Original Article
Isolation and characterization of CD105
+/CD90
+subpopulation in breast cancer MDA-MB-231 cell line
Xueliang Wang1, Yunjiang Liu2, Kaixuan Zhou3, Geng Zhang3, Feifei Wang4, Jingwen Ren4
1Department of Dermatologic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang 050011,
Hebei, P. R. China; 2Hebei Breast Disease Diagnosis and Treatment Center, The Fourth Hospital of Hebei Medical
University, Tianshan Road, Shijiazhuang 050035, Hebei, P. R. China; 3Research Center, The Fourth Hospital of
He-bei Medical University, Shijiazhuang 050011, HeHe-bei, P. R. China; 4Clinical laboratory, The Fourth Hospital of Hebei
Medical University, Shijiazhuang 050011, Hebei, P. R. China
Received February 10, 2015; Accepted April 12, 2015; Epub May 1, 2015; Published May 15, 2015
Abstract: Background: The epithelial-mesenchymal transition (EMT) generates cells with properties of stem cells, if that happened, the stem cell should be with mesenchymal property. This study aimed to identify a group of cells with mesenchymal stem cell (MSC)-like characteristics in breast cancer bone metastatic cell line MDA-MB-231, moreover, the relevance between breast cancer stem cells and the EMT was observed. CD105 and CD90, identified as the standards of MSCs, were used for the identification. Methods: The CD105+/CD90+ and CD105-/CD90-
sub-population of MDA-MB-231 cells were detected and sorted by flow cytometry. MSC-like characteristics in cell pro -liferation, migration and cell cycle were investigated here by MTT asaay, transwell migration assay, and PI staining respectively. The expression profiles of some stem cell-associated genes were also observed by quantitative real time PCR. Results: Around 0.99% and 90.77% of parental cells were identified as CD105+/CD90+ and CD105-/
CD90- cell subpopulations respectively. The CD105+/CD90+ cells exhibited stronger migratory capacity as
com-pared to parental and CD105-/CD90- cells, while less CD105+/CD90+ cells were arrested in the S phase. Besides,
pluripotent stem cell factors, like Oct-4, Nanog, Klf4 and Sox-2, were all upregulated in CD105+/CD90+ cells, with
also proliferation increase, as compared with other two populations. Conclusion: The CD105+/CD90+ subpopulation
from breast cancer MDA-MB-231 cells was proven to possess “mesenchymal stem cell-like” characteristics, and its high migratory ability might be associated with EMT. Moreover, using the surface markers of CD105 and CD90 for the identification of MSCs might provide new theoretical basis for the recurrence and metastasis of breast cancer.
Keywords: Breast neoplasm, CD105, CD90, cancer stem cell, epithelial mesenchymal transition, mesenchymal stem cells
Introduction
Cancer stem cells (CSCs) are believed to origi-nate from normal stem cells. Therefore, they possess similar biological characteristics and potential functions for chemoresistance, metastasis, and tumor recurrence. Endoglin (CD105) and thymus cell antigen 1 (Thy1,
CD90) have been identified as the standards of
mesenchymal stem cells (MSCs) by the International Association for Cell Therapy [1]. CD105 has an important role in angiogenesis
[2], and Saroufim had shown that tumoral
CD105 is an independent predictive marker for death risk and unfavourable prognosis in patients with clear-cell renal cell carcinomas
after curative resection [3]. Moreover, high expression of CD105 in breast tumor is associ-ated with poor overall and disease-free surviv-al, and can be an independent predictor. CD105 is also an important therapeutic target in meta-static breast cancer [4]. Yang showed that CD90 is a potential marker for liver CSCs [5]. Recent studies have demonstrated that epithe-lial–mesenchymal transition (EMT) has an important function in invasion and metastasis of most types of epithelial malignancies. The EMT is a latent embryonic process converting polarized and adjacent epithelial cells to mes-enchymal cells. This process, reversible by
-Characterization of CD105
+/CD90
+subpopulation in MDA-MB-231
ing the evolution, fusion, or generation of sec-ondary epithelia, which is essential to both morphogenesis and organogenesis [6]. EMT inducers, such as TWIST1 and SNAIL1, are
fre-quently detected at the stage of ductal carci -noma in situ long before metastatic dissemina-tion begins, thereby indicating a possible func-tion in tumor initiafunc-tion [7]. Expression of TWIST1 in luminal-committed mammary epithelial cells is helpful to promote breast carcinogenesis [8]. EMT induction has also been found to endow cells with stem-like properties [9]. Given that the combination of CD105 and CD90 is accept-ed to identify MSCs, the relevance between these two markers and EMT were observed in the present study, and the recurrence of breast cancer was also investigated.
