TERPOLYMER RESIN-II -THERMAL
AND METAL ION BINDING
PROPERTIES OF
RESORCINOL-THIOUREA-FORMALDEHYDE
TERPOLYMER RESIN
M. Karunakaran1, C.T. Vijayakumar2*, C. Magesh3, T Amudha4
1. Department of Chemistry, SSK College of Engineering & Technology, Navakkarai Post, Coimbatore - 641 105, Tamilnadu, India
2. Department of Polymer Technology, Kamaraj College of Engineering and Technology, S.P.G.C. Nagar, K. Vellakulam Post 625 701, Tamilnadu, India
3. Department of Chemistry, PPG Institute of Technology, Coimbatore - 641 035, Tamilnadu, India
4. Department of Chemistry, RVS College of Engineering and Technology, Coimbatore 641 402, Tamilnadu, India
Abstract
Terpolymer resin RTF-1 is prepared by the condensation of resorcinol (R), thiourea (T) and formaldehyde (F) in the presence of 2M HCl as catalyst at 1402C. The synthesised terpolymer resin is characterized by FTIR, 1
H-NMR and gel permeation chromatographic techniques. To establish the thermal stability of the resin, TGA analysis is performed. The Doyle, Horowitz & Metzger, Broido and Dharwadkar & Kharkhanavala methods are used to calculate the thermodynamic parameters, which include enthalpy of activation (H‡), entropy of activation (S‡), free energy of activation (G‡) and kinetic parameters like energy of activation (Ea) and pre-exponential factor (A) for various steps of thermal decomposition of RTF-1. Chelate ion exchange property of the terpolymer is studied for Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, and Cd2+ ions. Batch equilibrium method is employed in the study of the selectivity of metal ion uptake. This involved the measurement of the distribution of the given metal ion between the terpolymer sample and the solution containing the metal ion. The study is carried out over a wide pH range and in media of various ionic strengths.
Keywords: Resorcinol-thiourea-formaldehyde, Terpolymer, Thermal properties, Batch equilibrium method.
1. Introduction
The use of terpolymers in all spheres of life has been abundantly increased in recent years because of novelty and versatility. They occupy the pivotal position in the field of polymer science. The progress in this field has been extremely rapid, as they are generally useful in packaging, adhesives and coatings in electrical sensors, ion-exchangers, organometallic semiconductors, activators, catalyst and thermally stable materials [1-6]. Much of research work has being carried out on the synthesis and characterization of thiourea containing terpolymer. A literature survey reveals that terpolymers derived from substituted hydroxy or dihydroxy phenol/phenolic derivatives–formaldehyde resins with urea or thiourea or oxamide etc. shows improved ion-exchange properties, thermal resistance property, coordinating property, and good storage stability etc [7-9]. Jadho and co-workers [10] synthesized a terpolymer resin by condensation of 2,2-dihydroxybiphenyl and urea with formaldehyde in the presence of an acid catalyst and studied the chelating ion-exchange properties of this terpolymer for Fe3+,
Cu2+, Ni2+, Co2+, Zn2+, Cd2+, and Pb2+ ions. They reported that the polymer showed a higher selectivity for Fe3+ ion over any other ion. Masaram and co-workers [11] synthesised terpolymer chelating ion exchange resin derived from salicylic acid, hexamethylene diamine, and formaldehyde and is used for the separation of metal ion by selective adsorption in the resin column. The terpolymer resin showed a higher selectivity for Fe3+, Cu2+,
have been condensed with formaldehyde to produce heat and light stabilizers [9]. Joshi and Patel [12] have synthesized salicylic acid-urea-formaldehyde copolymers in view of the characteristics especially ion exchanging property and other industrial applications. Ion-exchange resins derived from p
-hydroxybenzaldehyde with resorcinol and formaldehyde with different mole ratios were reported [5]. These resins were characterized by physicochemical methods such as elemental analysis, and spectral data. Ion exchange properties were also carried out with bivalent metal ions and accounted. Anthranilic acid-formaldehyde-resorcinol terpolymer been proved to be a selective ion exchange resin for metal ions [13]. Condensation of resorcinol, aniline, orthophenylenediamine, and formaldehyde in presence of 4N HCl yields a copolymer resin possessing high thermal stability and ion exchange capacity [14]. Thermal and ion-exchange studies of terpolymer resins derived from o-cresol-urea-formaldehyde were also reported [15]. Anthranilic
acid-urea-formaldehyde resins were reported as useful sequestering agent for removing and separating ions from mixture of ions [16]. Thermal stability, electrical conductivity and ion exchange characteristics of anthranilic acid-thiourea-paraformaldehyde terpolymer were reported [17, 18]. Singru and co-workers [19] studied the electrical property, thermal stability, morphology, photoluminescence and ion-exchange properties of p
-cresol-melamine-formaldehyde terpolymer resin. Butoliya et al., [20] reported a terpolymer synthesized by the
condensation of 2,4-dihydroxybenzoic acid and melamine with formaldehyde in the presence of acid catalyst, and thermodynamic and kinetic parameters were calculated and reported. A number of terpolymers were synthesized by condensation of 4-hydroxybenzaldehyde oxime, formaldehyde and chloro, bromo, methoxy or methyl substituted acetophenones and their thermal stability was investigated by using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) [21].
