metal-organic papers
m510
Jian-Feng Liet al. [Zr(C12H28N2Si2)2] DOI: 10.1107/S1600536802015660 Acta Cryst.(2002). E58, m510±m512 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
Bis[(1
R
,2
R
)-(
ÿ
)-1,2-bis(trimethylsilylamino)-cyclohexane(2
ÿ
)-
j
2N,N
000]zirconium(IV)
Jian-Feng Li,aLin-Hong Weng,b
Shu-Ping Huang,aHong-Bo
Tongaand Dian-Sheng Liua*
aSchool of Chemistry and Chemical Engineering, The Shanxi University, Shanxi, People's Republic of China, andbDepartment of Chem-istry, The Fudan University, Shanghai, People's Republic of China
Correspondence e-mail: dsLiu@sxu.edu.cn
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C±C) = 0.012 AÊ
Rfactor = 0.047
wRfactor = 0.109
Data-to-parameter ratio = 14.9
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
The title complex, [(1R,2R)-(±)-1,2-(NSiMe3)2-C6H10]2Zr or [Zr(C12H28N2Si2)2], can be viewed as a tetrahedron located on a non-crystallographic twofold axis, which passes through the Zr atom and between the two N atoms of each cyclohexane-based ligand. The amide N atoms form two almost perpendicular planes with the central Zr atom, with a dihedral angle of 87.23 (18).
Comment
Group IV metal complexes containing bidentate bis(amide) or tetradentate bis(amidinate) ligands are promising systems for applications in catalysis because of their relationship to the well studied metallocene analogues Cp2MX2, bis(amide) (R2N)2MX2 (Minhas et al., 1996), and bis(amidinate) [N(R)C(R0)N(R)]
2MX2 (Bambirra et al., 2001; Foley et al., 2000). An attractive goal in this area is to develop chiral metal complexes for exploitation in stereoselective catalysis (Hagadorn & Arnold, 1998). (1R,2R)-Diaminocyclohexane has proved a useful building block for a broad range of chiral reagents (Larrow et al., 1994). Recently, we reported the chemistry of its lithium derivatives, including mono- and dili-thium (1R,2R)-(ÿ)-1,2-(NHSiMe3)2-C6H10, and grew single crystals suitable for X-ray diffraction analysis (Liet al., 2002). Using this approach for the preparation of transition-metal complexes, we now report the synthesis and structure of the zirconium bis(bisamide) title complex, (I).
The molecular structure of (I) is shown in Fig. 1. The two cyclohexane rings adopt chair conformations. The molecule of (I) has a non-crystallographic twofold axis, which passes through the Zr atom and between the pairs of N atoms, N1 and N2, and N3 and N4 (Fig. 2). The structure can be viewed as a tetrahedron, with the metal ion in the center, bonded to two bidentate ligands.
All the amide N atoms aresp2-hybridized (for example, the sum of the angles around N1 is 358.5). The geometry around
Zr is distorted tetrahedral, with the four ZrÐN bond distances in the range 2.058 (4)±2.077 (5) AÊ and similar to literature values (Leeet al., 2000). The ®ve-membered ZrÐNÐCÐCÐ N rings adopt an envelope conformation. From Table 1, we note that the intraannular NÐZrÐN angles, N1ÐZr1ÐN2 [86.8 (2)] and N3ÐZr1ÐN4 [86.72 (19)], are signi®cantly
smaller than the ideal tetrahedral value due to chelation, while the other NÐZrÐN angles between the two chelate rings are correspondingly larger. The N1ÐZr1ÐN2 and N3ÐZr1ÐN4 planes are almost perpendicular, the dihedral angle being 87.2 (2). In addition, the two NSiMe
3groups related by the
approximate C2 axis are symmetrically placed above and below the opposite NÐZrÐN plane, as in the complex
{()-trans-1,2-(NSiMe3)2±C6H10}TiI2(Tsuieet al., 1997).
