www.wjpr.net 1486
ANTIHYPERGLYCAEMIC ACTIVITY OF ETHANOLIC EXTRACT
OF GREWIA ASIATICA (L.) LEAVES IN ALLOXAN INDUCED
DIABETIC MICE
Jitendra Bhangale1*, Sanjeev Acharya2, Tushar Deshmukh3 1
Department of Pharmacology, Smt. N. M. Padalia Pharmacy College, Ahmedabad, 382210
Gujarat, India.
2
Department of Pharmacognosy, Institute of Pharmacy, Nirma University, Ahmedabad,
382481, India.
3
Department of Pharmacognosy, Tapi Valley Education Society’s, Hon’ble, Loksevak
Madhukarrao Chaudhari College of Pharmacy, Faizpur, 425 503, Maharashtra, India.
ABSTRACT
Grewia asiatica L. (Tiliaceae) is widely used in traditional system of
medicine to treat diabetes in India. The ethanolic extracts (100, 200
and 400 mg/kg) were taken to evaluate the antihyperglycemic activity
against alloxan induced diabetic mice. Oral administration of extract
for acute study at 4 h resulted in a significant reduction of serum
glucose level but the effect waned at 24 h. Repeated administration of
extract for 28 days caused significant reduction in serum glucose level
and body weight. Extract at dose of 400 mg/kg significantly
suppressed the rise in blood glucose after 30 min in the acute glucose
tolerance test. These results indicated that G. asiatica enhanced the
antihyperglycemic activity and the extracts should further be subjected
to bioactivity guided drug discovery to isolate a lead compound
responsible for this activity.
Keywords: Grewia asiatica, Alloxan, OGTT.
1. INTRODUCTION
Diabetes, a life long progressive disease, is the result of body’s inability to produce insulin or
use insulin to its full potential, and is characterized by high circulating glucose [1-2].
Diabetes mellitus (DM) is now considered as heterogeneous group of diseases characterized
Article Received on 01 May 2013,
Revised on 25 May 2013, Accepted on 20 June 2013
*Correspondence for Author:
Jitendra Bhangale,
Assistant Professor
Department of Pharmacology,
Smt. N. M. Padalia Pharmacy
College, Ahmedabad, 382210
Gujarat, India.
www.wjpr.net 1487
by chronic hyperglycaemia from whatever cause leading to complications involving
cardiovascular, renal, neurological and ophthalmic systems [3]. Currently available synthetic
oral antihyperglycaemic agents now available have not shown to alter the progressive β cell
failure and the current agents may be associated with an increased risk of unwanted effects on
prolonged use [4]. The patients are using herbal medicines which have less side effects, easy
availability and economic [5].
Grewia asiatica L. (Tiliaceae) is one of the most commonly plants in India. In Hindi it is
popularly known as Shukri, tadachi, dhaman, parusha; other common names include Phalsa,
shunkri (Bengali), Indian phalsa (English).The G. asiatica is available throughout the year.
The plant has been reported to possess antioxidant [6], antihyperglycaemic [7],
radioprotective [8-10], hepatoprotective [11], antifungal and antiviral activity [12].
In traditional folklore medicine, the fruit has been used as astringent, stomachic and cooling
agent [13]. When unripe, it has been reported to alleviate inflammation and was administered
in respiratory, cardiac and blood disorders, as well as in fever. The fruit was also beneficial
for food throat ailments. Root bark has been prescribed for rheumatism and its infusion used
as a demulcent. The leaves were applied on skin eruptions. Seeds of G. asiatica has been
used as antifertility agent and was reported to have anti-implantation and abortifacient
activities [14]. In traditional folkloric medicine in Bangladesh, G. asiatica plant is commonly
used for gonorrhoes by the Garo tribe and local traditional healers in Madhupur and tangail
district [15]. It is also used to treat lack of appetite, typhus, acidity, giddiness, diarrhoea,
hypertension, stimulant, anorexia. In India, G. asiatica is also used for gonorrhoea and as
astringent, demulcent, rheumatism, stomachic and tumour.
Number of Indian medicinal plants has been claimed for their antidiabetic activity in the
traditional system of medicine, but all of them have not been reported scientifically. Many
indigenous drugs have been claimed to have antidiabetic effect in Ayurvedic system of
medicine but they were not properly investigated [16].