Terpolymer resins having good thermal stability have enhanced the scope for development of some polymeric materials. The study of the thermal degradation of copolymer resins have recently become a subject of interest. The present work reports the synthesis and characterisation of a terpolymer resin (RTF-1) from resorcinol (R), thiourea (T) and formaldehyde (F). The purpose of the present study, is to explore the thermal degradation properties of a newly synthesized terpolymer resin and adsorption behaviour of seven metal ions Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+ on the newly synthesized terpolymer resin RTF-1 at different pH values, different concentrations of different electrolytes and at different shaking time intervals.
2. Experimental
2.1. Reagents and solutions
Resorcinol and thiourea were of Analytical Grade, received from Loba Chemicals, Mumbai and Formaldehyde (37% w/v) was purchased from s.d. Fine Chem., Ltd. Mumbai. Metal ion solutions were prepared by dissolving appropriate amount of metal nitrates in double distilled water and standardized by complexometric titration. All the chemicals were used as received without further purification.
2.2. Synthesis of RTF terpolymer:
The terpolymer (RTF-1) was synthesized by the condensation of resorcinol and thiourea with formaldehyde in the mole ratio of 1:1:2 in the presence of 2M HCl (200 mL ) as a catalyst. The mixture was heated at 140 ± 2C for 5 hours. The contents of the flask were shaken periodically to ensure homogeneous mixing. After the refluxing period was over the contents of the flask were poured into crushed ice with constant stirring and left overnight. The separated rosy red coloured resin was filtered off and washed several times with cold water followed by hot water and methanol for removing unreacted monomers. Finally the resin was purified by dissolving in 10% NaOH and reprecipitating with Conc. HCl/water (1:1V/V). The resin thus obtained was washed with cold water followed by hot water. The purified terpolymer resin was finely ground and dried in vacuum at 100°C. The yield of the resin was 79%. The details of the reaction and the structure of RTF-1 is shown in Scheme 1.
2.3. Characterization
2.4. Ion-exchange Studies
2.4.1. Determination of metal uptake in the presence of electrolytes of different concentrations
The ion exchange property of terpolymer resin RTF-1 was determined by the batch equilibrium method [17]. The polymer sample (25mg) was suspended in an electrolyte solution (25mL) of known concentration. The different electrolytes used here are NaCl, NaClO4, NaNO3 and Na2SO4. The pH of the suspension was adjusted to the required value by using either 0.l M HNO3 or 0.lM NaOH. The suspension was stirred for 24 h at room temperature. To this suspension 2mL of 0.1M solution of the metal ion was added and the pH was adjusted to the required value once again. The mixture was again stirred for 24 h and filtered. The solid was washed and the filtrate and washings were combined and the metal ion content was determined by titration against standard EDTA [Ethylene Diamine Tetra Acetic acid] at the same pH of experimental condition. The same titration has been carried out without polymer for blank reading. The amount of the metal ion uptake of the terpolymer was calculated from the difference between the blank experiment and the reading in the actual experiment. The experiment was repeated in the presence of several electrolytes. Metal ion, buffer and indicator used and colour change are given in Table 1.
The metal ion uptake can be determined as:
Metal ion adsorbed (uptake) by resin = (X-Y) Z m.mol/g
where Z (mL) is the difference between actual experimental reading and blank reading; X (mg) is metal ion in 2 mL 0.1 M metal nitrate solution before uptake; and Y (mg) is metal ion in 2 mL 0.1 M metal nitrate solution after uptake. By using this equation, the uptake of various metal ions by the resin can be calculated and expressed in terms of millimols per gram of the copolymer. The experiments were performed in the presence of seven different metal ions: Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+ and Cd2+.
2.5. Evaluation of the rate of metal uptake
In order to estimate the time required to reach the state of equilibrium under the given pH, experiments of the type described above were carried out, in which metal ion taken up by the chelating resins was estimated from time to time in the presence of 25mL of 1M NaNO3 solutions. It was assumed that, under the given conditions, the state of equilibrium was established within 24 h. The rate of metal uptake is expressed as the percentage of the amount of metal ions taken up after a certain time related to that in the state of equilibrium and it can be defined by the following relationship:
Metal ion adsorbed
Metal ion taken up at different times (%) = --- X 100 Metal ion adsorbed at equilibrium
By using this expression, the amount of metal adsorbed by terpolymer after specific time intervals was calculated and expressed in terms of percentage metal ion adsorbed. The distribution of each one of the seven metal ions, ie Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+ andCd2+, between the polymer phase and the aqueous phase was estimated at room temperature and in the presence of 1M NaNO3 solution.