Experimental
Li2[(1R,2R)-(ÿ)-1,2-(NSiMe3)2-C6H10] can be converted to its
sodium salt easily in hexane. When two equivalents of NatBu were
added to the clear solution of the dilithium salt at room temperature, a white precipitate formed immediately. The mixture was stirred overnight and ®ltered. The white precipitate which was isolated was dried in a vacuum to give Na2[(1R,2R)-(ÿ)-1,2-(NSiMe3)2-C6H10].
Treatment of Na2[(1R,2R)-(ÿ)-1,2-(NSiMe3)2-C6H10] with a half
equivalent of ZrCl4 in toluene at low temperature gave a yellow
solution. After ®ltering, the reaction afforded a colorless crystalline product in high yield. Spectroscopic analysis: 1H NMR (CDCl
3, ,
p.p.m.): 0.02 (s, 36H, SiMe3), 1.15 (m, 8H, CH), 1.59 (m, 8H, CH), 3.56 (m, 4H, CH);13C NMR (CDCl
3,, p.p.m.): 2.42, 25.83, 36.26, 65.95.
Crystal data [Zr(C12H28N2Si2)2]
Mr= 604.31
Trigonal,P31
a= 10.473 (5) AÊ
c= 28.166 (18) AÊ
V= 2676 (2) AÊ3
Z= 3
Dx= 1.125 Mg mÿ3
MoKradiation Cell parameters from 956
re¯ections
= 2.2±27.0
= 0.46 mmÿ1
T= 298 (2) K Block, colorless 0.350.300.10 mm Data collection
Bruker SMART CCD area-detector diffractometer
!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1997)
Tmin= 0.856,Tmax= 0.956 11 157 measured re¯ections
4626 independent re¯ections 4526 re¯ections withI> 2(I)
Rint= 0.038
max= 25.0
h=ÿ12!11
k=ÿ12!12
l=ÿ33!20 Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.047
wR(F2) = 0.109
S= 1.28 4626 re¯ections 310 parameters
H atoms treated by a mixture of independent and constrained re®nement
w= 1/[2(F
o2) + (0.0337P)2
+ 2.5967P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.003
max= 0.48 e AÊÿ3
min=ÿ1.04 e AÊÿ3
Absolute structure: Flack (1983), 1477 Friedel pairs
Flack parameter = 0.03 (6)
Table 1
Selected geometric parameters (AÊ,).
Zr1ÐN4 2.058 (4)
Zr1ÐN2 2.064 (5) Zr1ÐN1Zr1ÐN3 2.064 (5)2.077 (5)
N4ÐZr1ÐN2 119.06 (18)
N4ÐZr1ÐN1 123.59 (19)
N2ÐZr1ÐN1 86.8 (2)
N4ÐZr1ÐN3 86.72 (19)
N2ÐZr1ÐN3 123.3 (2)
N1ÐZr1ÐN3 121.71 (19)
N2ÐZr1ÐN1ÐC1 ÿ10.2 (4)
N1ÐZr1ÐN2ÐC6 ÿ12.9 (4)
N4ÐZr1ÐN3ÐC13 ÿ13.0 (4)
N3ÐZr1ÐN4ÐC18 ÿ10.5 (4)
N1ÐC1ÐC6ÐN2 ÿ44.0 (7)
N3ÐC13ÐC18ÐN4 ÿ45.9 (7)
All H atoms were initially located in a difference Fourier map. The methyl H atoms were then constrained to an ideal geometry, with CÐ H distances of 0.98 AÊ andUiso(H) = 1.5Ueq(C), but each group was
allowed to rotate freely about its CÐC bond. The position of the
Figure 2
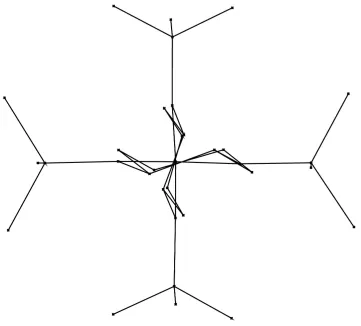
A diagram of the molecule of (I), viewed along the non-crystallographic twofold axis. All H atoms have been omitted for clarity.
Figure 1
View of the molecule of (I), showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are represented by small spheres of arbitrary radii.