The objective of the present investigation was to study the effect of ethanolic extract of G.
asiatica on serum glucose levels and on the oral glucose tolerance test (OGTT) in alloxan
www.wjpr.net 1488 2. MATERIALS AND METHODS
2.1. Drugs and chemicals
Fresh G. asiatica leaves were collected from local area of Jalgoan district, Maharashtra, India
in the months of July-October. This plant was identified and authenticated by Dr. J. Jayanthi,
Scientist C & HOD, Botanical Survey of India, Pune. Voucher specimens No.
(BSI/WC/Tech./2011/34(C)) have been kept in Botanical Survey of India, Pune, Maharashtra,
India. Glyburide (Ranbaxy Pharma. Ltd. India), alloxan monohydrate (Spectrochem, India),
glucose estimation kit (Accurex Biomedical Pvt. Ltd., India) and D-glucose (S.D.
Fine-Chem. Ltd, India) were purchased from respective companies.
2.2. Animals
Adult Swiss albino mice, weighing between 25-30 g were used and acclimatized to laboratory
conditions for one week. All animals were housed in well ventilated polypropylene cages at
12 h light/dark schedule with 25±2ºC and 55-65% relative humidity. The animals were fed
with commercial pellet rats chow and water ad libitum as a standard diet. Institutional animal
ethics committee approved the experimental protocol in accordance with CPCSEA.
2.3. Preparation of leaf extract
The leaves were collected and dried in shade and ground. Coarsely powdered leaves were
used for the study. Coarsely powdered plant material (1000 g) was subjected to hot
continuous extraction with Ethanol (60 – 800C) in a soxhlet extractor at a temperature of 45-500C to 40 cycles per batch for 2 batches. The extraction was continued until the solvent in the thimble becomes clear indicating the completion of the extraction. After each extraction
the solvent was distilled off and concentrated extract was transferred to previously weighed
petri dish and evaporated to dryness at room temperature to obtain dried extracts. After
completion of drying the petri dish was weighed again. The yield of extract was calculated by
subtracting original weight of empty petri dish. The yield was 5.8 g/100 g. The G. asiatica
extract was dissolved in distilled water to prepare the drug solution of concentration of 100
mg/ml and used for pharmacological studies.
2.4. Preliminary phytochemical studies
Preliminary qualitative phytochemical screening for the identification of the
www.wjpr.net 1489 2.5. Acute oral toxicity of the extract
Adult Albino mice (25-30 g) were divided into five groups containing ten mice each. The
mice were fasted for 6 h and access only water ad libitum before experimrntal study. Group I
received only vehicle (distilled water). Group II, III, IV and V animals received with different
doses of Ethanolic extract of G. asiatica i.e. 1000, 2000, 3000 and 4000 mg/kg respectively.
All the doses and vehicle were administered orally. The mice were observed continuously for
2 h for behavioral, neurological and autonomic profiles for any lethality or death for the next
48 h [18].
2.6. Induction of experimental diabetes
Animals were made diabetic by a single intravenous injection of aqueous alloxan
monohydrate (70 mg /kg i.v.) solution [19]. After 48 h, blood samples were collected and
serum glucose levels were determined to confirm the development of diabetes. Only those
animals which showed hyperglycaemia (blood glucose levels > 200 mg/dl) were used in the
experiment [20-21].
2.7. Collection of blood and determination of serum glucose
Blood samples from the experimental mice were collected by retro orbital plexus technique
using heparinised capillary glass tubes. The collected blood samples were analyzed for
glucose levels by the glucose oxidase peroxidase (GOD/POD) method as described earlier
[22] and serum glucose levels were expressed in mg/dl.
2.8. Effect of Ethanolic extract of G. asiatica (EtGA) on serum glucose in alloxan-induced diabetic mice
Diabetic Swiss albino mice of either sex were fasted overnight and divided into five groups
(n =10) viz; Group I - vehicle (distilled water, 10 ml/kg), Group II - glyburide (10 mg/kg),
Group III - EtGA (100 mg/kg), Group IV - EtGA (200 mg/kg) and Group V - EtGA (400
mg/kg). EtGA and glyburide were administered orally.
The acute study involved estimation of serum glucose levels at 0, 2, 4, 6 and 24 hour after
EtGA and glyburide administration. The animals had free access to feed and water after 6 h.
The subacute study involved repeated administration of EtGA and glyburide for 28 days
(once a day) at a prefixed time and serum glucose levels were estimated in samples
www.wjpr.net 1490
administration was stopped and a rest period of 7 days was given to the animals to study
effect of EtGA and glyburide treatment on serum glucose levels after 7 days [23]. The
animals had free access to feed and water during this period. During the study period of 35
days the mice were weighed daily and their body weights were recorded.
