases/European Respiratory Society guidelines, including (i) one or more cavities with or without a fungal ball present or nodules identified on computed tomography scan and (ii) direct evidence ofAspergillusinfection or positive result ofAspergillusprecipitating antibody assay (12). Patients with CPA were further classified into simple pulmonary aspergillosis (13), chronic cavitary aspergillosis (CCPA), and subacute invasive aspergillosis (SAIA) groups according to the guide-lines (12).
AspLFD.The newly improved, CE-markedAspergilluslateral flow device (AspLFD; OLM Diagnostics, Newcastle upon Tyne, United Kingdom) was used in accordance with the manufacturer’s protocol. Serum and BAL fluid samples stored at⫺80°C were thawed, vortexed, and centrifuged for 1 min at 14,000⫻g. Serum samples were mixed with sample buffer (provided by OLM) at a ratio of 1:2 and heated in the heat block at 100°C for 3 min. Amounts of 70l of treated serum and untreated BAL fluid samples were applied to the port of the cassette, and the results read 15 min later. To eliminate bias, two interpreters read the AspLFD test results without knowing the diagnosis. If the two readings did not match, a third interpreter blinded to clinical information determined the results.
Galactomannan assay,-D-glucan assay, andAspergillusprecipitating antibody.The Platelia Aspergillusenzyme immunoassay (EIA) (Bio-Rad, Tokyo, Japan) was used for the GM assay in serum and BAL fluid samples. The FSK1Aspergillusimmunodiffusion system (Microgen Bioproducts Ltd., United Kingdom) was used forAspergillusprecipitating antibody assays in serum samples according to the manufacturer’s instructions. The Fungitec G test MK-II (Nissui, Tokyo, Japan) was used for BDG assays in plasma samples according to the manufacturer’s instructions; briefly, amounts of 5l of plasma samples were mixed with the pretreatment solution and heated at 37°C for 10 min in a 96-well plate. After a heating step, amounts of 100l of reactive solutions were added to the samples and the mixtures stirred for 60 s and then heated again at 37°C for 30 min. The absorbance was measured at 405/492 nm. All the tests were performed by technicians who were blinded to the patients’ detailed conditions.
Statistical analysis.The differences in categorical variables or continuous numbers were analyzed by Fisher’s exact test or unpaired ttest. APvalue of⬍0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve analyses were performed on data from GM and BDG assays, and areas under the curves (AUCs), including 95% confidence intervals, were evaluated to assess the diagnostic ability to distinguish between CPA patients and control group patients with respiratory diseases. The Youden index was applied to determine the optimized cutoff in ROC curve analysis.
RESULTS
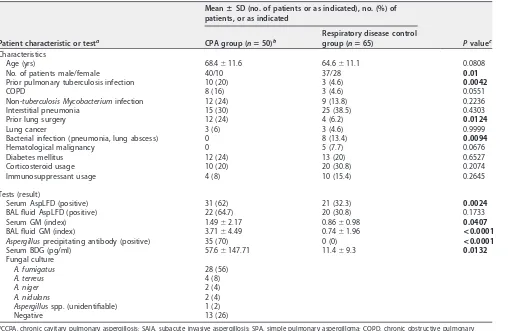
Patients’ characteristics.A total of 50 CPA patients and 65 disease control patients were enrolled, and the clinical characteristics of these patients are summarized in Table 1. The majority of CPA disease entities were CCPA (64%,n⫽32), followed by SAIA (20%, n⫽10) and SPA (16%,n⫽8). Bronchoscopy was performed in 41 of 50 CPA patients, and BAL stock samples were available in 34 cases. The underlying pulmonary diseases
on May 17, 2020 by guest
http://jcm.asm.org/
were interstitial pneumonia, nontuberculosis mycobacterium infection, prior pulmo-nary tuberculosis infection, chronic obstructive pulmopulmo-nary diseases, and diabetes mellitus. Steroid or immunosuppressant use was observed as a systemic immunosup-pressive factor, but no patient had neutropenia. A. fumigatus (56%) was the main pathogen isolated in sputum or BAL samples; 13 cases (26%) were culture negative.