Materials and methods
Cell culture
A human breast cancer cell line, MDA-MB-231, was obtained from Shanghai Institutes for Biological Science, Chinese Academy of Sci- ences (Shanghai, China). Cells were cultured in Leibovitz’s L-15 medium (GIBCO, USA) contain-ing 10% fetal bovine serum (FBS) and Penicillin/
Streptomycin, and at 37°C in a humidified 5%
CO2 atmosphere.
CD105 and CD90 expression analysis
At the logarithmic growth phase, cells were stained by CD105-RPE and CD90-FITC (BD PharMingen) antibodies. Their cell surface
marker expression was detected using flow
cytometry (BD FACSCalibur). Two groups of sub-populations, namely, CD105+/CD90+ and CD- 105-/CD90-, were sorted.
Cell proliferation analysis
The CD105+/CD90+ subpopulation, CD105-/ CD90- subpopulation, and MDA-MB-231 cell group (parental population) were cultured sepa-rately. Each population of cells was seeded in a 96-well culture plate with the same concentra-tion as that of the logarithmic growth phase. The sulforhodamine B (SRB) assay was used to compare and observe the proliferative capacity among the three subgroups [10]. The adherent cells were counted at different times for 9 d after seeding, and the growth curve was plotted.
Cell cycle analysis assay
Because stem cells are quiescent slow-cycling
cells, DNA content/cell cycle analysis of each population was performed, and the proportions of G0/G1, S, and G2/M phases were analyzed
by flow cytometry [11]. According to formers,
cells were trypsinized, washed with cold PBS
(phosphate-buffered saline), fixed with 70%
ethanol/PBS overnight at 4°C, and centrifuged.
The pellets were resuspended in 500 μl PBS in the presence of 50 μg/ml propidium iodide and
1 mg/ml DNase-free RNase A and incubated in the dark for 30 min at room temperature. Stained cells were washed once and resus-pended in PBS. The cell cycle data for individual
samples were acquired using the BD FACSCalibur flow cytometer (Becton-Dickinson,
San Jose, CA). The proportions of cells in G0/ G1, S, and G2/M phases were analyzed using dedicated software.
Cell migration and invasion assay
Cells (1×105) were seeded on Boyden cham-bers with 8.0 µm pore size (Transwell, Corning Life Sciences, Acton, MA, USA) in serum-free medium. Medium containing 20% FBS served as a chemoattractant in the lower chamber. After 24 h (for migration assays), non-invading cells were removed with cotton swabs. Invaded
cells were fixed with methanal, stained with
Giemsa solution and counted in five microscop
-ic fields for each well. Data are represented as
the mean ± SEM from three individual experi- ments.
RNA extraction, reverse transcription PCR, and quantitative real-time PCR
The expression of stemness genes Oct3/4,
Nanog, Sox2, and Klf4 were detected by quan -Table 1. Primers used for RT-PCR quantitation
Primers Sequences (5’-3’)
[image:2.612.92.289.82.230.2]titative real-time PCR in each cell subpopula-tion. Total RNA from cells was extracted using TRIzol (Invitrogen) and chloroform, and further
purified with an RNeasy kit (Qiagen).
App-roximately 500 ng of RNA was used for reverse transcription following the protocol of a SuperScript III First Strand Kit (Invitrogen). The resulting cDNA was diluted 12 times to perform real-time PCR with ABI SYBR Green PCR master mix (Kapa) in ABI Prism 7500 Real-Time PCR
System (PE Applied Bio systems). The quantita
-tive amount of stemness gene mRNA of the three populations was analyzed following the
manufacturer’s instructions, and acquired data
were analyzed by software 7500 version 2.0.6
(PE Applied Bio systems). All data were first nor -malized to that of internal control GAPDH, and then to the respective genes in the parental, double-negative, and double-positive cells. Error bars represent the standard deviation (SD) of at least three PCR experiments of each sample. Results were obtained in at least three
experiments. Primers used for RT-PCR quanti
-tation are listed in Table 1.
