EVALUATION OF ANTICANCER AND ANTIOXIDANT ACTIVITIES
OF LEAF EXTRACT OF DESMODIUM TRIQUETRUM
Dr. Siju E. N.*, Deepika K. T., Rahul K., Dr. Hariraj N. and Minil M.
College of Pharmaceutical Sciences, Govt. Medical College, Pariyaram, Kannur, Kerala,
India.
ABSTRACT
The Desmodium triquetrum (L.), (Tadehagi triquetrum), is a species of
flowering plant in the family Fabaceae. It belongs to the sub family
Faboideae. The antioxidant properties elicited by the extract may be
attributed to the presence of flavonoids. Flavonoids have been found to
possess antimutagenic and antimalignant effects. Moreover it has
protective effect against cancer by their effect on signal transduction in
cell proliferation and angiogenesis. Therefore the anticancer property
of D. triquetrum may be due to the presence of flavonoids. In vitro
antioxidant activity was evaluated by methods like reducing power
assay and DPPH assay. The extract showed good antioxidant activity
comparable to that of ascorbic acid. In vitro anticancer study of the methanolic extract of D.
triquetrum was evaluated by brine shrimp lethality assay, MTT assay, Alamar blue assay, and
Trypan blue assay in HeLa cells. The results indicated that the methanolic extract of D.
triquetrum possessed good anticancer property when compared to the standard drug 5-
Fluorouracil.
KEYWORDS: Desmodium Triquetrum, Antioxidant and Antimalignant Effects.
INTRODUCTION
Cancer is a class of diseases characterized by out-of-control cell growth. There are over 100
different types of cancer, and each is classified by the type of cell that is initially affected.
Cancer treatment depends on the type of cancer, the stage of the cancer (how much it has
spread), age, health status, and additional personal characteristics. Medicinal plants have long
played vital roles in the treatment of diseases all over the world. Recently, due to beneficial
effects of antioxidants, particularly natural antioxidants, in the treatment and prevention of
Volume 8, Issue 9, 1260-1283. Research Article ISSN 2277– 7105
Article Received on 14 June 2019,
Revised on 05 July 2019, Accepted on 26 July 2019,
DOI: 10.20959/wjpr20199-15524
*Corresponding Author Prof. Dr. Siju E. N.
College of Pharmaceutical
Sciences, Govt. Medical
College, Pariyaram, Kannur,
diseases, there has been a considerable interest in finding natural antioxidants from plant
sources. The studies on medicinal plants show that most of them possess significant
antioxidant activity.
Antioxidants are chemicals that interact with and neutralize free radicals, thus preventing
them from causing damage. Antioxidants are also known as “free radical scavengers.” Free
radicals are highly reactive chemicals that have the potential to harm cells. They are created
when an atom or a molecule (a chemical that has two or more atoms) either gains or losses an
electron (a small negatively charged particle found in atoms). Free radicals are formed
naturally in the body and play an important role in many normal cellular processes. At high
concentrations, however, free radicals can be hazardous to the body and damage all major
components of cells, including DNA, proteins, and cell membranes. The damage to cells
caused by free radicals, especially the damage to DNA, may play a role in the development of
cancer and other health conditions. The aim of this study is to establish the scientific
authenticity of antiproliferative and antioxidant activities of methanolic leaf extract of
Desmodium triquetrum (L.).
MATERIALS AND METHODS
Collection of plant material: The leaves of Desmodium triquetrum were obtained from
Pariyaram, Kannur Kerala (India) in the month of October 2016 and authenticated by Dr.
Ratheesh Narayanan M.K, Department of Botany, Payyanur College, Kannur, Kerala. A
voucher specimen (APSC/COL/10/2016) was deposited in the Department of Pharmacology,
Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala. After
authentication the plants were collected, cleaned and dried in shade at room temperature. The
dried leaves were pulverized in a mechanical grinder to obtain coarse powder.[1]
Preparation of extracts
Hydroalcoholic extract: The powdered plant (500g) was sieved through sieve No.10 and the
powder was subjected to defatting with petroleum ether for 6 hours. The filtered powder was
then subjected to cold maceration with methanol for 7 continuous days. The methanolic
extract was prepared by mixing with the help of a sonicator. It was then filtered through a
muslin cloth and marc was discarded. The filtrate was concentrated using a rotary vacuum
Pharmacognostic studies
Physicochemical parameters
1. Ash content
A. Total ash: About 2.0 g of powder of dried leaves of D. triquetrum was accurately weighed
and transferred to pre weighed silica crucible and was ignited with a flame of Bunsen burner,
for about 1 hour. The charred material was heated in muffle furnace for four hours at a
temperature not exceeding 4500C. The ash formed was white and free from carbon. It was
cooled and weighed on ash less filter paper.
% Total ash value = Weight of total ash/ Weight of crude drug taken X 100
B. Acid insoluble ash: The total ash obtained for powder of dried leaves D. triquetrum of
was boiled with 25 ml of dilute hydrochloric acid (2N HCl) for 5 mins. The contents of the
beaker were filtered and collected the insoluble matter on an ashless filter paper, washed with
hot water and ignited in a tared crucible at a temperature not exceeding 4500C for 4 h until a
constant weight was obtained. Cooled in a desiccator and weighed. Subtracted the weight of
the insoluble matters from the total weight of ash. The percentage of acid insoluble ash with
reference to the air-dried drug was calculated.
% Acid insoluble ash value = (Weight of total ash- Weight of acid insoluble ash)/Weight of
crude drug taken X 100
C. Water soluble ash: The ash obtained as described for the determination of total ash was
boiled for 5 min with 25 mL of water. The insoluble matter was collected on ash less filter
paper and washed with hot water. The insoluble ash was then transferred into silica crucible,
ignited for 15 min, and weighed. The procedure was repeated to get a constant weight. The
weight of insoluble matter was subtracted from the weight of the total ash. The difference of
weight was considered as water-soluble ash. The percentage of water soluble ash was
calculated with reference to the air dried sample.