Figure 3
metal-organic papers
m512
Jian-Feng Liet al. [Zr(C12H28N2Si2)2] Acta Cryst.(2002). E58, m510±m512amine H atom was re®ned freely along with an isotropic displacement parameter. All other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with CÐH distances in the range 0.95±1.00 AÊ andUiso(H) = 1.2Ueq(C).
Data collection:SMART(Siemens, 1996); cell re®nement:SAINT
(Siemens, 1996); data reduction:SAINT; program(s) used to solve structure:SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3 (Farrugia, 1997); software used to prepare material for publication:SHELXL97.
The authors thank the Natural Science Foundation of China (20171030 and 29872024, D-SL) and the Natural Science Foundation of ShanXi province (20011008, D-SL)
References
Bambirra, S., Meetsma, A., Hesson, B. & Teuben, J. H. (2001). Organo-metallics,20, 782±785.
Farrugia, L. J. (1997).ORTEP-3. University of Glasgow, Scotland. Flack, H. D. (1983).Acta Cryst.A39, 876±881.
Foley, S. R., Zhou, Y., Yap, G. P. A. & Richeson, D. S. (2000).Inorg. Chem.39, 924±929.
Hagadorn, J. R. & Arnold, J. (1998).Angew. Chem. Int. Ed.37, 1729±1731. Larrow, J. F., Jacobsen, E. N., Gao, Y., Hong, Y., Nie, X. & Zepp, C. M. (1994).
J. Org. Chem.59, 1939±1942.
Lee, C. H., La, Y.-H. & Park, J. W. (2000).Organometallics,19, 344±351. Li, J.-F., Weng, L.-H., Wei, X.-H. & Liu, D.-S. (2002).J. Chem. Soc. Dalton
Trans.7, 1401±1405.
Minhas, R. K., Scoles, L., Wong, S. & Gambarott, S. (1996).Organometallics,
15, 1113±1121.
Sheldrick, G. M. (1997).SHELXS97,SHELXL97 andSADABS. University of GoÈttingen, Germany.
Siemens (1996).SMARTandSAINT. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Tsuie, B., Swenson, D. C., Jordan, R. F. & Petersen, J. L. (1997).
supporting information
Acta Cryst. (2002). E58, m510–m512 [doi:10.1107/S1600536802015660]
Bis[(1
R
,2
R
)-(
−
)-1,2-bis(trimethylsilylamino)cyclohexane(2
−
)-κ
2N,N
′
]zirconium(IV)
Jian-Feng Li, Lin-Hong Weng, Shu-Ping Huang, Hong-Bo Tong and Dian-Sheng Liu
S1. Comment
Group 4 metal complexes containing bidentate bis(amide) or tetradentate bis(amidinate) ligands are promising systems for applications in catalysis because of their relationship to the well studied metallocene analogues Cp2MX2, bis(amide)
(R2N)2MX2 (Minhas et al., 1996), and bis(amidinate) [N(R)C(R′)N(R)]2MX2 (Bambirra et al., 2001; Foley et al., 2000). An
attractive goal in this area is to develop chiral metal complexes for exploitation in stereoselective catalysis (Hagadorn & Arnold, 1998). The (1R,2R)-DACH served as broad-range chiral reagents has been proved a useful building block (Larrow et al., 1994). Recently, we reported the chemistry of its lithium derivatives, including mono- and dilithium (1R,2R)-(-)-1,2-(NHSiMe3)2-C6H10, and grew single crystals suitable for X-ray diffraction analysis (Li et al., 2002). Using
this approach for the preparation of transition-metal complexes, we now reported the syntheses and structures of zirconium bis(amide) tetra-dentate complex, (I).
The molecular structure of (I) is shown in Fig. 1. The two cyclohexyl groups adopt chair conformations. The molecule of (I) is located on a crstallographic twofold axis, which passes through the Zr atom and the center of the N2—N1 and N3 —N4 bonds, and therefore has high symmetry (Fig. 2). The structure can be viewed as a tetrahedron, with the metal ion in the center bonded to two bidentate ligands.