Statistical analysis
Statistical data were analyzed by SPSS13.0 software. Data were processed as the mean ±
SD. Statistical significance was determined by
one-way ANOVA or Kruskal-Wallis H test. Mean
differences between groups were compared by
LSD and chi-square test (χ2 test). P < 0.05 was
considered statistically significant (*represents
P < 0.05, **represents P < 0.01). Results
Cells identified with CD105+/CD90+ were iso -lated from MDA-MB-231
In the present study, cultured cells in the expo-nential phase were used. Flow cytometry showed that cells of the CD105+/CD90+ sub-population accounted for 0.99%, whereas those of the CD105-/CD90- subpopulation accounted for 90.77% (Figure 1A). Then, two subgroups of cells, namely, CD105+/CD90+ and CD105-/CD90-, were sorted by flow cytometry. Cells of the two subpopulations were cultured in 24-well plates. The number of cells sorted was initially very low. Only scattered cells were observed on the plates 4 h after sorting, and the double-negative cells were greater than the double-positive cells (Figure 1B).
CD105+/CD90+ subpopulation showed higher proliferation
[image:3.612.91.520.76.273.2]After 72 h incubation, it can be found that every single double-positive cell had formed a small clone. These cells started to cover the well at 3
Figure 1. Flow cytometry analysis of mesenchymal stem cell markers CD105 and CD90 in MDA-MB-231 cells. A. Results from flow cytometry analysis, with series of gates set for CD105+ or CD90+ identification. R2, R3, R4 and R5
represented cell subpopulations identified as CD105+/CD90+ (0.99%), CD105+/CD90- cell subpopulation (4.93%),
CD105-/CD90- cell subpopulation (90.77%) and CD105-/CD90+ cell subpopulation (3.31%) respectively.
Characterization of CD105
+/CD90
+subpopulation in MDA-MB-231
d, indicating that they were in the exponential phase. After 6 d, the double-negative cells reached the exponential phase. The two sub-groups of cells were separately planted on six-well plates at the same conditions and initial cell concentrations. The results show that the time in which the double-positive subpopula-tion achieved the logarithmic phase was less than that of the double-negative subgroup (Figure 2C). The cell growth curves of the dou-ble-positive, double-negative, and parental populations revealed that the proliferation rate
of the double-positive population was signifi -cantly higher than that of the other two populations.
CD105+/CD90+ subpopulation possessed slow cycling characteristics
[image:4.612.91.521.72.466.2]For cell cycle analysis, exponential phase cells of the three populations were collected to show the proportions of cells at G0/G1, S, and G2/M phases. And, proportions in different phases of the three populations were compared, and the results were statistically analyzed. The results indicated that the double-positive population had more cells in the G0/G1 and G2/M phase than the double-negative and parental popula-tions, with less cells observed in the S phase (Figure 2A, 2B). Given that stem cells are quies -cent with slow cycling characteristics, this
Figure 2. Cell proliferation assay and cell cycle analysis for CD105+/CD90+ subpopulation, with CD105-/CD90- cell
result showed that the CD105+/CD90+ cells contained much more stem-like cells than the other two subpopulations.
CD105+/CD90+ cells exhibited stronger migra-tory capacity and cell adhesion
When cells of the three populations were attached to the lower chamber after 72 h, the number of adherent cells from the double-posi-tive, parental, and double-negative populations was also counted, while the double-positive population had the highest number of cells that passed through the membranes at 72 h, com-pared with the other two populations (Figure 3A, 3B). In addition, the number of cells that passed through the polycarbonate membranes after 24 h from the double-positive, parental, and double-negative populations was also cal-culated. Findings showed that the number of double-positive population cells that passed
through the membrane was the highest com-pared with that of the other two populations (Figure 3C), whereas that of the double-nega-tive population was the least. Moreover, clus-ters of double-positive cells on the lower cham-ber were observed to attach well, with normal cell formation. By contrast, only a few scattered cells were observed on the plates of the other two populations, and the cells demonstrated weak adhesion.
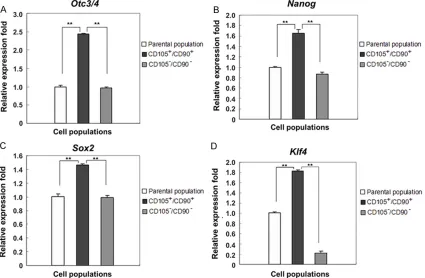
Stem cell-associated genes were overex -pressed in CD105+/CD90+ cells
Stem cell-associated genes, such as Oct-4,
Sox2, And Nanog, are recommended for identi-fying gastric CSCs [12]. Here, the expression of stemness genes Oct3/4, Nanog, Sox2, and also Klf4 were detected by quantitative
real-time PCR technology (RT-PCR). Among the three groups at the same imaging conditions, the
gra-Figure 3. Cell invasion and migration assay for CD105+/CD90+ subpopulation, with CD105-/CD90- cell
[image:5.612.91.522.75.383.2]Characterization of CD105
+/CD90
+subpopulation in MDA-MB-231
dation value of four genes expression was all the highest in the double-positive subpopula-tion, low in the parental populasubpopula-tion, and lowest in the double-negative population (Figure 4). For example, the expression level of Oct3/4 in the double-positive population was 2.448-fold higher than that in the parental population, and 2.517-fold higher than that in the double-nega-tive population (P < 0.05).