% Water soluble ash = (Weight of total ash- weight of water soluble ash)/Weight of crude
drug taken X 100
2. Loss on drying: About 2.0 g powder of D. triquetrum dried leaves of was accurately
weighed and transferred to pre-weighed glass petridish. The powder was distributed evenly.
The petridish was kept in the hot air oven, for about 2 hours, at 100 to 105°C. It was then
cooled in a desiccator and weighed. It was heated until a constant weight was obtained. The
% Loss on drying = Loss of weight of sample/Weight of sample X 100
3. Extractive values
A. Alcohol soluble extractive: About 1.0 g powder of dried leaves of D. triquetrum was
accurately weighed in stopper conical flask. To the flask, 10.0 ml of ethanol was added and
was allowed to stand for 18 hours with occasional shaking. The contents of the flask were
then filtered through Whatman No.1 filter paper in separate pre-weighed dry beakers and
filtrate was evaporated to dryness on a water bath. The dried residue was then weighed and
the percentage extractive value was calculated.
B. Water soluble extractive: About 1.0 g powder of the dried leaves of D. triquetrum was
accurately weighed in a stopper conical flask. To the flask, 10.0 ml of water was added and
was allowed to stand for 18 hours with occasional shaking. The contents of the flask was then
filtered through Whattman No.1 filter paper in pre-weighed dry beaker and the filtrate was
evaporated to dryness on a water bath. The dried residue was then weighed and the
percentage extractive value was calculated.
Phytochemical screening[3][4][5]
A) Test for Alkaloids: Dragendroff’s Test: Extract was treated with Dragendroff’s reagent
(potassium bismuth iodide solution). Formation of orange brown precipitate indicates the
presence of alkaloids.
• Hager’s Test: Extract was treated with Hager’s reagent (saturated picric acid solution).
Formation of a yellow colored precipitate indicates the presence of alkaloids.
• Mayer’s Test: Extracts was treated with Mayer’s reagent (potassium mercuric iodide
solution). Formation of a cream coloured precipitate indicates the presence of alkaloids.
•Wagner’s Test: Extract was treated with Wagner’s reagent (iodine potassium solution).
Formation of reddish brown precipitate indicates the presence of alkaloid.
B). Test for Carbohydrates
• Molisch Test: Extract was treated with Molisch’s reagent (α- naphthol in 95% ethanol) and
few drops of concentrated H2SO4 were added through the sides of the test tube. Appearance
• Fehling’s Test: A small portion of the extract was treated with Fehling’s reagent (copper sulphate in water) and Fehling’s reagent B (sodium potassium tartarate) and heated in a water
bath. Formation of red colour precipitate indicated the presence of reducing sugars.
• Barfoed’s Test: Extract was treated with Barfoed’s reagent (copper acetate in water and
glacial acetic acid), and heated in a water bath. Red coloured precipitate indicates the
presence of monosaccharides.
• Benedict’s Test: Extract was treated with Benedict’s reagent (copper sulphate + sodium
citrate + sodium carbonate in water) and heated for 10 minutes. Red coloured precipitate
indicates the presence of reducing sugars.
C) Tests for proteins and amino acids
• Biuret test: Extract was treated with 10% NaOH and a few drops of 1% CuSO4 solution.
Mix well. Pink to violet colour indicates the presence of proteins.
• Millon’s test: Extract was treated with 5 ml Millon’s reagent. White precipitate obtained
when warmed turns brick red or precipitate dissolves giving red colour indicates presence of
proteins.
• Xanthoprotein test (for protein containing tyrosine or tryptophan): Extract was treated
with 1 ml concentrated HNO3. White precipitate indicates presence of protein with a benzene
nucleus.
• Ninhydrin test: Extract was treated with 3 drops of 0.1% ninhydrin solution and heated in
boiling water bath for 10 min. Purple or deep blue color indicates presence of amino acids.
D) Test for Flavonoids
• Ferric Chloride Test: Extract was treated with few drops of ferric chloride solution.
Formation of blackish blue colour indicates the presence of flavonoids.
• Lead acetate Test: Extract was treated with lead acetate solution; yellow precipitate
indicates the presence of flavonoids.
• Alkaline reagent Test: Extract was treated with few drops of NaOH. Formation of intense
yellow colour which becomes colourless on addition of dilute acid indicates the presence of
• Ammonia Test: Expose a filter paper dipped in an alcoholic solution of the extract to the
vapours of ammonia. Appearance of a yellow colour shows presence of flavonoids.
• Extract was treated with 10 ml ethyl acetate in boiling water for 3 mins. This mixture was
filtered and filtrate is shaken with 1ml of 1% AlCl3. Light yellow colour indicates the
presence of flavonoids. The yellow solution turns colourless on adding dil. NaOH and HCl
which confirmed the presence of flavonoids.
E) Test for Saponins
• Froth Test: Diluted 1ml of extract with distilled water to 20ml and shaken in a graduated
cylinder for 15mins. Formation of 1 cm layer of foam indicates the presence of saponins.
• Foam Test: Extract (0.5g) was shaken with 2ml of water vigorously. If the foam produced
persists for 10 mins it indicates the presence of saponins.
• Haemolysis Test: Added solution of the extract to one drop of blood placed on glass slide
and observed for appearance of haemolytic zone.
F) Test for Triterpenoid
• Hirchorn Test: 1ml of extract was warmed with trichloroacetic acid. A yellow colour,
which changed to red was formed indicates the presence of triterpenoid.
G) Test for steroids
• Salkowski reaction: To 2 ml of extract, 2 ml chloroform and 2 ml concentrated H2SO4 were
added. Shook well, appearance of deep red-purple colour in chloroform layer and deep green
flouresence in acidic layer indicates the presence of steroids.
