Pseudoaneurysm overlying an osteochondroma: a noteworthy complication
Full text
Figure

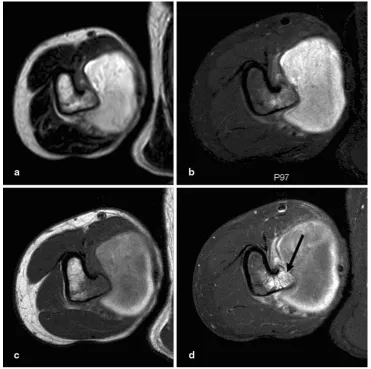

Related documents
female‘s (meaning adult women and girls) propensity for enrolling and continuing in the science, technology, engineering, and mathematics (STEM) fields at through university level..
(3) Tangential forces observed during the grinding operation are lower for laser dressed wheel but there is not much difference in the normal forces for both conventional as well
From the above evaluation of result of ANSYS analysis of conventional concrete and optical fibre concrete the deflection of optical fibre concrete is comparatively less to that
Minimum five years/60 months unique non-overlapping professional project management experience during which at least 7,500 hours were spent leading and directing the project*.
LOCAL PERCEPTIONS OF THE FAST TRACK LAND REFORM PROGRAMME (FTLRP) IN UMGUZA RESETTLEMENT SCHEME IN ZIMBABWE.. A research mini-thesis submitted in partial fulfilment of the
This study is aimed at giving a new contribution to the morphometric approach for the delineation of the boundaries of volcanic edifices, applied to 13 monogenetic volcanoes
We use a single-box model of the land biosphere to derive an estimate of the contemporary biomass burning contribution to the climate–carbon-cycle feedback using remote-sensing-
Mulquiney PJ, Kuchel PW: Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations:. computer simulation and metabolic