All the amide N atoms are sp2-hybridized (for example, the sum of the angles around N1 is 358.5°). The geometry
around Zr is distorted tetrahedral, with the four Zr—N bond distances in the range 2.058 (4)–2.077 (5) Å and similar to the literature values (Lee et al., 2000). The five-membered Zr—N—C—C—N ring adopts an envelope conformation. From Table 1, we note that the intraannular N—Zr—N′ angles, N1—Zr1—N2 [86.8 (2)°] and N3—Zr1—N4
[86.72 (19)°], are significantly smaller than the ideal tetrahedral value due to chelation, while the other two N—Zr—N′ angles between the two chelate rings, N1—Zr1—N3 [121.71 (19)°] and N2—Zr1—N4 [119.06 (18)°] are
correspondingly larger. The N1—Zr1—N2 and N3—Zr1—N4 planes are almost vertical and the dihedral angle is 92.8°. In addition, the two NSiMe3 groups related by the crystallographically imposed C2 axis are symmetrically placed above
and below the opposite N—Zr—N′ plane, similar to the complex {(±)-trans-1,2-(NSiMe3)2-C6H10}TiI2 (Tsuie et al.,
1997).
S2. Experimental
Li2[(1R,2R)-(-)-1,2-(NSiMe3)2-C6H10] can be translated to its sodium salt easily in hexane. When two equivalents of
NaBut was added to the clear solution of dilithium salt at room temperature, a white precipitate formed immediately. The
mixture was stirred overnight and filtered. The white precipitate which was isolated was dried in a vacuum to give Na2[(1R,2R)-(-)-1,2-(NSiMe3)2-C6H10]. Treatment of Na2[(1R,2R)-(-)-1,2-(NSiMe3)2-C6H10] with a half equivalent of
ZrCl4 in toluene at low temperature gave a yellow solution. After filtering, the reaction afforded a colorless crystalline
product in high yield. Spectroscopic analysis, 1H NMR (CDCl
supporting information
sup-2
Acta Cryst. (2002). E58, m510–m512
(m, 8H, CH), 3.56 (m, 4H, CH); 13C NMR (CDCl
3, δ, p.p.m.): 2.42, 25.83, 36.26, 65.95.
S3. Refinement
All H atoms were initially located in a difference Fourier map. The methyl H atoms were then constrained to an ideal geometry, with C—H distances of 0.98 Å and Uiso(H) = 1.5Ueq(C), but each group was allowed to rotate freely about its C
[image:5.610.129.483.196.474.2]—C bond. The position of the amine H atom was refined freely along with an isotropic displacement parameter. All other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H distances in the range 0.95–1.00 Å and Uiso(H) = 1.2Ueq(C).
Figure 1
Figure 2
supporting information
sup-4
[image:7.610.128.483.70.320.2]Acta Cryst. (2002). E58, m510–m512