AspLFD.The sensitivity and specificity of theAspergillus-specific lateral-flow device (AspLFD) were 62% and 67.7%, respectively, in serum samples and 66.7% and 69.2%, respectively, in BAL fluid samples (Table 2). In serum samples, the concordance rates of the LFD test with the GM test (cutoff index, 1.54) and the BDG test (cutoff, 19.3 pg/ml) were 65.2 and 60.0%, respectively. In BAL fluid samples, the concordance rate of the LFD test with the GM test (cutoff index, 0.6) was 64.3%. Cause analysis of AspLFD false-positive cases in the control group was performed; the patients with AspLFD false-positive results were significantly older and had higher prevalences of a cavitary lesion(s) in the chest CT than the patients with negative results in the control group (Table 3). In addition, these patients showed significantly higher GM indices than patients with negative AspLFD results. The diagnostic performances for the use of AspLFD in combination with GM or BDG tests are summarized in Table 2.
[image:3.585.43.551.80.411.2]GM antigen, -D-glucan, and Aspergillus precipitating antibody assays. The sensitivity and specificity of the serum GM antigen assay were 64% and 43.1%, respectively, at a cutoff of 0.5 and 34% and 72.3% at a cutoff of 1.0. The optimized cutoff index of this assay from the ROC curve was 1.54, with a sensitivity of 22% and a specificity of 92.3%, while the BAL fluid GM antigen assay showed higher sensitivity and TABLE 1Characteristics of patients enrolled in this study and results for each test
Patient characteristic or testa
MeanⴞSD (no. of patients or as indicated), no. (%) of patients, or as indicated
Pvaluec CPA group (nⴝ50)b
Respiratory disease control group (nⴝ65)
Characteristics
Age (yrs) 68.4⫾11.6 64.6⫾11.1 0.0808
No. of patients male/female 40/10 37/28 0.01
Prior pulmonary tuberculosis infection 10 (20) 3 (4.6) 0.0042
COPD 8 (16) 3 (4.6) 0.0551
Non-tuberculosis Mycobacteriuminfection 12 (24) 9 (13.8) 0.2236
Interstitial pneumonia 15 (30) 25 (38.5) 0.4303
Prior lung surgery 12 (24) 4 (6.2) 0.0124
Lung cancer 3 (6) 3 (4.6) 0.9999
Bacterial infection (pneumonia, lung abscess) 0 8 (13.4) 0.0094
Hematological malignancy 0 5 (7.7) 0.0676
Diabetes mellitus 12 (24) 13 (20) 0.6527
Corticosteroid usage 10 (20) 20 (30.8) 0.2074
Immunosuppressant usage 4 (8) 10 (15.4) 0.2645
Tests (result)
Serum AspLFD (positive) 31 (62) 21 (32.3) 0.0024 BAL fluid AspLFD (positive) 22 (64.7) 20 (30.8) 0.1733
Serum GM (index) 1.49⫾2.17 0.86⫾0.98 0.0407
BAL fluid GM (index) 3.71⫾4.49 0.74⫾1.96 <0.0001
Aspergillusprecipitating antibody (positive) 35 (70) 0 (0) <0.0001
Serum BDG (pg/ml) 57.6⫾147.71 11.4⫾9.3 0.0132 Fungal culture
A. fumigatus 28 (56)
A. terreus 4 (8)
A. niger 2 (4)
A. nidulans 2 (4)
Aspergillus spp. (unidentifiable) 1 (2)
Negative 13 (26)
aCCPA, chronic cavitary pulmonary aspergillosis; SAIA, subacute invasive aspergillosis; SPA, simple pulmonary aspergilloma; COPD, chronic obstructive pulmonary
disease; AspLFD,Aspergillus-specific lateral-flow device; BAL, bronchoalveolar lavage: GM, galactomannan; BDG,-D-glucan. bCategories of CPA and proportions of patients were as follows: CCPA, 32 (64%); SAIA, 10 (20%); and SPA, 8 (16%).
cAPvalue of⬍0.05 (Fisher’s exact test or unpairedttest) was considered significant. Values in boldface are significant.