Table 1: Details regarding EDTA titration
Metal ions Buffer Indicator Colour change
Fe3+ Dil.HNO
3/dil.NaOH Variamine Blue Blue-yellow
Co2+ Hexamine Xylenol Orange Red-yellow
Ni2+ Aq.NH
3/NH4Cl Murexide Yellow-violet
Cu2+ Dil.HNO3/dil.NaOH Fast Sulphon Black F Purple-green
Zn2+ Aq.NH
3/NH4Cl Eriochrome Black T Wine red-blue
Cd2+ Hexamine Xylenol Orange Red-yellow
Pb2+ Hexamine Xylenol Orange Red-yellow
2.6 Evaluation of the distribution of metal ions at different pH
The distribution of each one of the seven metal ions, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+ and Cd2+ , between the polymer phase and the aqueous phase was estimated in the presence of a 1M NaNO3 solution. The experiments were carried out as described earlier at pH 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, and 6. The distribution ratio Dis defined by the following relationship:
Amount of metal ion on resin Volume of solution (mL) D = --- X ---
Amount of metal ion in solution Weight of resin (g)
3. Results and Discussion 3.1. FTIR Spectral Study
The spectrum of RTF-1 resin is depicted in Fig.1 and the spectral data are presented in Table 2. A broad absorption band appeared in the region 3379-3200 cm-1 may be assigned to the stretching vibrations of phenolic
hydroxyl (-OH) groups exhibiting intermolecular hydrogen bonded polymeric association [22]. The methylene bridge associated with resorcinol can be identified by the peak at 2923 cm-1 [23]. The peaks appeared at 1458,
1292 and 1163 cm-1 is due to methylene bridge coupled with aromatic ring [24, 25]. A peak appeared at 1386
cm-1 can be assigned to in plane bending vibration of phenolic –OH [25]. A sharp strong peak at 1508 cm-1 may be ascribed to N-H bending of secondary amide group [26]. 1,2,3,5 tetra substituted aromatic ring may be identified by the peaks at 1217 and 975 cm-1 [27]. A strong peak appeared at 1080 cm-1 may be due to C=S stretching vibration [28].
Table 2: FT-IR spectral data for terpolymer RTF-1
Vibration mode
Frequency (cm-1)
Reported Observed
Phenolic –OH group with inter molecular polymeric association
3750-3150 3379-3166
stretching of Methylene group - (C-H)
2910-2930 2923
- CH2 – Bridge 1460, 1291, 1158-1180 1458, 1292, 1163
Phenolic – OH in plane bending 1370- 1385 1386
N –H bending -Secondary amide 1479 – 1510 1508
1,2,3,5 substituted aromatic ring 1220 & 974 1217 & 975
C=S stretching vibration 1200-1050 1080
3.2. 1H NMR Spectral Study
The 1H NMR spectrum of RTF-1 resin is shown in Fig. 2 and the spectral data are presented in Table 3. The chemical shift (δ) ppm observed is assigned on the basis of the data available in the literature. A peak at 1.3 can be allotted to S-H group [29]. A Peaks at 3.0-4.0 () ppm may be due to the presence of methylene protons of Ar-CH2-NH- moiety [28]. The protons of –NH- bridge can be identified from the peak at 4.5 () ppm. Signals appeared at () ppm values 6.7-6.9 can be assigned to aromatic protons. The protons of Ar-OH or –NH-CS- group involved in proton exchange reaction gave peak at 7.9 () ppm.
Table 3: 1H spectral data for terpolymer RTF-1 resin
Nature of proton Expected chemical shift
(δ) ppm Chemical shift (for RTF-1 resin δ) ppm
S-H proton 1.2 – 1.6 1.3
Methylenic protons of Ar -CH2 - NH - 3.5-6 3.5 - 4
Aromatic proton (Ar-H) (asymmetrical
substitution pattern) 6.2–8.5 6.8-7.0
Fast/intermediate proton exchange reaction of phenolic -OH group/ -NH-CS- group or Ar-OH phenolic hydrogen bonded
8-12 7.9
3.3 Molecular weight measurement
The molecular weight of polymer sample was determined using gel permeation chromatographic technique. The number average molecular weight, weight average molecular weight, and z - average molecular weight of RTF-1 were calculated as RTF-1RTF-1968, RTF-1483RTF-1 and RTF-18RTF-123 respectively.
On the basis of FTIR, 1H NMR spectral data, molecular weight measurements and the nature and reactive sites of the monomers, the most probable structure proposed for the RTF-1 terpolymer is shown in Scheme 1.