H) Tests for tannins and phenolic compounds To 2-3ml test solution, added few drops of
following solutions and was looked for respective coloration or precipitate: 5% ferric chloride
solution: Deep blue black colour. Lead acetate solution: White precipitate. Gelatin solution:
White precipitate. Bromine water: Discoloration of bromine water. Acetic acid solution: Red
color solution. Potassium dichromate: Red precipitate. Dilute iodine solution: Transient red
I) Tests for glycosides[4]
General test for glycoside: Part A: To 2-3 ml of extracts dil. H2SO4 was added and heated
on a water bath for 1- 2 min. Neutralize with 10% NaOH, check with litmus paper and to
resulting solution add Fehling’s solution A & B. Intense red precipitate indicates presence of
glycosides.
Part B: To 2-3 ml of extract, water was added and heated. According to need, NaOH was
added for neutralization and also added equal quantity of water. To the resulting solution
added Fehling’s solution A & B. Increased red precipitate indicates absence of glycosides.
Compare A and B.
I. Test for cardiac glycosides
•Legal’s test: To aqueous or alcoholic test solution, added 1 ml pyridine and 1 ml sodium
nitroprusside. Pink to red colour indicates presence of cardenolides.
•Test for deoxysugars (Kellar Killani test): To 2 ml extract added glacial acetic acid, one
drop of 5% FeCl3 and concentrated H2SO4. Reddish brown colour at junction of the two
liquids and bluish green colour in the upper layer indicates presence of deoxysugars.
II. Test for anthraquinone glycosides
Borntrager’s Test: Extract the sample with ether or any water immiscible organic solvent by
heating and filter. Add NaOH or NH3 and make it alkaline. Pink, red or violet colour in
aqueous layer indicates presence of anthraquinone glycosides.
Elemental analysis
The macro elements such as Sodium (Na), Potassium (K) and Calcium (Ca) were determined
using a flame photometer (Systronics), where as the elements such as Iron (Fe), Copper (Cu),
Manganese (Mn), Zinc (Zn), Cobalt (Co), Nickel (Ni) and Cadmium (Cd) were determined
using flame Atomic Absorption Spectrophotometer (AAS model-400 Perkin Elmer). The
toxic heavy metals such as Arsenic (As), Lead (Pb) and Mercury (Hg) were determined using
a hydride generator attached to AAS.
Digestion and preparation of sample
The raw drugs were washed with distilled water and dried at 120 º C in an electric oven till a
constant weight was obtained. The dried material was then ground to powder. The powdered
and 5ml of HNO3 was also added into an empty flask, which served as blank. The flasks
were covered with watch glasses and heated to reflux on an electric hot plate at 80ºC to
100ºC. After heating for one hour the contents of flask were treated with additional 5ml of
HNO3 followed by 2 ml of 30% H2O2 and gently swirled till the clear solution was obtained.
Diluted with deionized water and filtered (Whatman no. 42) into volumetric flasks marked as
sample solution.
Elemental analysis using Atomic Absorption Spectrophotometer (AAS)
The elemental analysis of digested samples was done by AAS. The elements like Fe, Cu, Mn,
Ni, Zn, Co and Cd were analyzed. In this method, the sample in the form of a homogeneous
liquid was introduced into flame, where thermal and chemical reactions create “free” atoms
capable of absorbing, emitting or fluorescing at characteristic wavelength. Flame
spectroscopy can be subdivided into the different processes, to give us flame emission,
atomic absorption spectroscopy and atomic fluorescence spectroscopy. In AAS the majority
of free atoms in the commonly used flames were in the ground state, but the flame did not
have enough energy to excite these atoms. A light source emitting a narrow spectral line of
the characteristic energy is used to excite the free atoms formed in the flame. The decrease in
energy (absorption) is then measured. The absorption is proportional to the concentration of
free atoms in the flame, given by the Lambert-Beer law. 28
Absorbance = log10 10 /It = K x C x L
Where,
I0 = Intensity of incident radiation.
It = Intensity of transmitted radiation.
C = Concentration of sample (free atoms).
K = Constant (can be determined experimentally). L = Path length.
Working standard solutions of Fe, Cu, Mn, Ni, Zn, Co and Cd were prepared from stock
standard solution of 1000ppm. The standards are then analyzed and their absorbance
recorded. The calibration can be performed in the concentration mode in which case the
concentration of the sample is read off directly. Calibration of the instrument was repeated
periodically during operation. A blank was also taken and necessary correction was made
In vitro determination of antioxidant activity
a) Reducing power assay[6]
Reducing power activity is often used to evaluate the ability of natural antioxidants to donate
electron. Many reports have revealed that there is a direct correlation between antioxidant
activities and reducing power of certain plant extracts.
Procedure
Extracts were prepared in different concentrations (10-100) and 1 ml of each in distilled
water were mixed with phosphate buffer (2.5 ml, 2 M, pH 6.6) and potassium ferricyanide
(2.5 ml, 1%). The mixture was incubated at 50 0C for 20 min. A portion (2.5 ml) of
trichloroacetic acid (TCA, 10%) was added to the mixture which was then centrifuged at
1500 rpm for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5
ml) and FeCl3 (0.5 ml, 0.1%), and the absorbance was measured at 700 nm. Increased
absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was
used as standard.
Reducing power (%) =[(Ac-At)/Ac] ×100 Ac: Absorbance of the control
At: Absorbance of the extracts/standard.
b) Estimation of radical scavenging activity (RSA) using DPPH assay[7]
The antioxidant potential of the extracts were determined via scavenging activity of stable 2,
2-diphenyl-1-picrylhydrazyl (DPPH) free radical. DPPH, a stable free radical at room
temperature, produces a violet colour in methanol. When the free radical reacts with an
antioxidant, its free radical property is lost due to chain breakage and its colour changes to
light yellow.