Figure 3
A packing diagram of the title complex.
bis[(1R,2R)-(-)-1,2-bis(trimethylsilylamino)cyclohexane-κ2N,N′]zirconium
Crystal data
[Zr(C12H28N2Si2)2]
Mr = 604.31
Trigonal, P31
a = 10.473 (5) Å
c = 28.166 (18) Å
V = 2676 (2) Å3
Z = 3
F(000) = 972
Dx = 1.125 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 956 reflections
θ = 2.3–27.0°
µ = 0.46 mm−1
T = 298 K Block, colorless 0.35 × 0.30 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1997)
Tmin = 0.856, Tmax = 0.956
11157 measured reflections 4626 independent reflections 4526 reflections with I > 2σ(I)
Rint = 0.038
θmax = 25.0°, θmin = 2.3°
h = −12→11
k = −12→12
l = −33→20
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.047
wR(F2) = 0.109
S = 1.28 4626 reflections 310 parameters
1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0337P)2 + 2.5967P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.003
Δρmax = 0.48 e Å−3
Δρmin = −1.04 e Å−3
Absolute structure: Flack (1983), 0000 Friedel pairs
Absolute structure parameter: 0.03 (6)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Zr1 0.27699 (5) 0.22031 (6) 0.23036 (2) 0.04014 (14) Si1 0.3511 (2) 0.4663 (2) 0.14258 (8) 0.0591 (5) Si2 0.0084 (2) −0.1279 (2) 0.25372 (8) 0.0604 (5) Si3 0.2034 (2) 0.3955 (2) 0.31761 (8) 0.0642 (5) Si4 0.5477 (2) 0.1437 (2) 0.20739 (7) 0.0529 (4) N1 0.2373 (5) 0.2965 (5) 0.16764 (18) 0.0419 (11) N2 0.0851 (5) 0.0247 (5) 0.21697 (19) 0.0499 (12) N3 0.3166 (5) 0.3367 (6) 0.29350 (19) 0.0463 (12) N4 0.4678 (5) 0.2158 (5) 0.24396 (17) 0.0407 (11) C1 0.1160 (7) 0.1704 (6) 0.1428 (2) 0.0454 (14)
H1 0.1602 0.1233 0.1242 0.054*
C2 0.0296 (8) 0.2101 (8) 0.1083 (3) 0.0620 (18)
H2A 0.0971 0.2826 0.0859 0.074*
H2B −0.0188 0.2540 0.1257 0.074*
C3 −0.0865 (8) 0.0746 (9) 0.0812 (3) 0.074 (2)
H3A −0.1439 0.1029 0.0613 0.088*
H3B −0.0376 0.0379 0.0607 0.088*
C4 −0.1880 (9) −0.0458 (9) 0.1146 (3) 0.084 (3)
H4A −0.2529 −0.1333 0.0964 0.101*
H4B −0.2487 −0.0146 0.1314 0.101*
C5 −0.1031 (8) −0.0839 (8) 0.1507 (3) 0.071 (2)
H5A −0.1718 −0.1575 0.1727 0.085*
H5B −0.0506 −0.1253 0.1344 0.085*
C6 0.0061 (6) 0.0528 (7) 0.1781 (2) 0.0501 (15)
H6 −0.0503 0.0939 0.1928 0.060*
C7 0.4256 (11) 0.4550 (11) 0.0833 (4) 0.101 (3)
H7A 0.4770 0.4010 0.0863 0.152*
H7B 0.4923 0.5527 0.0720 0.152*
H7C 0.3457 0.4056 0.0613 0.152*
supporting information
sup-6
Acta Cryst. (2002). E58, m510–m512
H8A 0.1895 0.5420 0.1112 0.152*
H8B 0.3372 0.6813 0.1269 0.152*