Scheme 1: Synthesis of RTF-1 terpolymer resin
OH
H H
O
H2N NH2
S
H N
H N
S
Resorcinol Formaldehyde Thiourea
*
OH HO
* Catalyst
5h 140 °C
OH HO
OH
3.4. Thermogravimetric Analysis
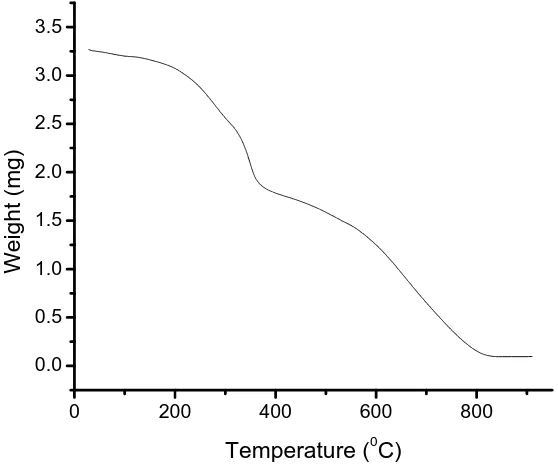
The thermogram recorded in the temperature range 30 to 900 C of the resin RTF-1 is presented in Fig. 3 and respective TG data are presented in Table 4. It reveals that the resin undergoes two degradation steps. In the first step, decomposition starts at 198 ºC and extends up to 310 ºCinvolving 47 % weight loss. The second step decomposition, which begins at 582 ºC and extends up to 813 ºC, with 96.4% weight loss and is most probably due to the random cleavage of polymeric resin affording simpler degradation products [30]. It is very difficult to draw any conclusion from the magnitude of the thermal activation because the decomposition mechanism is expected to be quite complex [6].
Fig. 3: Thermogravimetric curve for RTF-1
Table 4: TG data for terpolymer RTF-1 resin
Sample Name Td (C)
20 % 40 % 60 % 80 % 90 %
RTF - 1 286 355 580 694 752
The thermo gravimetric analysis has proved to be a useful analytical technique in evaluating kinetic parameters such as energy of activation (Ea), enthalpy of activation (H‡), entropy of activation (S‡), free energy of activation (G‡), and pre-exponential factor (A), which provide valuable quantitative information regarding the stability of the material. Various methods have been proposed by different investigators to estimate the kinetic and thermodynamic parameters of thermal degradation reaction. In this paper, the activation energy (Ea) of the thermal degradation of the terpolymer resin was calculated by C.D. Doyle [31], Horowitz and Metzger [32], Broido [33], and Dharwadkar & Kharkhanavala methods [34, 35] and their linear plots are depicted in Fig. 4-7 respectively. From the linear plots, the calculated energy of activation (Ea) and pre-exponential factor (A) values is listed in Table 5. It is observed that the calculated activation energy values with different methods are nearly equal and the calculated activation energy for first and second stages is 36 ± 3 kJmol-1 and 43 ± 1 kJmol -1 respectively.
0 200 400 600 800
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5
Weight (
m
g)
Stage 1 Stage 2
Fig. 4: Doyle Plot
Stage 1 Stage 2
Fig. 5: Horowitz & Metzger Plot
1.60 1.62 1.64 1.66 1.68 1.70 1.72 1.74 1.76 -0.65 -0.60 -0.55 -0.50 -0.45 -0.40 -0.35 log (-ln( 1-a ) 1000/T
-50 -40 -30 -20 -10 0 10 20 30 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 ln (l n (1 /(1 -a )))
0.95 1.00 1.05 1.10 1.15 1.20 -0.1 0.0 0.1 0.2 0.3 0.4 0.5 log( -l n (1-a) ) 1000/T
-100 -50 0 50 100 150
Enthalpy of activation (H‡), entropy of activation (S‡) and free energy of activation (G‡) were calculated by using C.D. Doyle and Dharwadkar & Kharkhanavala methods respective equations [31, 34-36] and the values are presented in Table 5. The variations of the calculated values are maybe due to the approximation used to evaluate the respective kinetic equations.
Stage 1 Stage 2
Fig. 6: Broido Plot
1.45 1.50 1.55 1.60 1.65 1.70 1.75 1.80 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 ln (-ln (1 -a )) 1000/T
0.90 0.95 1.00 1.05 1.10 1.15 1.20 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 ln (-ln( 1 -a) ) 1000/T
Stage 1 Stage 2
Fig. 7: Dharwadkar & Kharkhanavala Plot
-45 -30 -15 0 15 30 45
-1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 ln (l n(1/ (1-a )) )
-60 0 60 120 180
Table 5: Kinetic and thermodynamic parameters for the thermal decomposition reactions for terpolymer RTF-1 resin
Stage T range
(ºC)
TMax
(ºC)
Parameters
Approximation methods
Doyle Horowitz & Metzger Broido Dharwadkar &
Kharkhanavala
1 198-310 345
Ea (kJ mol-1)
A (s-1)
S‡ (J mol-1 K-1)
H‡(kJ mol-1)
G‡(kJ mol-1)
34.7
4.9
-4.8 X 102
29.5
3.2 X104
38.1
32.9
39.4
34.3
35.9
12.4
- 2.3X 102
30.8
2.9 X 104
2 582-813 653
Ea (kJ mol-1)
A (s-1)
S‡ (J mol-1 K-1)
H‡(kJ mol-1)
G‡(kJ mol-1)
42.2
3.3
-3.9X102
36.9
3.7 X 104
42.8
35.1
44.3
36.6
43.2
3.1
-2.84 X 102
35.4
3.08 X 104
3.5. Ion-exchange Properties
Batch equilibrium technique was used to study ion-exchange properties of RTF-1 terpolymer resin. Seven metal ions Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+ andCd2+, in the form of aqueous metal nitrate solution were used. The results of the batch equilibrium study carried out with the terpolymer RTF-1 was presented in Table 6. The ion-exchange study was carried out using three experimental variables:
1. electrolyte and its ionic strength, 2. uptake time and
3. pH of the aqueous medium.