Procedure
Different concentrations of 25-200 mg/ml of extracts were added, in equal volume, to 0.1
mM ethanolic DPPH solution. The mixture was shaken vigorously and allowed to stand for
20 min in the dark at room temperature. Absorbance was monitored at 517 nm. DPPH
solution without extract served as the control. α- tocopherol was used as the standard for the
concentration range as considered for the sample. DPPH radical scavenging activity % was
calculated for the sample and the standard using the following formula,
% scavenging activity= (Absorbance of control – Absorbance of sample)/Absorbance control
Cytotoxicity assay[8]
a) Preliminary screening by brine shrimp lethality assay: Brine shrimp lethality assay is a
convenient method for general screening for toxicity of the extracts or compounds towards
brine shrimp (Artemia salina) and it can give an indication regarding possible cytotoxicity of
the test samples.
Procedure: Artificial sea water was prepared by dissolving 38g of NaCl (3.8%) in 1000 ml
of distilled water and was filtered off to obtain a clear solution. The dried cysts of the brine
shrimps were hatched in artificial sea water with constant aeration and light for 48 hours. The
extract was dissolved in sea water and transferred to test tubes to obtain concentrations of
1.25, 2.5, 5, 10, 20 and 40 mg/ ml in 5 ml artificial sea water with 20 nauplii in each test tube.
Standard drug vincristine sulphate was used as positive control at concentrations of 0.312,
0.625, 1.25, 2.5, 5 and 10 mg/ml. Experiments were conducted in triplicate and the average
value was noted. Artificial seawater was used as the control. After 24 h incubation at
25-30°C, the number of viable nauplii was counted using a magnifying glass. The percent (%)
mortality was calculated using the following formula
% Mortality = Nt/No x 100
Where, Nt = Number of dead nauplii after 24 hrs of incubation, N0 = Number of total nauplii
transferred (n = 20).
The percentage of mortality was plotted against concentration. Using the linear regression
equation of the graph, the concentration that would kill 50% of the larvae i.e median lethal
concentration (LC50) was determined using GraphPad Prism software.
b) Cytotoxicity evaluation in HeLa cells by MTT assay[9]
Cell line: The human cervical cancer cell lines (HeLa) was obtained from National Centre for
Cell Science (NCCS), Pune and grown in Eagles Minimum Essential Medium containing
10% fetal bovine serum (FBS). The cells were maintained at 370C, 5% CO2, 95% air and
100% relative humidity. Maintenance cultures were passaged weekly, and the culture
medium was changed twice a week.
Cell treatment procedure
The monolayer cells were detached with Trypsin-Ethylenediaminetetraacetic acid (EDTA) to
make single cell suspensions. Viable cells were counted using a hemocytometer and diluted
microlitres per well of cell suspension were seeded into 96-well plates at plating density of
10,000 cells/well and incubated to allow for cell attachment at 370C, 5% CO2, 95% air and
100% relative humidity. After 24 hours the cells were treated with serial concentrations of the
test samples. They were initially dissolved in neat dimethylsulfoxide (DMSO) and an
aliquot of the sample solution was diluted to twice the desired final maximum test
concentration with serum free medium. Additional four serial dilutions were made to provide
a total of five sample concentrations. Aliquots of 100 μl of these different sample dilutions
were added to the appropriate wells already containing 100 μl of medium, resulting in the
required final sample concentrations. Following sample addition, the plates were incubated
for an additional 48 h at 370C, 5% CO2, 95% air and 100% relative humidity. The medium
containing without samples were served as control and triplicate was maintained for all
concentrations.
MTT assay
3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide (MTT) is a yellow water
soluble tetrazolium salt. A mitochondrial enzyme in living cells, succinate-dehydrogenase,
cleaves the tetrazolium ring, converting the MTT to an insoluble purple formazan. Therefore,
the amount of formazan produced is directly proportional to the number of viable cells. After
48 h of incubation, 15μl of MTT (5mg/ml) in phosphate buffered saline (PBS) was added to
each well and incubated at 370C for 4h. The medium with MTT was then flicked off and the
formed formazan crystals were solubilized in 100μl of DMSO. Measured the absorbance at
570 nm using micro plate reader. The % cell inhibition was determined using the following
formula.
% Cell Inhibition = 100- Abs (sample)/Abs (control) x 100.
% Viability= Abs (sample)/ Abs (control) x 100
Using the linear regression graph plotted between % cytotoxicity and concentration, IC50
was determined using GraphPad Prism software.[10]
c) Assay Chemistry and Redox Principle[11]
Alamar Blue monitors the reducing environment of the living cell. It is a blue non-fluorescent
dye that is reduced to the pink-colored, highly fluorescent resorufin by mitochondrial
reductases. Resazurin solution is highly dichromatic based on Kreft's dichromaticity index
(DI). The dye acts as an intermediate electron acceptor in the electron transport chain without
Blue is +380 mV at pH 7.0, 25 °C. Alamar Blue, therefore, can be reduced by NADPH (Eo =
320 mV), FADH (Eo = 220 mV), FMNH (Eo = 210 mV), NADH (Eo = 320mV), as well as
the cytochromes (Eo = 290 mV to +80 mV). As the indicator dye accepts electrons, it
changes from the oxidized, non-fluorescent, blue state to the reduced, fluorescent, pink state.