H8C 0.2186 0.5854 0.1651 0.152*
C9 0.5089 (9) 0.5618 (9) 0.1851 (4) 0.093 (3)
H9A 0.4733 0.5771 0.2148 0.140*
H9B 0.5807 0.6552 0.1721 0.140*
H9C 0.5533 0.5020 0.1902 0.140*
C10 −0.1699 (9) −0.1626 (11) 0.2808 (4) 0.094 (3) H10A −0.1545 −0.0758 0.2973 0.142* H10B −0.2046 −0.2433 0.3027 0.142* H10C −0.2419 −0.1864 0.2562 0.142* C11 −0.0191 (13) −0.3010 (10) 0.2233 (4) 0.118 (4) H11A −0.0725 −0.3153 0.1943 0.177* H11B −0.0740 −0.3842 0.2438 0.177*
H11C 0.0751 −0.2913 0.2165 0.177*
C12 0.1440 (9) −0.0853 (10) 0.3022 (3) 0.077 (2)
H12A 0.2396 −0.0532 0.2888 0.115*
H12B 0.1145 −0.1721 0.3210 0.115*
H12C 0.1479 −0.0085 0.3219 0.115*
C13 0.4331 (6) 0.3223 (7) 0.3186 (2) 0.0448 (14)
H13 0.3847 0.2280 0.3356 0.054*
C14 0.5178 (8) 0.4452 (8) 0.3560 (3) 0.0645 (18)
H14A 0.5729 0.5398 0.3403 0.077*
H14B 0.4483 0.4501 0.3776 0.077*
C15 0.6230 (10) 0.4127 (9) 0.3838 (3) 0.076 (2)
H15A 0.6792 0.4926 0.4059 0.092*
H15B 0.5666 0.3230 0.4020 0.092*
C16 0.7270 (10) 0.3951 (11) 0.3514 (3) 0.089 (3)
H16A 0.7859 0.3656 0.3699 0.107*
H16B 0.7932 0.4893 0.3367 0.107*
C17 0.6456 (8) 0.2808 (9) 0.3127 (3) 0.0659 (19)
H17A 0.5878 0.1843 0.3271 0.079*
H17B 0.7166 0.2774 0.2914 0.079*
C18 0.5441 (6) 0.3184 (6) 0.2845 (2) 0.0441 (14)
H18 0.6046 0.4177 0.2715 0.053*
C19 0.1210 (12) 0.3026 (12) 0.3753 (3) 0.106 (3)
H19A 0.0340 0.2094 0.3693 0.159*
H19B 0.0955 0.3637 0.3937 0.159*
H19C 0.1912 0.2868 0.3924 0.159*
C20 0.2951 (12) 0.5980 (10) 0.3254 (4) 0.109 (3)
H20A 0.3817 0.6308 0.3446 0.163*
H20B 0.2286 0.6229 0.3408 0.163*
H20C 0.3225 0.6451 0.2950 0.163*
C21 0.0532 (10) 0.3464 (12) 0.2739 (4) 0.103 (3)
H21A 0.0940 0.3691 0.2424 0.155*
H21B 0.0048 0.4018 0.2805 0.155*
H21C −0.0168 0.2429 0.2761 0.155*
H22A 0.7276 0.3729 0.1757 0.116*
H22B 0.7607 0.2495 0.1591 0.116*
H22C 0.8034 0.3143 0.2105 0.116*
C23 0.4228 (10) 0.0661 (11) 0.1551 (3) 0.087 (3)
H23A 0.3229 0.0070 0.1659 0.131*
H23B 0.4503 0.0062 0.1369 0.131*
H23C 0.4309 0.1452 0.1357 0.131*
C24 0.5645 (11) −0.0086 (10) 0.2349 (4) 0.094 (3)
H24A 0.6541 0.0319 0.2530 0.140*
H24B 0.5664 −0.0715 0.2104 0.140*
H24C 0.4818 −0.0648 0.2554 0.140*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-8
Acta Cryst. (2002). E58, m510–m512
Geometric parameters (Å, º)
Zr1—N4 2.058 (4) C9—H9A 0.9600
Zr1—N2 2.064 (5) C9—H9B 0.9600
Zr1—N1 2.064 (5) C9—H9C 0.9600
Zr1—N3 2.077 (5) C10—H10A 0.9600
Si1—N1 1.721 (5) C10—H10B 0.9600
Si1—C8 1.870 (8) C10—H10C 0.9600