on May 17, 2020 by guest
http://jcm.asm.org/
specificity of 72.7% and 83.1%, respectively, at a cutoff index of 0.6 optimized from the ROC curve (Table 2). The AUCs of the ROC curves were 0.561 and 0.824, respectively (Fig. 1A and B). The serum BDG assay showed low sensitivity (40.6%) and high specificity (90.7%) at the 20-pg/ml cutoff value, which is the optimized cutoff value for TABLE 2Diagnostic performances of the AspLFD, GM, and BDG tests
Sample type, testa
Value (%) for:
Sensitivity Specificity
Positive predictive value
Negative predictive value Serum (n⫽115)
AspLFD 62 67.7 59.6 69.8
GM (COI, 0.5) 64 43.1 46.3 60.8
GM (COI, 1.0) 34 72.3 48.5 58.8
GM (COI, 1.54) 22 92.3 70.6 61.2
BDG (cutoff, 19.3 pg/ml) 48 90.8 80 69.4
BDG (cutoff, 20.0 pg/ml) 46 90.7 79.3 68.6
Aspergillusprecipitating antibody 70 81.3
AspLFD and GM (COI, 1.54) 22 95.3 71.4 61.3
AspLFD or GM (COI, 1.54) 62 61.5 57.4 68.8
AspLFD and BDG (cutoff, 19.3 pg/ml) 32 96.9 88.8 64.9 AspLFD or BDG (cutoff, 19.3 pg/ml) 78 61.5 60.9 78.4
BAL (n⫽98)
AspLFD 66.7 69.2 52.4 80.3
GM (COI, 0.5) 84.8 78.5 66.7 71.8
GM (COI, 0.6) 72.7 83.1 71.1 77.1
GM (COI, 1.0) 57.6 90.1 76 80.8
AspLFD and GM (COI, 0.6) 57.5 95.3 86.3 81.5
AspLFD or GM (COI, 0.6) 66.7 56.9 44 77.1
aAspLFD,Aspergillus-specific lateral-flow device test; GM, galactomannan; BAL, bronchoalveolar lavage; BDG,-D-glucan; COI, cutoff index.
TABLE 3Cause analysis of false-positive serum AspLFD results in control group
Patient characteristic or testa
MeanⴞSD (no. of patients with serum LFD result or as indicated), no. (%) of patients with serum LFD result, or as indicated
Pvalueb False positive (nⴝ21) Negative (nⴝ44)
Characteristics
Age (yrs) 68.7⫾8.5 62.7⫾11.7 0.0375
No. of patients male/female 14/7 21/23 0.189
Prior pulmonary tuberculosis infection 2 (9.5) 0 (0) 0.101
COPD 1 (4.8) 2 (4.5) ⬎0.999
Non-tuberculosis Mycobacteriuminfection 4 (19) 5 (11.4) 0.4546
Interstitial pneumonia 6 (28.6) 19 (43.2) 0.289
Prior lung surgery 0 (0) 4 (9) 0.306
Lung cancer 1 (4.8) 2 (4.5) ⬎0.999
Cavitary legion 10 (47.6) 6 (13.6) 0.0051
Bacterial infection (pneumonia, lung abscess) 4 (19) 5 (11.4) 0.443
Hematological malignancy 1 (4.8) 4 (9) ⬎0.999
Diabetes mellitus 5 (23.8) 8 (18.1) 0.7416
Corticosteroid usage 4 (19) 16 (36.4) 0.25
Immunosuppressant usage 3 (14.3) 7 (15.9) ⬎0.999
Penicilliumspp. culture positive in BAL fluid 3 (14.3) 3 (6.8) 0.378
Tests (result)
BAL fluid AspLFD (positive) 6 (28.6) 13 (29.5) ⬎0.999
Serum GM 1.27⫾1.35 0.6705⫾0.71 0.0209
Serum GM (positive; COI, 1.54) 3 (14.3) 2 (4.5) 0.3181
BAL fluid GM 1.2⫾2.4 0.506⫾1.74 0.1907
BAL fluid GM (positive; COI, 0.6) 8 (38.1) 3 (6.8) 0.0033
Serum BDG (pg/ml) 10.6⫾5.9 11.8⫾10.6 0.6373
Serum BDG (positive; cutoff, 19.3 pg/ml) 2 (9.5) 4 (9) ⬎0.999
aCOPD, chronic obstructive pulmonary disease; BAL, bronchoalveolar lavage; AspLFD,Aspergillus-specific lateral-flow device test; GM, galactomannan; COI, cutoff index;
BDG,-D-glucan.
bAPvalue of⬍0.05 (Fisher’s exact test or unpairedttest) was considered significant. Values in boldface are significant.
on May 17, 2020 by guest
http://jcm.asm.org/
[image:4.585.43.548.423.712.2]Candidiasis. The optimized CPA cutoff value here was 19.3 pg/ml, with 48% sensitivity and 90.8% specificity. Its AUC for the ROC curve was 0.721, which was superior to that of the serum GM assay. TheAspergillusprecipitating antibody assay showed a sensitivity of 70%.