In addition to mitochondrial reductases, other enzymes (such as the diaphorases (EC 1.8.1.4,
dihydrolipoamine dehydrogenase, NAD(P)H:quinone oxidoreductase (EC 1.6.99.2) and
flavin reductase located in the cytoplasm and the mitochondria may be able to reduce Alamar
Blue. The increase in dead cells reduces the ability of cells to convert resazurin to resorufin
which is correlated from the decrease in fluorescent intensity. Fluorescence signals are
measured at an excitation wavelength at 530– 560 nm and an emission wavelength at 590 nm
and correlated with untreated control cells.[12]
d) Cytotoxicity evaluation in HeLa cells by Trypan blue assay[13][14][15]
Trypan blue is a vital stain used to selectively color dead tissues or cells blue. Trypan blue is
recommended in dye exclusion procedures for viable cell counting based on the principle that
live (viable) cells actively pump out the dye by efflux mechanism whereas dead (non-viable)
cells do not. Hence in this assay, white transparent cells are viable cells and blue cells taking
up the dye are dead cells.[16]
Procedure[17][18]
700 μl of a cell suspension was transferred to 24 well plates and incubated for 24 hrs in 5%
CO2. After incubation, 300 μl of varying concentrations of extract and standard (25-100
μg/ml) was added and incubated for 24 hrs. 100 μl of cell suspension was taken in an Eppendorf tube and to that 100 μl of 0.4% trypan blue solution was added and mixed
thoroughly. It was allowed to stand for 5-15 minutes. A small amount of trypan blue-cell
suspension mixture was transferred to both chambers of a hemocytometer using a Pasteur
pipette. All the chambers were filled by capillary action and not overfilled. From chamber 1
of the hemocytometer, the cells in the 1 mm center square and four 1 mm corner squares were
counted. Non-viable cells stained with blue color. Viable and non-viable cells were counted
separately.
% Cytotoxicity = number of non-viable cells (stained)
Using the linear regression graph plotted between % cytotoxicity and concentration, IC50
was determined using GraphPad Prism software.
RESULTS
Pharmacognostic studies
Percentage yield
The percentage yield of the methanolic extract was found to be 7.93% w/w.
Physicochemical parameters
Table. 1: Physicochemical parameters of Desmodium triquetrum (L.) Preliminary
phytochemical screening.
Sl No. Physicochemical parameter Value (%)
1 Total ash 4.9
2 Acid insoluble ash 3.5
3 Water soluble ash 3.2
4 Loss on drying 7.1
[image:13.595.143.454.288.389.2]5 Water soluble extractive 11.9 6 Alcohol soluble extractive 15.01
Table. 2: Phytochemical constituents of Desmodium triquetrum (L.).
Sl No. Constituents Hydroalcoholic extract
1 Alkaloids -
2 Carbohydrates +
3 Proteins and Amino acids -
4 Flavonoids +
5 Saponins +
6 Triterpenoids -
7 Steroids -
8 Tannins and Phenolic compounds +
9 Glycosides -
(+): Present (-): Absent
Elemental analysis: The macronutrients like Sodium (Na), Potassium (K), Calcium (Ca) and
the micronutrient like Iron (Fe), Copper (Cu), Manganese (Mn), Zinc (Zn) and Cobalt (Co) in
extracts were well within normal ranges. These elements act as antioxidants and help in tissue
regeneration and cellular repair. Also the toxic elements like Nickel (Ni), Cadmium (Cd),
Lead (Pb), Mercury (Hg) and Arsenic (As) were estimated and results are tabulated in Table
[image:13.595.79.489.414.578.2]Table. 3: Elements present in methanolic extract of Desmodium triquetrum (L.).
Sl. No. Elements Methanolic extract (ppm)
1 Sodium (Na) 124
2 Pottassium (K) 213
3 Calcium (Ca) 80
4 Iron (Fe) 0.0821
5 Copper (Cu) 0.1567
6 Manganese (Mn) 0.0417
7 Zinc (Zn) 0.2984
8 Cobalt (Co) 0.0491
9 Nickel (Ni) 0.1862
10 Cadmium (Cd) 0.2461
11 Arsenic (As) 0.0184
12 Lead (Pb) 0.1451
13 Mercury (Hg) 0.0002
In Vitro Determination of Antioxidant Activity
Reducing power assay: The methanolic extract showed concentration dependent activity
with an IC50 value of 16.91 μg/ml. Ascorbic acid was found to have an IC50 value of 40.93
[image:14.595.73.525.420.617.2]μg/ml. The findings are tabulated in Table 4 and depicted in Figure 1.
Table. 4: In vitro determination of antioxidant activity by Reducing power assay.
Groups Concentration
(μg/ml)
Absorbance at 700 nm
Reducing
power (%) IC50 (μg/ml)
Control - 1.3684±0.002
40.93 Ascorbic
acid
12.5 0.8022±0.002 41.37
25 0.5483±0.001 59.93
50 0.3257±0.004 76.19
100 0.2240±0.002 83.63 200 0.1357±0.003 90.08
Methanolic extract
12.5 0.8901±0.002 34096
16.91
25 0.6612±0.001 51.76
50 0.5801±0.002 57.61
Figure. 1: In vitro antioxidant activity of D. triquetrum (L.) by Reducing power assay.
Standard : Ascorbic acid, MEDT : Methanolic extract of Desmodium triquetrum.
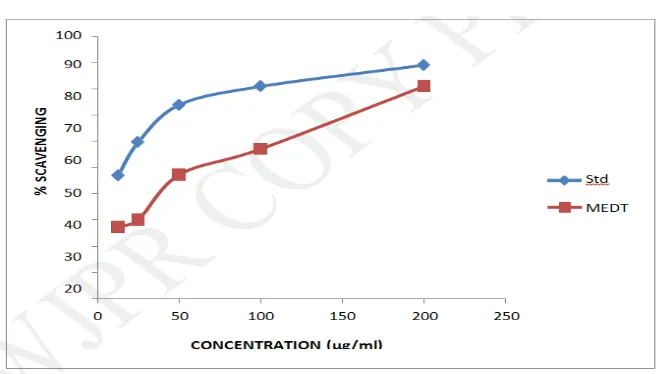
Estimation of radical scavenging activity (RSA) using DPPH assay
The methanolic extract showed concentration dependent activity with an IC50 value of 82.66
μg/ml. Ascorbic extract was found to have an IC50 value of 26.13 μg/ml. The findings are
tabulated in Table 5 and depicted in Figure 2.