Among these three variables, two were kept constant and only one was varied at a time to evaluate its effect on metal uptake capacity of the terpolymer. Moreover the amount of metal ions taken up by a given amount of terpolymer depends on the nature and concentration of the electrolyte present in the solution. From results given in Table 6 reveal that, in presence of chloride, perchlorate, and nitrate ions, for example, the uptake of Fe3+, Cu2+, and Ni2+ ions by the terpolymer sample increases with increasing concentration of the electrolyte, whereas in presence of sulphate ions the amount of the above mentioned ions taken up by the terpolymer sample decreases with increasing concentration of the electrolyte. On the other hand in the presence of sulphate ions the amount of the above mentioned ions taken up by the terpolymer decreases with increasing concentration of the electrolyte. The reaction between electrolyte, metal ion and terpolymer is explained as
electrolyte + metal ion + terpolymer electrolyte ligand-metalion chelate + terpolymer-metal ion chelate
Table 6: Evaluation of effect of electrolytes on metal iona uptake by RTF-1terpolymer
Metal Ion Electrolyte (mol. l-1)
pH Weight (in mg) of metal ion uptake in presence of
NaCl NaClO4 NaNO3 Na2SO4
Fe3+
0.01
2. 5
0.52 1.21 1.22 2.46
0.05 1.13 2.11 1.48 2.13
0.10 1.77 2.49 1.12 1.71
1.0 2.29 2.83 2.56 1.24
Co2+
0.01
5.0
2.12 1.88 1.70 2.06
0.05 1.68 1.46 1.32 1.61
0.10 1.31 1.14 0.99 1.25
1.0 0.93 0.81 0.58 0.83
Ni2+
0.01
5
1.41 1.16 1.94 2.88
0.05 2.01 1.85 2.29 2.44
0.10 2.85 2.54 2.72 2.07
1.0 3.49 2.97 3.02 1.16
Cu2+
0.01
5
1.72 1.89 2.03 3.27
0.05 2.21 2.33 2.58 2.49
0.10 2.56 2.69 2.93 1.62
1.0 3.52 3.76 4.19 1.16
Zn2+
0.01
5
2.37 2.45 1.71 2.03
0.05 2.08 2.10 1.27 1.48
0.10 1.61 1.56 1.06 1.09
1.0 0.80 1.01 0.80 0.62
Cd2+
0.01
5
1.55 1.58 1.86 1.24
0.05 1.18 1.32 1.55 0.85
0.10 1.01 0.99 1.27 0.39
1.0 0.59 0.53 0.72 0.14
Pb2+
0.01
5.5
1.54 1.42 1.77 ---
0.05 1.13 1.16 1.25 ---
0.10 0.86 1.01 1.08 ---
1.0 0.38 0.35 0.43 ---
a[Mn+ (NO
3)n ] = 0.1 mol/L; Volume = 2 mL; Volume of electrolyte solution = 25 mL; Weight of the resin = 25 mg; Time = 24 h at room temperature
3.6. Rate of Metal Uptake
The rate of metal adsorption was determined to find out the shortest period of time within which equilibrium could be established while operating as close to equilibrium conditions as possible. Fig. 8 shows the dependence of the rate of metal ion uptake on the nature of the metal. The rate refers to the change in concentration of the metal ions in the aqueous solution which is in contact with the given polymer. The results show that the time taken for the uptake of the different metal ions at a given stage depends on the nature of the metal ion under
given conditions. It is found that Fe
3+ions require about 4h for the establishment of
require about 6 hours for the establishment of equilibrium. The experimental results reveal that the rate of metal ion uptake follows the order, Fe3+ > Co2+ ≈ Ni2+ ≈ Cu2+ ≈ Zn 2+ > Cd2+ ≈ Pb2+ for the terpolymer.