Positivity rates for non-fumigatus Aspergillusspp.The positivity rates of each test in nine CPA patients with disease caused by non-fumigatus Aspergillusspp. are sum-marized in Table 4. The sensitivity of the LFD for serum samples was 66.7%, similar to the result forA. fumigatus(62.9%). The BDG assay (cutoff, 19.3 pg/ml) showed results similar to those of the LFD. However, the GM assay (cutoff index, 1.54) and the
[image:5.585.47.406.72.430.2]FIG 1Receiver operating characteristic curves of galactomannan (GM) antigen tests in serum and bronchoalveolar lavage (BAL) fluid and-D-glucan test in serum. Areas under the curves (95% confidence interval) for serum GM (A), bronchoalveolar GM (B), and serum-D-glucan test (C) were 0.561 (0.4545 to 0.6679), 0.824 (0.7322 to 0.9163), and 0.721 (0.6259 to 0.8156), respectively.
TABLE 4Positivity rate of each test in patients with CPA caused by non-fumigatus Aspergillusspp.
Testa No. of patients positive/no. tested (%) Serum AspLFD 6/9 (66.7)
BAL fluid AspLFD 5/8 (62.5) Serum GM (COI, 1.54) 2/9 (22.2) BAL fluid GM (COI, 0.6) 3/9 (33.3) Serum BDG (cutoff, 19.3 pg/ml) 6/9 (66.7)
Aspergillusprecipitating antibody 2/9 (22.2)
aAspLFD,Aspergillus-specific lateral-flow device test; BAL, bronchoalveolar lavage; GM, galactomannan; BDG,
-D-glucan.
on May 17, 2020 by guest
http://jcm.asm.org/
[image:5.585.40.374.648.720.2]Aspergillusprecipitating antibody assay both showed a low sensitivity of 22.2% in this population.
DISCUSSION
In this study, the serum AspLFD assay showed better performance than the serum GM or BDG assay for the diagnosis of CPA. Moreover, its sensitivity was not inferior to that of theAspergillusprecipitating antibody assay, and unlike theAspergillus precipi-tating antibody assay, it did not show any species specificity. The AspLFD also showed a high false-positive rate of 32% in the respiratory disease control group; however, since two factors, higher age and cavitary lesions, observed as false-positive factors in the cause analysis are also the common risk factors of CPA (12, 14), these cases could be undiagnosed CPA cases. The sensitivity and specificity of the AspLFD for serum samples were similar to those from a recent study in nonneutropenic invasive pulmonary aspergillosis (IPA) patients (15).
On the other hand, the diagnostic performance of the AspLFD in BAL fluid samples was similar to its performance in serum samples and, moreover, slightly inferior to that of the GM assay in BAL fluid samples. In addition, given its nature as a POC test, the utility of the AspLFD for BAL fluid samples might be limited. A recent study assessed the performance of the AspLFD in BAL fluid samples and reported that the LFD was positive in only 7% of CPA patients (16), which was an extremely low sensitivity compared to recent studies for the diagnosis of IPA (15, 17). However, the sensitivity of the BAL fluid GM index was also lower (41% at a cutoff value of 0.5) in that study (16) than in our study or other previous studies (18–20). The proportion of each disease entity of CPA in that study did not differ from those in our study. However, the volume of lavage fluid used for BAL was not described in the study; it is possible that a difference in lavage fluid volume affected the result of each test. Standardization of the method for bronchial washing is desirable; however, it might be difficult, as the amount of fluid recovered varies depending on the location of the focus of infection in the lungs. Due to the nature of the lateral-flow device’s qualitative test, there could be a difference in interpreting the positive line.