Si1—C7 1.870 (9) C11—H11A 0.9600
Si1—C9 1.874 (9) C11—H11B 0.9600
Si2—N2 1.728 (5) C11—H11C 0.9600
Si2—C12 1.857 (9) C12—H12A 0.9600
Si2—C10 1.877 (8) C12—H12B 0.9600
Si2—C11 1.892 (10) C12—H12C 0.9600
Si3—N3 1.726 (5) C13—C18 1.524 (9)
Si3—C20 1.852 (9) C13—C14 1.554 (8)
Si3—C21 1.857 (10) C13—H13 0.9800
Si3—C19 1.868 (9) C14—C15 1.522 (10)
Si4—N4 1.721 (5) C14—H14A 0.9700
Si4—C24 1.859 (8) C14—H14B 0.9700
Si4—C22 1.864 (8) C15—C16 1.501 (12)
Si4—C23 1.864 (8) C15—H15A 0.9700
N1—C1 1.473 (7) C15—H15B 0.9700
N2—C6 1.488 (8) C16—C17 1.524 (11)
N3—C13 1.481 (8) C16—H16A 0.9700
N4—C18 1.497 (7) C16—H16B 0.9700
C1—C2 1.521 (8) C17—C18 1.527 (8)
C1—C6 1.553 (9) C17—H17A 0.9700
C1—H1 0.9800 C17—H17B 0.9700
C2—C3 1.533 (10) C18—H18 0.9800
C2—H2A 0.9700 C19—H19A 0.9600
C2—H2B 0.9700 C19—H19B 0.9600
C3—C4 1.505 (12) C19—H19C 0.9600
C3—H3A 0.9700 C20—H20A 0.9600
C3—H3B 0.9700 C20—H20B 0.9600
C4—C5 1.530 (11) C20—H20C 0.9600
C4—H4A 0.9700 C21—H21A 0.9600
C4—H4B 0.9700 C21—H21B 0.9600
C5—C6 1.522 (9) C21—H21C 0.9600
C5—H5A 0.9700 C22—H22A 0.9600
C5—H5B 0.9700 C22—H22B 0.9600
C6—H6 0.9800 C22—H22C 0.9600
C7—H7A 0.9600 C23—H23A 0.9600
C7—H7B 0.9600 C23—H23B 0.9600
C7—H7C 0.9600 C23—H23C 0.9600
C8—H8A 0.9600 C24—H24A 0.9600
C8—H8B 0.9600 C24—H24B 0.9600
N4—Zr1—N2 119.06 (18) Si1—C9—H9C 109.5 N4—Zr1—N1 123.59 (19) H9A—C9—H9C 109.5
N2—Zr1—N1 86.8 (2) H9B—C9—H9C 109.5
N4—Zr1—N3 86.72 (19) Si2—C10—H10A 109.5 N2—Zr1—N3 123.3 (2) Si2—C10—H10B 109.5 N1—Zr1—N3 121.71 (19) H10A—C10—H10B 109.5 N1—Si1—C8 113.9 (4) Si2—C10—H10C 109.5 N1—Si1—C7 113.3 (4) H10A—C10—H10C 109.5 C8—Si1—C7 107.7 (5) H10B—C10—H10C 109.5 N1—Si1—C9 105.1 (3) Si2—C11—H11A 109.5 C8—Si1—C9 108.0 (5) Si2—C11—H11B 109.5 C7—Si1—C9 108.7 (5) H11A—C11—H11B 109.5 N2—Si2—C12 105.8 (3) Si2—C11—H11C 109.5 N2—Si2—C10 112.3 (4) H11A—C11—H11C 109.5 C12—Si2—C10 108.4 (4) H11B—C11—H11C 109.5 N2—Si2—C11 113.1 (4) Si2—C12—H12A 109.5 C12—Si2—C11 107.1 (5) Si2—C12—H12B 109.5 C10—Si2—C11 109.8 (5) H12A—C12—H12B 109.5 N3—Si3—C20 113.6 (4) Si2—C12—H12C 109.5 N3—Si3—C21 106.1 (3) H12A—C12—H12C 109.5 C20—Si3—C21 106.2 (5) H12B—C12—H12C 109.5 N3—Si3—C19 112.1 (4) N3—C13—C18 112.4 (5) C20—Si3—C19 109.5 (5) N3—C13—C14 113.5 (5) C21—Si3—C19 109.0 (5) C18—C13—C14 108.8 (5) N4—Si4—C24 113.8 (4) N3—C13—H13 107.3 N4—Si4—C22 112.2 (3) C18—C13—H13 107.3 C24—Si4—C22 109.0 (4) C14—C13—H13 107.3 N4—Si4—C23 105.9 (3) C15—C14—C13 109.9 (6) C24—Si4—C23 107.6 (5) C15—C14—H14A 