Fig. 8: Rates of metal ion uptake by terpolymer RTF-1
3.7. Distribution Ratios of Metal Ions at Different values of pH
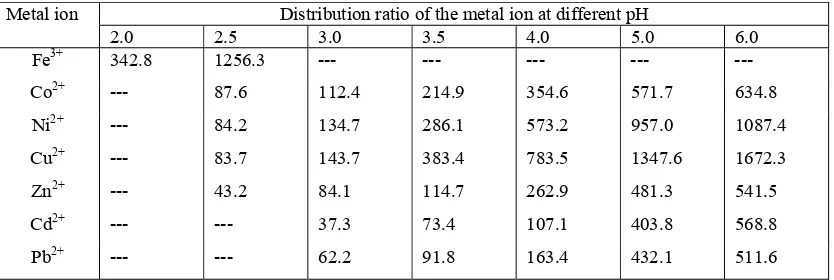
The effect of pH on the amount of metal ion distributed between two phases can be explained by the results given in Table 7. The results indicate that the relative amount of metal ions taken up by the terpolymer increases with increasing pH of the medium [40] .The study was carried out to a definite pH value for the particular metal ion to prevent hydroxide formation of the metal ions at higher pH. In case of Fe3+ the highest working pH was 2.5. The results indicate that the selectivity of terpolymer sample for Fe3+, Cu2+, and Ni2+ ions is higher than that for other metal ions. Whereas the selectivity of terpolymer resin sample for Pb2+ ion is the lowest. The other metal ions under study viz., Co2+, Zn2+, and Cd2+ ions have shown intermediate selectivity. The lower distribution ratio of Fe3+ is due to steric hindrance [41]. Amongst the other metal ions, Ni2+ and Cu2+ ions are taken up by the terpolymers more selectively. The other four metal ions Co2+, Zn2+, Cd2+, and Pb2+ have a low distribution ratio D over the pH range 4 – 6. This is due to low stability constants, i.e., due to the weak ligand stabilisation energy of the metal complexes [42]. In this study, the observed order of distribution ratio of divalent ions are found to be Cu2+ > Ni2+> Zn2+ > Co2+ > Pb2+ > Cd2+, which matches well with the results reported. The results of this study help in selecting the optimum pH for the selective uptake of a metal ion from a mixture of different ions. For example, for the separation of Cu2+ and Fe3+ions, the optimum pH is 2.5, at which the distribution ratio D for Cu2+ is 83.7
and that for Fe3+ is 1256.3. Hence, the results of this study were helpful in selecting the optimum pH for a selective uptake of a particular metal ion from a mixture of ions.
2 4 6
40 60 80 100
M
e
ta
l io
n
u
p
tak
e
(%
)
Time (h)
Table 7:Distribution ratio ‘D’aof different metal ionsb as a function of the pH of RTF-1 terpolymer
Metal ion Distribution ratio of the metal ion at different pH
2.0 2.5 3.0 3.5 4.0 5.0 6.0
Fe3+ 342.8 1256.3 --- --- --- --- ---
Co2+ --- 87.6 112.4 214.9 354.6 571.7 634.8
Ni2+ --- 84.2 134.7 286.1 573.2 957.0 1087.4
Cu2+ --- 83.7 143.7 383.4 783.5 1347.6 1672.3
Zn2+ --- 43.2 84.1 114.7 262.9 481.3 541.5
Cd2+ --- --- 37.3 73.4 107.1 403.8 568.8
Pb2+ --- --- 62.2 91.8 163.4 432.1 511.6
a
D =
b
[M
n+ (NO3)n ] = 0.1 mol/L; Volume = 2 mL; NaNO3 0.5 mol/L ;Volume 25mL; room temperature; Time = 24 h (equilibrium state)
4. Conclusion
The terpolymer (RTF-1) was synthesized via condensation of resorcinol and thiourea with formaldehyde and the FT-IR and 1H-NMR study confirmed its structure. The number average, weight average, and z-average molecular weights of the terpolymer RTF-1 were 11968, 14831 and 18123 respectively. TGA results demonstrated that the terpolymer undergoes two degradation steps and its activation energy values are 36 ± 3 kJmol-1 and 43 ± 1kJmol-1 respectively. An ion-exchange property of RTF-1 terpolymer resin was studied through ‘Batch equilibrium technique’. The metal ions Fe3+, Cu2+, and Ni2+ formed weak chelate with electrolyte and strong chelate with terpolymer ligand and thus the metal ion uptake capacity increased where as the metal ions Co2+, Zn2+, Cd2+, and Pb2+ formed strong chelate with the electrolytes. Hence the uptake of Co2+, Zn2+, Cd2+, and Pb2+ ions decreased with increasing concentration of these electrolytes. The determined rate of metal ion uptake for the terpolymer follows the order, Fe3+ > Co2+ ≈ Ni2+ ≈ Cu2+ ≈ Zn 2+ > Cd2+ ≈ Pb2+. Further the increase in the pH of the medium facilitates the increase of metal ion uptake by the terpolymer.