Discussion
Cancer stem cells (CSCs), important targets for therapeutic approaches, are a small subset of cells found in tumors that can self-renew and differentiate, initiate tumors, and induce tumor growth, recurrence, and metastasis [13]. Numerous biomarkers in breast cancer stem cell lines have been analyzed by researchers. Breast CSCs have been described to be immune-phenotypically characterized by CD- 44+CD24-/low ESA+Lin- [14], while not all breast CSCs have the same immunophenotype. Another study shows that the high expression of CD34 is associated with the progenitor cell of T-cell acute lymphocytic leukemia (T-ALL) [15]. Similary, CD34 is not expressed in each
cell line and also not essential for the self-renewal and multi-differentiation potential of CSCs [16]. The mechanism about how cells maintain complete systems during long-term culture in vitro, similar to the body, remains unknown.
Diagnostic use of CD105 indicates the impor-tant role in controlling the clinical signs of the breast cancer; moreover, it may be used in the
field of diagnostic follow-up, determining the
response to treatment and prognosis of the dis-ease [17]. CD90, another commonly used stem cell markers, is proven to be differentially expressed in some breast cancer cell lines and might be related to their malignancy grade [18]. Here, we selected this two cell surface mark-ers, CD105 and CD90, that expressing on the surface of MSCs, to analyze MDA-MB-231 breast cancer cells, derived from breast cancer patients with bone metastasis. Then, CD105+/ CD90+ subpopulation was characterized, and the relevance between breast cancer stem cells and the EMT was also observed.
Long-lived CSCs with indefinite proliferative
[image:6.612.98.523.74.352.2]potential are likely derived from transformed
adult stem cells, and are thought to share many characteristics with their parental population,
including a quiescent slow-cycling phenotype
[19]. Previous studies showed that stem cells
have the characteristics of quiescent cells,
such as corneal stem cells in mice and hair fol-licle stem cells. Stem cells in malignant tumor tissues with strong vitality are few and mostly in
the quiescent state, but have a key function in
regulating tumor growth, and can self-renew and proliferate under special stimulation [20]. This ability can help in protecting the consump-tion of immature cells in the body to confer malignant tumors with resistance to apoptosis and hinder traditional tumor therapies, such as radiotherapy and chemotherapy. This ability may also be the mechanism of tumor recur-rence and metastasis.
Cell cycles analysis of the three populations by
flow cytometry showed that most of the
CD105+/CD90+ cells were detected in the G0/
G1 phase, with significant differences observed
between the parental group and double-nega-tive group, indicating CD105+/CD90+ cells to be
quiescent slow-cycling cells. Additionally, as
higher rate of angiogenesis and cellular prolif-eration have been observed in malignant tumors compared to the benign tumors [21], the cell proliferative and migratory abilities were also assessed using cell growth curves and transwell assay. Taken together, results showed that the CD105+/CD90+ cells had high-er prolifhigh-erative and migratory abilities than the other two types of cells.
The epithelial-mesenchymal transition (EMT) is a process in which epithelial cells
trans-differ-entiate and acquire an invasive mesenchymal
phenotype. It has a central function in embryo-genesis and mesoderm differentiation into multiple tissue types during development [22]. The emergence of embryonic stem cell-associ-ated genes in high-grade undifferenticell-associ-ated can-cers suggests that aberrant regulations of EMT may have a function in CSC characteristics [23]. Many stem cells are found present in tumor microenvironment such as cancer stem cells (CSCs), mesenchymal stem cells (MSCs), all of which might be the inducers of EMT in tumor cells [24], meanwhile, clinical evidence has demonstrated that regulators of EMT in cancer cells was correlated with poor clinical
outcomes and tumor aggressiveness.
Quan-titative real-time PCR revealed that the CD105+/
CD90+ cells have highly expressed embryonic stem cell-associated genes, indicating the rel-evance between the two markers and EMT. In conclusion, the CD105+/CD90+ subpopula-tion of breast cancer MDA-MB-231 cells pos-sessed “mesenchymal stem cell-like” charac-teristics, and its high migratory ability may be associated with EMT. Moreover, using the sur-face markers of CD105 and CD90 for the
iden-tification of MSCs might provide new theoreti
-cal basis for the recurrence and metastasis of breast cancer.