This study has several limitations, including the small size of the patient cohort and the fact that it is a single-center retrospective study. Another limitation is that Asper-gillusIgG antibody ELISAs were not used for comparison, as these are not commercially available in Japan. The essential limitation in this research area is the difficulty of CPA diagnosis, as gold standards of diagnosis, such as histopathological findings and fungal culture tests, are difficult to satisfy; additionally, the utility of theAspergillusantibody test for non-fumigatus Aspergillusspp. might be limited.
In conclusion, to the best of our knowledge, this is the first study to evaluate the performance of the AspLFD in serum for the diagnosis of CPA. Given the results reported here, the performance of AspLFD was not sufficiently good but was tolerable for the diagnosis of CPA; moreover, the best advantage of this test over other serodiagnostic tests is its short turnaround time, the most important requirement for point-of-care test. The AspLFD has potential as a screening test for CPA in clinics or resource-limited countries, but further prospective studies with large numbers of patients are required.
ACKNOWLEDGMENTS
Newly improved LFD tests used in this study were purchased from OLM Diagnostics in 2018. OLM Diagnostics was not involved in planning the study design, in data collection, analysis, or interpretation, or in the writing of the manuscript.
T. Takazono and K. Izumikawa planned the research design and drafted the manuscript. Y. Ito and K. Nishimura tested the patients’ samples. M. Tashiro, T. Saijo, K. Yamamoto, Y. Imamura, and T. Miyazaki collected patient’s clinical data. K. Yanagihara and H. Mukae revised the manuscript. All authors read and approved the submitted version.
The authors declare no conflicts of interest associated with the manuscript.
on May 17, 2020 by guest
http://jcm.asm.org/
REFERENCES
1. Kitasato Y, Tao Y, Hoshino T, Tachibana K, Inoshima N, Yoshida M, Takata S, Okabayashi K, Kawasaki M, Iwanaga T, Aizawa H. 2009. Comparison of Aspergillus galactomannan antigen testing with a new cut-off index and Aspergillus precipitating antibody testing for the diagnosis of chronic pulmonary aspergillosis. Respirology 14: 701–708.https://doi.org/10.1111/j.1440-1843.2009.01548.x. 2. Kohno S, Izumikawa K, Ogawa K, Kurashima A, Okimoto N, Amitani R,
Kakeya H, Niki Y, Miyazaki Y, Japan Chronic Pulmonary Aspergillosis Study Goup (JCPASG). 2010. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: a multicenter trial in Japan. J Infect 61:410 – 418.https://doi.org/10.1016/j.jinf.2010.08.005.
3. Nam HS, Jeon K, Um SW, Suh GY, Chung MP, Kim H, Kwon OJ, Koh WJ. 2010. Clinical characteristics and treatment outcomes of chronic necro-tizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis 14:e479 – e482.https://doi.org/10.1016/j.ijid.2009.07.011.
4. Shin B, Koh WJ, Jeong BH, Yoo H, Park HY, Suh GY, Kwon OJ, Jeon K. 2014. Serum galactomannan antigen test for the diagnosis of chronic pulmonary aspergillosis. J Infect 68:494 – 499.https://doi.org/10.1016/j .jinf.2014.01.005.
5. Baxter CG, Denning DW, Jones AM, Todd A, Moore CB, Richardson MD. 2013. Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin Microbiol Infect 19:E197–E204.https://doi.org/10.1111/1469-0691.12133.
6. Fujiuchi S, Fujita Y, Suzuki H, Doushita K, Kuroda H, Takahashi M, Yamazaki Y, Tsuji T, Fujikane T, Osanai S, Sasaki T, Ohsaki Y. 2016. Evaluation of a quantitative serological assay for diagnosing chronic pulmonary aspergillosis. J Clin Microbiol 54:1496 –1499.https://doi.org/ 10.1128/JCM.01475-15.
7. Page ID, Baxter C, Hennequin C, Richardson MD, van Hoeyveld E, van Toorenenbergen AW, Denning DW. 2018. Receiver operating character-istic curve analysis of four Aspergillus-specific IgG assays for the diag-nosis of chronic pulmonary aspergillosis. Diagn Microbiol Infect Dis 91:47–51.https://doi.org/10.1016/j.diagmicrobio.2018.01.001.
8. Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 3:E57.https://doi.org/10.3390/jof3040057.