References
[1] D.T. Masram, K.P. Kariya, N.S. Bhave, (2010): Electrical conductivity study of resin synthesized from salicylic acid, butylenediamine and formaldehyde. Arch. Apll. Sci. Res. 2 (2), pp. 153-161
[2] D.T. Masram, N.S. Bhave, K.P. Kariya, (2010): Kinetics study of thermal degradation of resin derived from salicylaldehyde, ethylenediamine and formaldehyde. E - J. Chem. 7(2), pp. 564-568
[3] S.S. Katkamwar, A.B. Zade, S.S. Rahangdale, W. B. Gurnule, (2009): Terpolymer resin–III: Synthesis and characterization of 8-hydroxyquinoline-dithiooxamide-formaldehyde terpolymer resins. J. Appl. Polym. Sci. 113, pp. 3330-3335
[4] R.N. Singru, W.B. Gurnule, (2009): Chelation ion-exchange study of copolymer resin derived from 8-hydroxyquinoline 5-sulphonic acid, oxamide, and formaldehyde. J. Appl. Polym. Sci. 116, pp. 3356–3366
[5] V. V. Hiwase, A.B. Kalambe, K.M. Khedkar, S.D. Deosarkar, (2010): Ion exchange properties of resins derived from p -hydroxybenzaldehyde, resorcinol and formaldehyde. E - J. Chem. 7(1), pp. 287-294
[6] B.A. Shah, A.V. Shah, B.N. Bhandaria, R.R. Bhatt, (2008): Synthesis, Charecterization and Chelation Ion-Exchange Studies of a Resin CopolymerDerived from 8-Hydroxyquinoline-Formaldehyde-Catechol. J. Iran. Chem. Soc.5(2), pp. 252-261
[7] H. Patel, M. Patel, K. Patel, R. Patel, (2007): Novel acrylic copolymers: synthesis, characterization and antimicrobial studies. e-Polymers. 125, pp. 1-11
[8] P.E.P. Michael, J.M. Barbe, H.D. Juneja, L.J. Paliwal, (2007): Synthesis, characterization and thermal degradation of 8-hydroxyquinoline-guanidine-formaldehyde terpolymer. Eur. Polym. J. 43(12), pp. 4995-5000
[9] R.N. Singru A.B. Zade, W.B. Gurnule, (2008): Synthesis, characterization, and thermal degradation studies of copolymer resin derived from p-cresol, melamine, and formaldehyde. J. Appl. Polym. Sci. 109, pp. 859-868
[10] M. M. Jadhao, L. J. Paliwal, N.S. Bhave, (2005): Chelation ion-exchange properties of 2,2-dihydroxybiphenyl-urea-formaldehyde (2:1:3) resin. Indian J. Chem. 44A, pp. 1206-1210
[11] D.T. Masram,K.P. Kariya, N.S. Bhave, (2007): Synthesis of resin I: salicylic acid, hexamethylene diamine and formaldehyde and its ion-exchange properties. e-Polymers. 75, pp. 1-12
[12] R.M. Joshi, M.M. Patel, (1983): p-Hydroxybenzoic acid-urea-formaldehyde copolymers and their ion-exchange properties. J. Macromol. Sci. Part B 19(5), pp. 705-722
[14] R.K. Samal, B.K, Senapati, T.B. Behuray, (1998): Synthesis and characterization of some novel copolymer resins. III. J. Appl. Polym. Sci. 68, pp. 2183-2187
[15] M. Karunakaran, C. Magesh. (2010): Thermal and ion-exchange studies on chelating terpolymer resins derived from o-cresol urea formaldehyde, Arabian J. Chem. In Press.
[16] C. Magesh, C.T. Vijayakumar, M. Karunakaran, (2010):Anthranilic acid-urea-formaldehyde terpolymer resin and their ion-exchange properties, Int. J. Chem. Appl. 2(1), pp. 21-32
[17] M. Karunakaran, A. Burkanudeen, (2003): Chelation ion-exchange properties of anthranilic acid-thiourea-para formaldehyde copolymers, Oriental.J. Chem. 19(1), pp. 225-228
[18] R,S. Azarudeen, M.A. Riswan, D. Jayakumar, A. Burkanudeen, (2009): An eco-friendly synthesis of a terpolymer resin:
characterization and chelation ion-exchange property. Iran. Polym. J. 18 (10), pp. 821-832.