2.9. Effect of EtGA on oral glucose tolerance test (OGTT) in normal and diabetic mice
The diabetic animals were fasted overnight before commencing the experiment. Nondiabetic
and diabetic mice were divided into five groups (n = 10) viz; Group I - vehicle (distilled
water, 10 ml/kg), Group II - glyburide (10 mg/kg), Group III - EtGA (100 mg/kg), Group IV
- EtGA (200 mg/kg) and Group V - EtGA (400 mg/kg).
The mice of all the groups were loaded with D-glucose (2.5 g/kg, p.o.) solution after half an
hour of drug administration [24-26]. Blood samples were withdrawn by the retro orbital
plexus technique before drug administration and at 30, 60, and 120 minutes after glucose
loading. The serum glucose was estimated immediately thereafter.
2.10. Statistical analysis
Data was expressed as mean ± SEM and statistical analysis was carried out by two-way
ANOVA with post hoc Dunnett’s test performed using GraphPad InStat version 3.00 for
Windows 95, GraphPad Software, San Diego California USA, www.graphpad.com. The
significance level was considered at 2α=0.05.
3. RESULTS & DISCUSSION
Plant have played a major role in the introduction of new therapeutic agents. Throughout the
world, number of medicinal plant has been claimed for the treatment of diabetes [27]. G.
asiatica is used as a medicine for the treatment of diabetes mellitus. Glyburide is a potent,
second-generation, oral sulfonylurea antidiabetic agent used as an adjunct to diet to lower
blood glucose levels in patients with diabetes mellitus. The hypoglycaemic action of
glyburide is due to stimulation of pancreatic islet cells, which results in an increase in insulin
secretion. The effects of sulfonylurea are initiated by binding to and blocking on ATP
sensitive K+ channel, which have been cloned. The drugs thus resemble physiological secretagogues (e.g. glucose, leucine) which also lower the conductance of this channel.
www.wjpr.net 1491
sensitive Ca+2 channel. Prolonged administration of glyburide also produces extrapancreatic effects that contribute to its hypoglycaemic activity [28].
The EtGA was found to be safe at all the doses used and there was no mortality found up to
the dose of 5000 mg/kg of EtGA when administered orally. Therefore, we have selected 500
mg/kg as the therapeutic dose and made variations by taking 100 mg/kg as lower dose and
400 mg/kg as higher dose.
A single administration of EtGA( 400 mg/kg) as well as glyburide (10 mg/kg) significantly
reduced serum glucose levels at 4 h and EtGA (200 and 400 mg/kg) and glyburide (10
mg/kg) significantly reduced serum glucose levels at 6 h.
The reduction in serum glucose from basal value (before) at 6 h after glyburide and EtGA
(200 and 400 mg/kg) were 127.11, 172.63 and 213.54 respectively. The onset of the
antihyperglycaemic effect of glyburide was at 2 h and EtGA (400 mg/kg) was at 4 h; the peak
effect was 6 h but the effect waned at 24 h. EtGA (400 mg/kg) resulted in lowered serum
glucose at 24 h. (Table 1)
In the subacute study, repeated administration (once a day for 28 days) of EtGA and
glyburide caused significant reduction in the serum glucose level as compared to vehicle
treated group. On the 21st day, EtGA (200 and 400 mg/kg) and glyburide showed significant reduction in the serum glucose level as compared to vehicle treated group. On the 35th day, the reductions in serum glucose level of glyburide and EtGA (100, 200 and 400 mg/dl) were
268.62, 94.16, 171.88 and 234.57 respectively. (Table 2) The body weight of vehicle treated
diabetic animals decreased during the study period. Glyburide and EtGA (400 mg/kg)
prevented the decreased in body weight of diabetic animals (Table 3).
Subacute treatment for 35 days with the EtGA in the treated doses brought about
improvement in body weights indicating its beneficial effect in preventing loss of body
weight in diabetic animals [29]. The ability of EtGA to prevent body weight loss seems to be
due to its ability to reduced hyperglycaemia.