Acknowledgements
This work was supported by the Key Scientific
and Technological Projects of Hubei Province, China (NO. 20120124) and China Medical Foundation.
Disclosure of conflict of interest
None.
Address correspondence to: Dr. Yunjiang Liu, Hebei Breast Disease Diagnosis and Treatment Center, The Fourth Hospital of Hebei Medical University, Tianshan Road, Shijiazhuang 050035, Hebei, P. R. China. Tel: 86095287; Fax: +86-311-86077634; E-mail: L_yunj@163.com
References
[1] Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D and Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cyto- therapy 2006; 8: 315-317.
[2] Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res 2011; 31: 2283-2290.
[3] Saroufim A, Messai Y, Hasmim M, Rioux N, Iacovelli R, Verhoest G, Bensalah K, Patard JJ, Albiges L, Azzarone B, Escudier B and Chouaib S. Tumoral CD105 is a novel independent prognostic marker for prognosis in clear-cell renal cell carcinoma. Br J Cancer 2014; 110: 1778-1784.
Characterization of CD105
+/CD90
+subpopulation in MDA-MB-231
[5] Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT and Fan ST. Significance of CD90+ cancer stem cells in hu -man liver cancer. Cancer Cell 2008; 13: 153-166.
[6] Thiery JP, Acloque H, Huang RY and Nieto MA. Epithelial-mesenchymal transitions in develop-ment and disease. Cell 2009; 139: 871-890. [7] Geradts J, de Herreros AG, Su Z, Burchette J,
Broadwater G and Bachelder RE. Nuclear Snail1 and nuclear ZEB1 protein expression in invasive and intraductal human breast carci-nomas. Hum Pathol 2011; 42: 1125-1131. [8] Morel AP, Hinkal GW, Thomas C, Fauvet F,
Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, Pourchet J, Puisieux I, Browne GJ, Spicer DB, Lachuer J, Ansieau S and Puisieux A. EMT in-ducers catalyze malignant transformation of mammary epithelial cells and drive tumorigen-esis towards claudin-low tumors in transgenic mice. PLoS Genet 2012; 8: e1002723. [9] Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A,
Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J and Weinberg RA. The epithelial-mesen-chymal transition generates cells with proper-ties of stem cells. Cell 2008; 133: 704-715. [10] Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR. New colorimetric cyto-toxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990; 82: 1107-1112. [11] Jayat C and Ratinaud MH. Cell cycle analysis by
flow cytometry: principles and applications. Biol Cell 1993; 78: 15-25.
[12] Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M and Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the perito-neum in gastric carcinoma. Cancer Sci 2009; 100: 1397-1402.
[13] Reya T, Morrison SJ, Clarke MF and Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105-111.
[14] Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ and Clarke MF. Prospective identi-fication of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100: 3983-3988.
[15] le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, Rosemann A, Irving J, Stam RW, Shultz LD, Harbott J, Jurgens H, Schrappe M, Pieters R and Vormoor J. In child-hood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic matura-tion have stem cell properties. Cancer Cell 2008; 14: 47-58.
[16] Yamazaki H, Nishida H, Iwata S, Dang NH and Morimoto C. CD90 and CD110 correlate with cancer stem cell potentials in human T-acute lymphoblastic leukemia cells. Biochem Bio- phys Res Commun 2009; 383: 172-177. [17] Ali AM, Ueno T, Tanaka S, Takada M, Ishiguro
H, Abdellah AZ and Toi M. Determining circulat-ing endothelial cells uscirculat-ing CellSearch system during preoperative systemic chemotherapy in breast cancer patients. Eur J Cancer 2011; 47: 2265-2272.
[18] Lobba AR, Forni MF, Carreira AC and Sogayar MC. Differential expression of CD90 and CD14 stem cell markers in malignant breast cancer cell lines. Cytometry A 2012; 81: 1084-1091. [19] Moore N and Lyle S. Quiescent, slow-cycling
stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol 2011; 2011.
[20] Dean M, Fojo T and Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5: 275-284.
[21] Tadbir AA, Pardis S, Ashkavandi ZJ, Najvani AD, Ashraf MJ, Taheri A, Zadeh MA and Sardari Y. Expression of Ki67 and CD105 as proliferation and angiogenesis markers in salivary gland tu-mors. Asian Pac J Cancer Prev 2012; 13: 5155-5159.
[22] Kalluri R and Weinberg RA. The basics of epi-thelial-mesenchymal transition. J Clin Invest 2009; 119: 1420-1428.
[23] Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A and Weinberg RA. An embry-onic stem cell-like gene expression signature in poorly differentiated aggressive human tu-mors. Nat Genet 2008; 40: 499-507.