Table. 5: In vitro determination of antioxidant activity by DPPH assay.
Groups Concentration
(μg/ml)
Absorbance at
700 nm % Scavenging
IC50 (μg/ml)
Control - 0.1364±0.001
26.13 Ascorbic acid
12.5 0.0724±0.002 46.92
25 0.0549±0.001 59.75
50 0.0356±0.004 73.9
100 0.0260±0.002 80.94 200 0.0149±0.003 89.08
Methanolic extract
12.5 0.9991±0.002 27.41
82.66
25 0.9536±0.001 30.13
50 0.7193±0.002 47.28
[image:15.595.145.455.72.252.2]Figure. 2: In vitro antioxidant activity of D. triquetrum (L.) by DPPH assay. Standard :
Ascorbic acid, MEDT : Methanolic extract of Desmodium triquetrum.
Cytotoxicity Assay
Preliminary screening by brine shrimp lethality assay
The metanolic extract showed concentration dependent mortality with an LC50 value of 12.21
μg/ml. 5- Fluorouracil was found to have an LC50 value of 3.25 μg/ml. The findings are
tabulated in Table 6 and depicted in Figure 3.
Table. 6: In vitro cytotoxic activity of Desmodium triquetrum (L.) by brine shrimp
lethality assay.
Groups Concentration
(μg/ml) % Mortality LC50 (μg/ml)
Control - 0
Methanolic extract
1.25 17.25±0.047
12.21
2.5 29.30±0.073
5 36.86±0.443
10 60.29±0.040
20 78.17±0.333
40 87.58±0.997
5- Fluorouracil
0.312 9.67±0.471
3.25 0.625 18.70±0.359
1.25 36.03±0.177
2.5 59.08±0.376
5 78.24±0.217
10 100
Figure. 3: In vitro cytotoxic activity of Desmodium triquetrum (L.) by brine shrimp
lethality assay. Test: Methanolic extract of Desmodium triquetrum. Std : 5- Fluorouracil.
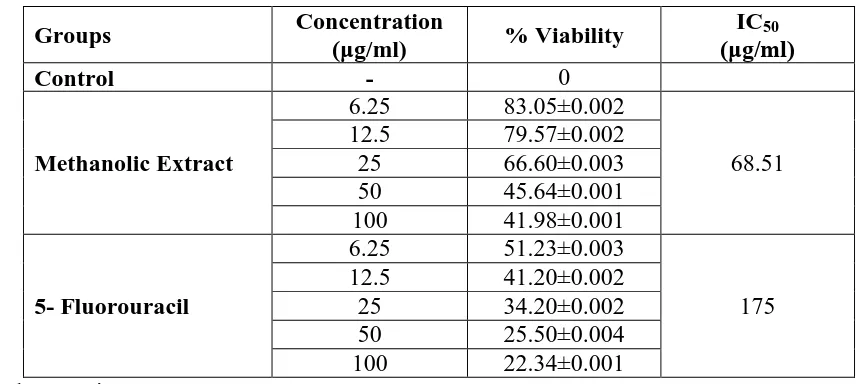
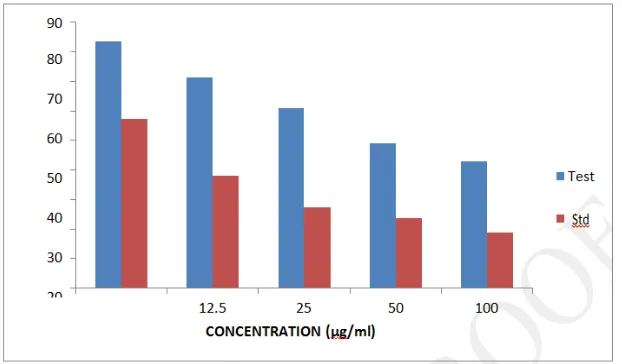
Cytotoxicity evaluation in HeLa cells by MTT assay: The methanolic extract showed
concentration dependent cytotoxicity on HeLa cells with an IC50 value of 68.51 μg/ml. 5-
[image:17.595.145.453.69.250.2]Fluorouracil was found to have an IC50 value of 175 μg/ml. The findings are tabulated in
Table 7 and depicted in Figure 4.
Table. 7: In vitro cytotoxic activity of Desmodium triquetrum (L.) by MTT assay.
Groups Concentration
(μg/ml) % Viability
IC50 (μg/ml)
Control - 0
Methanolic Extract
6.25 83.05±0.002
68.51 12.5 79.57±0.002
25 66.60±0.003
50 45.64±0.001
100 41.98±0.001
5- Fluorouracil
6.25 51.23±0.003
175 12.5 41.20±0.002
25 34.20±0.002
50 25.50±0.004
[image:17.595.82.508.425.619.2] [image:17.595.78.508.426.618.2]Figure. 4: In vitro cytotoxic activity of Desmodium triquetrum (L.) by MTT assay.
Test: Methanolic extract of Desmodium triquetrum, Std: 5- Fluorouracil.
Control 6.25 μg/ml 12.5 μg/ml
25 μg/ml 50 μg/ml 100 μg/ml
Figure. 5: Cell death in MEDT by MTT at various concentrations
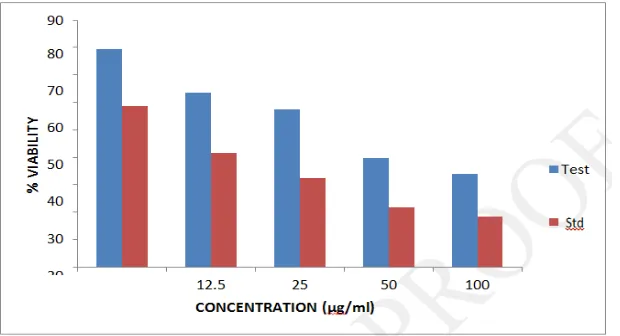
Cytotoxicity evaluation in HeLa cells by Alamar Blue assay
The methanolic extract showed concentration dependent cytotoxicity on HeLa cells with an
IC50 value of 68.60 μg/ml. 5- Fluorouracil was found to have an IC50 value of 15.51 μg/ml.