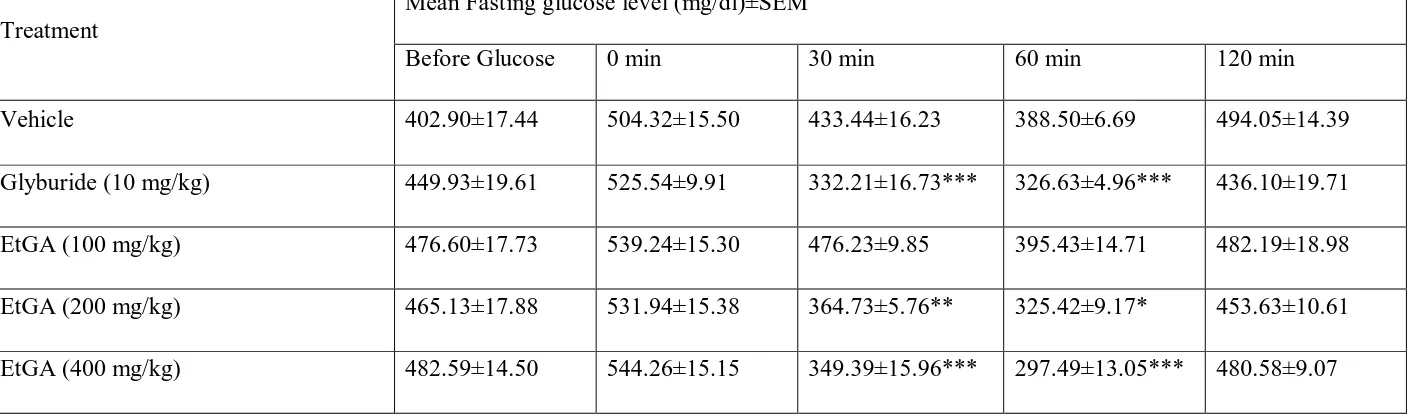
In the oral glucose tolerance test, administration of glucose load (2.5 g/kg) increased serum
glucose levels significantly after 30 min in non diabetic and alloxan treated diabetic mice.
www.wjpr.net 1492
the glucose threshold within 60 min, which was then reversed at 120 min after glucose
loading nondiabetic (Table 4) as well as alloxan induced diabetic animals (Table 5).
EtGA significantly enhanced glucose utilization in OGTT in both nondiabetic and diabetic
animals. From the data obtained OGTT, it is clear that administration of EtGA effectively
prevented the increase in serum glucose level without causing a hypoglycaemic state. The
effect may be due to restoration of the delayed insulin response. The results of both acute and
subacute study hypothesized that the late onset of action and prolonged duration of action of
EtGA may results from improved pancreatic cytoarchitecture. In this context, other medicinal
plants, such as Cassia auriculata [24], Pleurotus pulmonarius [25] have been reported to
possess similar effects.
Flavonoids are potent antioxidant and known to modulate the activities of various enzymes
due to their interaction with various biomolecules [30]. Apart from flavonoids, alkaloids,
tannins and phenolics are the other bioactive principles reported to possess
antihyperglycaemic activity [31]. Flavonoids regenerate the damaged ß cells in the alloxan
diabetic rats [32].
Preliminary phytochemical analysis indicated that, the leaves extracts of G. asiatica contain
alkaloids, flavonoids, tannins, sterols, carbohydrates and glycosides (Table 6).
The traditional medicinal plants with various active principles and properties have been used
since ancient times by physicians and laymen to treat a great variety of human diseases such
as diabetes, coronary heart disease and cancer. The beneficial multiple activities like
manipulating carbohydrate metabolism by various mechanisms, preventing and restoring
integrity, function of β-cells, insulin releasing activity, improving glucose uptake and
utilization and the antioxidant properties present in medicinal plants offer exciting
opportunity to develop them into novel therapeutics [33].
Antihyperglycaemic activity of ethanolic extract of G. asiatica may probably be due to the
www.wjpr.net 1493 Table 1: Effect of EtGA on serum glucose level in alloxan-induced diabetic mice (Acute study).
Treatment
Mean fasting glucose level (mg/dl)±SEM
0 h 2 h 4 h 6 h 24 h
Vehicle 441.19±15.10 450.17±9.58 458.83±14.45 462.52±16.81 468.17±15.93
Glyburide (10 mg/kg) 441.67±5.25 360.18±17.62* 336.95±19.39*** 228.54±21.01*** 369.31±18.18**
EtGA (100 mg/kg) 482.44±15.05 476.72±14.46 450.16±19.70 434.32±20.71 464.12±25.85
EtGA (200 mg/kg) 477.85±17.82 450.52±13.63 417.72±18.16 350.74±18.48** 433.01±28.29
EtGA (400 mg/kg) 489.58±15.35 397.61±16.29 350.13±19.73** 316.95±29.98*** 372.62±23.23*
n = 10, data was analyzed by two-way ANOVA with post hoc Dunnett’s test using Graphpad Instat software, *P<0.05, **P<0.01, ***P<0.001 as
www.wjpr.net 1494 Table 2: Effect of EtGA on serum glucose level in alloxan-induced diabetic mice (Subacute study).