9. Denning DW, Page ID, Chakaya J, Jabeen K, Jude CM, Cornet M, Alastruey-Izquierdo A, Bongomin F, Bowyer P, Chakrabarti A, Gago S, Guto J, Hochhegger B, Hoenigl M, Irfan M, Irurhe N, Izumikawa K, Kirenga B, Manduku V, Moazam S, Oladele RO, Richardson MD, Tudela JLR, Rozaliyani A, Salzer HJF, Sawyer R, Simukulwa NF, Skrahina A, Sriruttan C, Setianingrum F, Wilopo BAP, Cole DC, Getahun H. 2018. Case definition of chronic pulmonary aspergillosis in resource-constrained settings. Emerg Infect Dis 24:1312.https://doi.org/10.3201/eid2408.17-1312. 10. Thornton CR. 2008. Development of an immunochromatographic
lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol 15:1095–1105.https://doi.org/10.1128/CVI.00068-08. 11. White PL, Parr C, Thornton C, Barnes RA. 2013. Evaluation of real-time
PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol 51:1510 –1516.https://doi.org/10.1128/JCM.03189-12.
12. Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, on behalf of the European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45– 68. https://doi.org/10.1183/13993003.00583-2015.
13. Chong GM, van der Beek MT, von Dem Borne PA, Boelens J, Steel E, Kampinga GA, Span LF, Lagrou K, Maertens JA, Dingemans GJ, Gaajetaan GR, van Tegelen DW, Cornelissen JJ, Vonk AG, Rijnders BJ. 2016. PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bron-choalveolar lavage: a multicentre validation of the AsperGenius assay(R) in 201 patients with haematological disease suspected for invasive aspergillosis. J Antimicrob Chemother 71:3528 –3535.https://doi.org/10 .1093/jac/dkw323.
14. Smith NL, Denning DW. 2011. Underlying conditions in chronic pulmo-nary aspergillosis including simple aspergilloma. Eur Respir J 37: 865– 872.https://doi.org/10.1183/09031936.00054810.
15. Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. 2018. Point-of care diagnosis of invasive aspergillosis in in non-neutropenic patients: Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycoses 62:230 –236. https://doi.org/10.1111/myc.12881.
16. Salzer HJF, Prattes J, Flick H, Reimann M, Heyckendorf J, Kalsdorf B, Obersteiner S, Gaede KI, Herzmann C, Johnson GL, Lange C, Hoenigl M. 2018. Evaluation of galactomannan testing, the Aspergillus-specific lateral-flow device test and levels of cytokines in bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. Front Microbiol 9:2223.https://doi.org/10.3389/fmicb.2018.02223.
17. Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. 2 November 2018. Bronchoalveolar lavage Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test for diagnosis of invasive pulmonary aspergillosis in patients with hematological malig-nancies. J Infecthttps://doi.org/10.1016/j.jinf.2018.10.014.
18. Park SY, Lee SO, Choi SH, Jeong JY, Sung H, Kim MN, Choi CM, Hong SB, Oh YM, Shim TS, Lim CM, Koh Y, Kim DS, Kim YS, Woo JH, Kim SH. 2011. Serum and bronchoalveolar lavage fluid galactomannan assays in pa-tients with pulmonary aspergilloma. Clin Infect Dis 52:e149 – e152. https://doi.org/10.1093/cid/cir027.
19. Izumikawa K, Yamamoto Y, Mihara T, Takazono T, Morinaga Y, Kurihara S, Nakamura S, Imamura Y, Miyazaki T, Nishino T, Tsukamoto M, Kakeya H, Yanagihara K, Mine M, Yasuoka A, Tashiro T, Kohno S. 2012. Bronchoalveolar lavage galactomannan for the diagnosis of chronic pulmonary aspergillosis. Med Mycol 50:811– 817.https://doi.org/10.3109/13693786.2012.682228. 20. Urabe N, Sakamoto S, Sano G, Suzuki J, Hebisawa A, Nakamura Y,
Koyama K, Ishii Y, Tateda K, Homma S. 2017. Usefulness of two Aspergillus PCR assays and Aspergillus galactomannan and beta-D -glucan testing of bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol 55:1738 –1746. https://doi.org/10.1128/JCM.02497-16.