FORMULATION AND CHARACTERIZATION OF DRUG IN ADHESIVE TRANSDERMAL PATCHES OF BUFLOMEDIL HYDROCHLORIDE
Full text
Figure

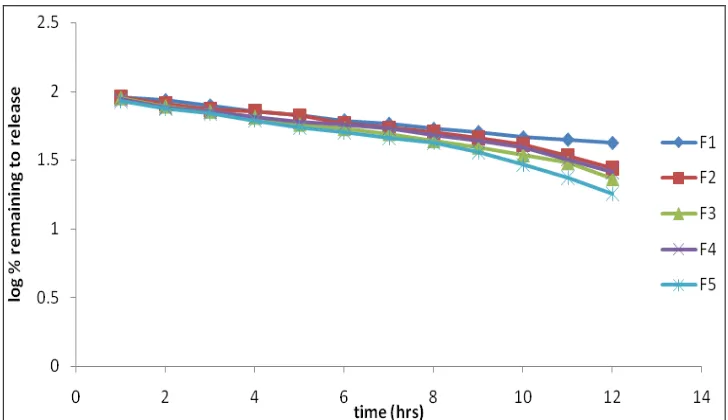
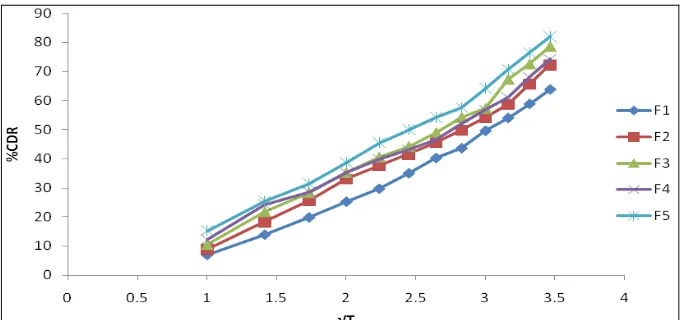

Related documents
The most important contributions of this paper are (i) the proposal of a concern mining approach for KDM, what may encourage other groups to start researching on
To support safe self-movement of the disabled and the aged, we developed an electric wheelchair that realizes the functions of detecting both the potential hazards in a
A Representative images and quantification of the proportion of microglia cells (Iba-1 + cells, red) adhered to myelin (MBP + , green) relative to the number of myelin fibers in
The objective of this study was to develop Fourier transform infrared (FTIR) spectroscopy in combination with multivariate calibration of partial least square (PLS) and
In AODV protocol, when a node receives a route reply packet (RREP), it checks the sequence number value in routing table; if it is greater than the one in the RREP, the RREP packet
[2] Recently published management guidelines for jaundiced near- term and term infants by the American Academy of Pediatrics (AAP) are based on the premise that
These have been commoner with sap from plants infected with the Datura strain than with the type strain, but even with the Datura strain precipitation end-points of 1/64