Treatment
Mean fasting glucose level (mg/dl)±SEM
0 day 7 day 14 day 21 day 28 day After day 7 rest period
Vehicle 441.19±15.10 483.52±19.30 501.74±17.16 514.50±16.45 529.14±13.07 528.86±12.21
Glyburide (10 mg/kg) 441.67±5.25 344.29±17.81*** 292.16±27.87*** 245.47±30.44*** 192.69±22.24*** 172.85±21.82***
EtGA (100 mg/kg) 482.44±15.05 448.31±20.21 430.29±24.53 454.97±20.97 398.40±19.33*** 388.28±32.42***
EtGA (200 mg/kg) 477.85±17.82 421.96±16.44 392.72±17.53** 348.38±22.83*** 330.64±15.69*** 305.97±27.44***
EtGA (400 mg/kg) 489.58±15.35 389.56±17.58* 366.84±19.11*** 330.08±19.95*** 317.94±29.15*** 255.01±27.72***
n = 10, data was analyzed by two-way ANOVA with post hoc Dunnett’s test using Graphpad Instat software, *P<0.05, **P<0.01, ***P<0.001 as
www.wjpr.net 1495 Table 3: Effect of EtGA on body weight in alloxan-induced diabetic mice
Treatment
Mean body weight (g)±SEM
0 7 14 21 28 After day 7 rest period
Vehicle 30.50±0.43 27.50±0.62 27.00±0.26 26.00±0.45 22.00±1.06 18.00±0.82
Glyburide (10 mg/kg) 30.00±0.58 29.00±0.82 31.00±0.89** 30.00±0.68** 29.00±0.82*** 30.00±1.37***
EtGA (100 mg/kg) 29.00±0.26 28.00±0.68 27.00±0.52 27.00±1.15 27.00±1.15 25.00±1.06
EtGA (200 mg/kg) 30.00±0.52 30.00±0.37 29.00±0.86 28.00±1.24 28.00±1.41*** 26.00±0.77***
EtGA (400 mg/kg) 25.00±0.37 30.00±0.73 29.00±0.52 30.00±0.89** 30.00±0.73*** 31.00±1.13***
n = 10, data was analyzed by two-way ANOVA with post hoc Dunnett’s test using Graphpad Instat software, **P<0.01, ***P<0.001 as
www.wjpr.net 1496 Table 4: Effect of alcoholic extract of EtGA on oral glucose tolerance test (OGTT) in nondiabetic mice
Treatment
Mean Fasting glucose level (mg/dl)±SEM
Before glucose 0 min 30 min 60 min 120 min
Vehicle 129.31±10.31 334.68±14.66 261.31±9.98 219.01±7.91 158.71±10.40
Glyburide (10 mg/kg) 121.52±11.32 315.30±9.48 191.95±8.06*** 165.64±9.98*** 172.86±11.18
EtGA (100 mg/kg) 113.98±6.28 299.09±10.08 225.86±8.75 183.68±8.57 149.20±5.80
EtGA (200 mg/kg) 113.12±7.41 327.25±6.34 224.57±7.42* 156.80±4.56*** 160.46±7.26
EtGA (400 mg/kg) 117.63±7.17 333.39±14.78 201.78±7.12*** 142.11±5.70*** 154.73±7.71
n = 10, data was analyzed by two-way ANOVA with post hoc Dunnett’s test using Graphpad Instat software, *P<0.05, **P<0.01, ***P<0.001 as
www.wjpr.net 1497 Table 5: Effect of EtGA on oral glucose tolerance test (OGTT) in diabetic mice
Treatment
Mean Fasting glucose level (mg/dl)±SEM
Before Glucose 0 min 30 min 60 min 120 min
Vehicle 402.90±17.44 504.32±15.50 433.44±16.23 388.50±6.69 494.05±14.39
Glyburide (10 mg/kg) 449.93±19.61 525.54±9.91 332.21±16.73*** 326.63±4.96*** 436.10±19.71
EtGA (100 mg/kg) 476.60±17.73 539.24±15.30 476.23±9.85 395.43±14.71 482.19±18.98
EtGA (200 mg/kg) 465.13±17.88 531.94±15.38 364.73±5.76** 325.42±9.17* 453.63±10.61
EtGA (400 mg/kg) 482.59±14.50 544.26±15.15 349.39±15.96*** 297.49±13.05*** 480.58±9.07
n = 10, data was analyzed by two-way ANOVA with post hoc Dunnett’s test using Graphpad Instat software, *P<0.05, **P<0.01, ***P<0.001 as
www.wjpr.net 1498 Table 6: Phytochemical screening of the ethanolic extract of G. Asiatica
Sr. No. TEST Inference
1 Alkaloids
+ve
2 Flavonoids
+ve
3 Saponins
-ve
4 Tannins
-ve
5 Sterols
+ve
6 Carbohydrates
-ve
7 Test for glycosides
+ve
REFERENCES
1. Grover JK, Yadav S, Vats V. Medicinal plants of India with antidiabetic potential. J
Ethnopharmacol 2002; 81(1): 81-100.