[19] R.N. Singru, W.B. Gurnule, V.A. Khati, A.B. Zade, J.R. Dontulwar, (2010): Eco-friendly application of p-cresol–melamine– formaldehyde polymer resin as an ion-exchanger and its electrical and thermal study. Desalination. 263(1-3), pp. 200-210
[20] S. S. Butoliya, W. B. Gurnule, A. B. Zade, (2010): Study of non-isothermal decomposition and kinetic analysis of 2,4-dihydroxybenzoic acid-melamine-formaldehyde copolymer, E - J. Chem. 7(3), pp. 1101-1107
[21] N.P.S. Chauhan, R. Ameta, R. Ameta, S.C. Ameta, (2010): Synthesis and Characterization of p-Hydroxybenzaldehyde Oxime based Terpolymers and their Biological Activities. Malays. Polym. J. 5(2), pp.162-180
[22] T.K. Pal, R.B. Kharat, (1989): Synthesis and characterization of salicylic acid-dithiobiuret-trioxane resins. J. Ind. Chem. Soc. 66, pp.283-286
[23] B.A. Shah, A.V. Shah, R.R. Bhatt, (2007): Studies of Chelation Ion-exchange Properties of Copolymer Resin Derived from Salicylic Acid and its Analytical Applications Iran. Polmy. J. 16(3), pp. 173-184
[24] H.B. Pancholi, M.M. Patel, (1991): Ion-exchange properties of 2-hydroxy-4-methoxyacetophenone-thiourea-trioxane polymer. High. Perform. Polym. 3, pp. 257-262
[25] W.B. Gurnule, P.K. Rahangdale, L.J. Paliwal, R.B. Kharat, (2003): Synthesis, characterization and ion-exchange properties of 4-hydroxyacetophenone, biuret and formaldehyde terpolymer resins. React. Funct. Polym. 55, pp. 255-265
[26] H.B. Pancholi, M.M. Patel, (1991): Synthesis and characterization of 2-hydroxy 4-methioxyacetophenone-Thiourea-Trioxane polymers. High. Perform. Polym. 3, pp. 25 -31
[27] R.K. Samal, B.K. Senapati, T.B. Behuray, (1966): Synthesis and characterization of aniline-doped mixed copolymer resins. II, J. Appl. Polym. Sci. 62, pp. 655-660
[28] T. K. Pal, R. B. Kharat, (1989): Salicylic acid-biuret-trioxane terpolymer resins and their ion-exchange properties. Angew. Makromol. Chemie.113, pp.55-68.
[29] R.M. Silverstein, G.C. Bassle, T.C. Morrill, (1991): Spectrometric identification of organic compounds, 5th ed.; Wiley: Singapore. [30] M.M. Jadhao, L.J. Paliwal, N.S. Bhave, (2010): Resins IV: Preparation and characterization of terpolymer resins prepared from
biphenol, thiourea and formaldehyde. J. Appl. Polym. Sci. 118, pp. 1245-1251
[31] G.S. Learmonth, T. Wilson, (1964): Thermal degradation of resins. J. Appl. Polym. Sci. 8, pp. 2873-2881
[32] C.D. Doyle, (1961) : Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 5, pp. 285-292
[33] H.H.Horowitz, G.A. Metzger, (1963): New analysis of thermogravimetric traces. Anal Chem. 35, pp. 1464-1468
[34] A.Broido, (1969): A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. Part A-2. 7, pp. 1761-1773
[35] S.R. Dharwadkar, M.D. Kharkhanavala, (1969):Calculation of activation energy for decomposition reactions from thermogravimetric analysis.Thermal Anal., Vol. 2.Academic Press, New. York, pp.1049-1069
[36] P. Sivasamy, C.T. Vijayakumar, K. Lederer, A. Kramer, (1992):A kinetic analysis of thermogravimetric data of radically polymerised
N-phenylmaleimide. Thermochim. Acta. 208, pp. 283-291
[37] M. Nathan, Sulexna, (2006): Di- and triorganotin(IV) derivatives of 5-amino-3H-1,3,4-thiadiazole-2-thione as precursors for SnS/SnO2: Thermal studies and related kinetic parameters. Mater. Res. Bull. 41, pp. 78-91
[38] B.A. Shah, A.V. Shah, M.S. Shah, (2006): Synthesis, characterization, and analytical applications of o-substituted benzoic acid chelating resin. Iran. Polym. J. 15, pp. 809-819
[39] M.M. Jadhao, L.J. Paliwal, N.S. Bhave, (2009): Ion-exchange properties of 2,2’-dihydroxybiphenyl-urea-formaldehyde terpolymer resins. Desalination. 247, pp. 456-465
[40] S.S. Rahangdale, A.B. Zade, W.B. Gurnule, (2009): Chelation ion exchange properties of 2, 4dihydroxyacetophenonebiuret -formaldehyde terpolymer resin. E - J. Chem., 6, pp. 835-843
[41] S.S. Butoliya, A.B. Zade, W.B., Gurnule, (2009). Terpolymer resin VIII: Chelation ion-exchange properties of
2,4-dihydroxybenzophenone-oxamide-formaldehyde terpolymer resins. J. Appl. Polym. Sci. 113, pp. 1-9
[42] R.C. DeGeiso, L.G. Donaruma, E.A. Tomic, (1962): Chelation ion exchange properties of a salicylic acid-formaldehyde polymer. Anal. Chem. 34, pp. 845-847
[43] S.L. Davydova, N.A. Plate, (1975): Problems of complex formation with macromolecular ligands. Coord. Chem. Rev. 16, pp. 195-225