[image:18.595.114.480.314.578.2]Table. 8: In vitro cytotoxic activity of Desmodium triquetrum (L.) by Alamar blue assay.
Groups Concentration (μg/ml) % Viability IC50 (μg/ml)
Control - 0
Methanolic Extract
6.25 83.58455± 0.001
68.60 12.5 71.41229± 0.003
25 60.83597± 0.002 50 48.99303± 0.002 100 42.82457± 0.004
5- Fluorouracil
6.25 57.26409± 0.002
15.51 12.5 38.17606± 0.002
25 27.35909± 0.001 50 23.59721± 0.003 100 18.89804± 0.002 Values are in Mean ± SEM, n=3
Figure. 6: In vitro cytotoxic activity of Desmodium triquetrum (L.) by Alamar blue assay.
Test: Methanolic extract of Desmodium triquetrum, Std: 5- Fluorouracil.
Cytotoxicity evaluation in HeLa cells by Trypan blue assay
The methanolic extract showed concentration dependent cytotoxicity on HeLa cells with an
IC50 value of 49.91μg/ml. 5- Fluorouracil was found to have an IC50 value of 4.7 μg/ml. The
[image:19.595.145.456.301.483.2]Table. 9: In vitro cytotoxic activity of Desmodium triquetrum (L.)by trypan blue assay.
Groups Concentration (μg/ml) % Viability IC50 (μg/ml)
Control - 0
Methanolic Extract
6.25 79.36±0.004
49.91
12.5 63.51±0.003
25 57.45±0.004
50 39.80±0.003
100 33.84±0.005
5- Fluorouracil
6.25 58.67±0.003
4.7
12.5 41.65±0.003
25 32.26±0.002
50 21.67±0.001
100 18.49±0.001
[image:20.595.145.455.292.460.2]Values are in Mean ± SEM, n=3
Figure. 7: In vitro cytotoxic activity of Desmodium triquetrum (L.) by trypan blue assay.
Test: Methanolic extract of Desmodium triquetrum. Std: 5- Fluorouracil.
DISCUSSION
The leaves of D. triquetrum were collected and methanolic extract was prepared. The
percentage yield was found to be 7.93%. The physicochemical parameters like total ash, acid
insoluble ash, water soluble ash, loss on drying, water and alcohol soluble extractives were
studied. The methanolic extract showed positive results for the phytochemicals like
carbohydrates, flavonoids, saponins, tannins and phenolic compounds.
Lately the presence of heavy metals in medicines has been a cause of concern in the the
global scenario. Especially after the recent episodes where few Indian Ayurvedic
formulations have been shown to contain heavy metals in doses more than that of the
permissible limits as advised by W.H.O. and F.A.O. of U.S.A. In this study, percentage of
limits. Macronutrients like Iron (Fe), Copper (Cu), Manganese (Mn), Zinc (Zn) and Cobalt
(Co) are also well within the limit. These elements act as an antioxidants and help in tissue
regeneration and cellular replay, which was an additional advantage for the proposed study.
Toxic elements like Nickel (Ni), Cadmium (Cd), Lead (Pb), Mercury (Hg) and Arsenic (As)
are well within the limit and this ensures the safety of the study.
The antioxidant activity of methanolic leaf extract of D. triquetrum were studied using
methods like reducing power assay and DPPH assay. The extract exhibited a concentration
dependent antioxidant activity with an IC50 value of 16.91 μg/ml. Ascorbic acid which was used as the standard gave an IC50 value of 40.93 μg/ml by the reducing power assay. D.
triquetrum showed concentration dependent radical scavenging activity in DPPH assay with
IC50 value of 82.66 μg/ml. Ascorbic acid had an IC50 value of 26.13 μg/ml.
The present study clearly indicated that methanolic extract of D. triquetrum have appreciable
in vitro cytotoxic potential against HeLa (cervical cancer) cells in MTT assay and Almar blue
assay. The presence of flavonoids, polyphenols, and saponins (in isolation or in combination)
in the extract might be responsible for exhibiting anticancer effect. There are reports
indicating biological interactions of flavonoids, polyphenols, or phenolic compounds with
proteins, enzymes, and other biological processes in the cells that make them toxic to the cell
or serve as growth inhibitors. Flavonoids have been extensively studied because of their
numerous biological activities and have shown to have a chemopreventive role in cancer
through their effects on signal transduction in cell proliferation and angiogenesis.
Preliminary phytochemical screening revealed the presence of flavonoids, saponins and
phenols which, may be attributed as the cause of exhibiting antiproliferative property by D.
triquetrum. In the present study the methanolic extract of D. triquetrum was compared to that
of 5- Fluorouracil as standard in all the four methods adopted.
Brine shrimp lethality assay is a convenient method for general screening for toxicity of the
extracts or compounds towards brine shrimp (Artemia salina) and it can give an indication
regarding possible cytotoxicity of the test samples. The LC50 of methanolic extract by brine
shrimp lethality assay was found to be 25.23 μg/ml against the LC50 value of 10.11 μg/ml by
The colorimetric assay of MTT measures the reduction of
3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase. The MTT
that enters the cells and passes into mitochondria gets reduced to an insoluble, coloured
(purple) formazan product. Since redcution of MTT can only occur in metabolically active
cells, the level of activity is a measure of the viability of the cells.
The MTT assay showed a concentration dependent decrease in the % viability of HeLa cells
by the methanolic extract of D. triquetrum which is evident in the photographs provided. The
viability of cells decreased with increasing concentration. 5- Fluorouracil had an IC50 value
of 175 μg/ml, whereas methanolic extract of D. triquetrum possessed an IC50 value of 68.5111 μg/ml.