109.7 C22—Si4—C23 108.0 (4) C13—C14—H14A 109.7 C1—N1—Si1 125.0 (4) C15—C14—H14B 109.7 C1—N1—Zr1 108.0 (4) C13—C14—H14B 109.7 Si1—N1—Zr1 125.5 (3) H14A—C14—H14B 108.2 C6—N2—Si2 125.2 (4) C16—C15—C14 111.5 (7) C6—N2—Zr1 107.4 (4) C16—C15—H15A 109.3 Si2—N2—Zr1 125.5 (3) C14—C15—H15A 109.3 C13—N3—Si3 126.6 (4) C16—C15—H15B 109.3 C13—N3—Zr1 106.4 (4) C14—C15—H15B 109.3 Si3—N3—Zr1 125.1 (3) H15A—C15—H15B 108.0 C18—N4—Si4 124.7 (4) C15—C16—C17 112.1 (7) C18—N4—Zr1 107.6 (3) C15—C16—H16A 109.2 Si4—N4—Zr1 126.1 (3) C17—C16—H16A 109.2 N1—C1—C2 115.0 (5) C15—C16—H16B 109.2 N1—C1—C6 111.8 (5) C17—C16—H16B 109.2 C2—C1—C6 108.6 (5) H16A—C16—H16B 107.9
N1—C1—H1 107.0 C16—C17—C18 110.9 (6)
supporting information
sup-10
Acta Cryst. (2002). E58, m510–m512
C6—C1—H1 107.0 C18—C17—H17A 109.5
C1—C2—C3 111.7 (6) C16—C17—H17B 109.5
C1—C2—H2A 109.3 C18—C17—H17B 109.5
C3—C2—H2A 109.3 H17A—C17—H17B 108.0
C1—C2—H2B 109.3 N4—C18—C13 111.1 (5)
C3—C2—H2B 109.3 N4—C18—C17 113.8 (5)
H2A—C2—H2B 107.9 C13—C18—C17 108.6 (5)
C4—C3—C2 111.4 (6) N4—C18—H18 107.7
C4—C3—H3A 109.3 C13—C18—H18 107.7
C2—C3—H3A 109.3 C17—C18—H18 107.7
C4—C3—H3B 109.3 Si3—C19—H19A 109.5
C2—C3—H3B 109.3 Si3—C19—H19B 109.5
H3A—C3—H3B 108.0 H19A—C19—H19B 109.5
C3—C4—C5 112.0 (6) Si3—C19—H19C 109.5
C3—C4—H4A 109.2 H19A—C19—H19C 109.5
C5—C4—H4A 109.2 H19B—C19—H19C 109.5
C3—C4—H4B 109.2 Si3—C20—H20A 109.5
C5—C4—H4B 109.2 Si3—C20—H20B 109.5
H4A—C4—H4B 107.9 H20A—C20—H20B 109.5
C6—C5—C4 110.6 (6) Si3—C20—H20C 109.5
C6—C5—H5A 109.5 H20A—C20—H20C 109.5
C4—C5—H5A 109.5 H20B—C20—H20C 109.5
C6—C5—H5B 109.5 Si3—C21—H21A 109.5
C4—C5—H5B 109.5 Si3—C21—H21B 109.5
H5A—C5—H5B 108.1 H21A—C21—H21B 109.5
N2—C6—C5 114.6 (6) Si3—C21—H21C 109.5 N2—C6—C1 111.3 (5) H21A—C21—H21C 109.5 C5—C6—C1 109.4 (6) H21B—C21—H21C 109.5
N2—C6—H6 107.0 Si4—C22—H22A 109.5
C5—C6—H6 107.0 Si4—C22—H22B 109.5
C1—C6—H6 107.0 H22A—C22—H22B 109.5
Si1—C7—H7A 109.5 Si4—C22—H22C 109.5
Si1—C7—H7B 109.5 H22A—C22—H22C 109.5
H7A—C7—H7B 109.5 H22B—C22—H22C 109.5
Si1—C7—H7C 109.5 Si4—C23—H23A 109.5
H7A—C7—H7C 109.5 Si4—C23—H23B 109.5
H7B—C7—H7C 109.5 H23A—C23—H23B 109.5
Si1—C8—H8A 109.5 Si4—C23—H23C 109.5
Si1—C8—H8B 109.5 H23A—C23—H23C 109.5
H8A—C8—H8B 109.5 H23B—C23—H23C 109.5
Si1—C8—H8C 109.5 Si4—C24—H24A 109.5
H8A—C8—H8C 109.5 Si4—C24—H24B 109.5
H8B—C8—H8C 109.5 H24A—C24—H24B 109.5
Si1—C9—H9A 109.5 Si4—C24—H24C 109.5
Si1—C9—H9B 109.5 H24A—C24—H24C 109.5
H9A—C9—H9B 109.5 H24B—C24—H24C 109.5