The alamar blue assay is used to assess the cell viability. It is based on the ability of the
membrane of viable cells to exclude the dye, while nonviable cells are stained blue. Almar
blue as a dye cannot enter cells through an intact membrane and therefore stains only cells,
which have punctured membranes.
In almar blue assay, a concentration dependent decrease in % viability was observed with the
methanolic extract of D. triquetrum showing IC50 value of 68.6046 μg/ml and 5- Fluorouracil showing an IC50 value of 15.5128 μg/ml.
The trypan blue assay is used to assess the cell viability. It is based on the ability of the
membrane of viable cells to exclude the dye, while nonviable cells are stained blue. Trypan
blue as a dye cannot enter cells through an intact membrane and therefore stains only cells,
which have punctured membranes. Since trypan blue has a macromolecular nature, the holes
in the membrane must be pretty big to let the stain molecules pass inside the cell – in other
words cell death, which is shown by membrane disintegration, must be fairly far progressed
to be detectable by the trypan blue method.60,61 In trypan blue assay, a concentration
dependent decrease in % viability was observed with the methanolic extract of D. triquetrum
showing IC50 value of 49.91 μg/ml and 5- Fluorouacil showing an IC50 value of 4.4 μg/ml.
CONCLUSION
The antioxidant properties elicited by the extract may be attributed to the presence of
flavonoids. Flavonoids have been found to possess antimutagenic and antimalignant effects.
proliferation and angiogenesis. Therefore the anticancer property of D. triquetrum may be
due to the presence of flavonoids. It is further suggested that in vivo studies and
characterization of fractionated extracts may be conducted in this extract, to establish its
effect. So it can be concluded that this species should be conserved and explored further.
ACKNOWLEDGEMENT
The authors Dr. Siju E N*, Mr. Rahul K, Dr.Hariraj N, Mr.Minil M are thankful to all
respected teaching and non teaching staff of the Academy of Pharmaceutical sciences,
Pariyaram who helped in various aspects. We are also thankful to Dr. Ratheesh Narayanan
MK, Assistant Professor, Department of Botany, Payyannur College for his help in the
authentification of plant for performing our work. We extend our thanks to Biogenics &
Molecular Research Center, for their help in various invitro studies.
REFERENCES
1. G.A. Kalyani, Purnima Ashok, A.D. Taranalli, C.K. Ramesh, V. Krishna, A.H.M
Viswanatha Swamy. Anti-inflammatory and in vitro antioxidant activity of Desmodium
triquetrum (L.). Indian pharmaceutical journal, 2011; 43(6): 740-741.
2. Gino A. Kurian, Thejeshvi Rajamani, Pavithra Ramanarayanan, Jose Paddikkala. A
comparative study on in vitro and in vivo antioxidant activities of aqueous extract of
Desmodium gangeticum (Leguminosae) root. International Journal of Green Pharmacy,
2009; 1(1): 324-331.
3. Khandelwal KR. Practical Pharmacognosy techniques and experiments, 2 ed. Pune: Nirali
Prakshan, 2000; 30-38: 149-156.
4. Prashanth T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K. Phytochemical Screening
and Extraction: A Review. Internationale Pharmaceutica Sciencia, 2011; 1(1): 98-106.
5. Anees AS and Seemi S. Natural Products Chemistry Practical Manual, 1st edition. New
Delhi: CBS Publishers and Distributors, 2008; 212-213.
6. G.A. Kalyani, Purnima Ashok, A.D. Taranalli, C.K. Ramesh, V. Krishna, A.H.M
Viswanatha Swamy. Anti-inflammatory and in vitro antioxidant activity of Desmodium
triquetrum (L.). Indian pharmaceutical journal, 2011; 43(6): 740-741.
7. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice Evans C. Antioxidant
activity applying an improved ABTS radical cation decolorization. Free Radic Biol Med.,
8. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to
proliferation and cytotoxicity assays. Journal of Immunological Method, 1983; 65: 55-63.
9. Eble MJ, Hensley FW, Flentje M. A modified computer assessed colorimetric microtitre
assay (MTT) to assess in vitro radiosensitivity of V79 and Hela cells. Int. J Radiant Biol.,
1994; 65: 193-201.
10.Karl Buch, Tanja Peters, Thomas Naworth, Markus Sanger. MTT assay to monitor drug
toxicity. Radiation Oncology, 2012; 36(2): 1186-1748.
11.J Wilson, T Prognan. Investigation of Alamar blue (resazurin) fluorescent dye for the
assessment of mammalian cell cytotoxicity. Eur. J. Biochem, 200; 267: 5421-5426.
12.SephranN. Rampersad. Multiple applications of Alamar blue as an indicator of metabolic
function and cellular health in cell viability biaassays. Sensors, 2012; 12(9):
12347-12360.
13.White MJ, Di Caprio, MJ Greenberg. Assessment of neuronal viability with alamar blue
in cortical and granule cell cultures. Neuroscience Meth, 1996; 70: 723-727.
14.Eric J, Feron MD, Alfons Van Lommel. Trypan blue staining of Epiretinal Membranes in
proliferative Vitreoretinopathy. Clinical Sciences, 2002; 120(2): 141-144.
15.P Gain, G Thuret, C Chiquet. Value of two mortality assessment techniques for organ
cultured corneal endothelium: trypan blue versus TUNEL technique.
16.AM Noel. A new fluorimetric assay for cytotoxicity measurements in vitro. Int. J. Oncol,
1993; 3: 473-476.
17.White MJ, Di Caprio, MJ Greenberg. Assessment of neuronal viability with alamar blue
in cortical and granule cell cultures. Neuroscience Meth, 1996; 70: 723-727.
18.Rice Evan CA. Formation of free radicals and mechanisms of action in normal
biochemical processes and pathological states. In: Free radical damage and its control.