2. Vetrichelvan T, Jegadeesan M. Anti-diabetic activity of alcoholic extract of Aerva lanata
(L.) Juss. Ex Schultes in rats. J Ethnopharmacol 2002; 80: 103-107.
3. Chakkarwar PN, Majrekar NA. Insulin glargine: A long acting insulin analog. J Postgrad
Med 2005; 51(1): 68-71.
4. Edwin E, Sheeja E, Chaturvedi M, Sharma S, Gupta VB. A comparative study on
antihyperglycemic activity of fruits and barks of Ficus bengalensis (L.). Adv Pharmacol
Toxicol 2006;7(3): 69-71.
5. Shah SN, Bodhankar SL, Bhonde R, Mohan V. Hypoglycemic activity of the combination
of active ingredients isolated from Trigonella foenumgraecum in alloxan induced diabetic
mice. Pharmacologyonline 2006a; 1: 65-82.
6. Gupta MK, Lagarkha RS, Sharma Dk, Singh PK, Ansari RHS. Antioxidant activity of the
successive extracts of Grewia asiatica leaves. Asian J chem 2007; 19(5): 3417-3420.
7. Patil PS, Patel MM, Bhavsar CJ. Comparative antidiabetic activity of some herbal plants
extracts. Pharm Sci Monit 2010; 1(1):12-19.
8. Ahaskar M, Sharma KV, Singh S, Sisodia R. Raadioprotective effect of fruit extract of
grewia asiatica in Swiss albino mice against lethal dose of γ irradiation. Asian J exp Sci
www.wjpr.net 1499
9. Sharma KV, Sisodia R. Evaluation of the free radical scavenging activity and
radioprotective efficacy of Grewia asiatica fruit. J Radiol Prot 2009; 29: 429-443.
10. Sisodia R, Singh S. Biochemical, behavioural and quantitative alterations in cerebellum
of Swiss albino mice following irradiation and its modulation by Grewia asiatica. Int J
Radiat Biol 2009; 85(9): 787-795.
11. Sharma KV, Sisodia R. Hepatoprotective efficacy of Grewia asiatica fruit against
oxidative stress in Swiss albino mice. Iran J Radiat Res 2010; 8(2): 75-85.
12. Sangita K, Avijit M, Shilpa P, Shivkanya J. Studies of the antifungal and antiviral activity
of methanolic extract of leaves of Grewia asiatica. Pharm J 2009; 1(3): 221-223.
13. Kirtikar KR, Basu BD. Indian medicinal plants, vol I, 2nd edn,. Published by Lalit Mohan
Basu, Allahbad; 1989.
14. Pokharkar RD, Saraswat RK, Kotkar S. Survey of plants having antifertility activity from
Western Ghat area of Maharashtra State. J herb Med Toxicol, 2010; 4(2): 71-75.
15. Hossan MS, Hanif A, Agarwala B, Sarwar MS, Karim M, Taufiq-Ur-Rahman M, Jahan
R, Rahmatullah M. Traditional use of medicinal plants in Bangladesh to treat urinary tract
infections and sexually transmitted disease. Ethnobot Res Appl 2010; 8: 61-74.
16. Rangari VD. Pharmacognosy and Phytochemistry. 1stedn, Career Publications, Nashik;
2004.
17. Harborne JB. Phytochemical methods, 3rd edn, Chapman and hall, London; 1998.
18. Ravichandran V, Suresh B, Sathishkumar MN, Elango K, Srinivasan R. Antifertility
activity of hydroalcoholic extract of Ailanthus excels (Roxb): An ehanomedicines used by
tribals of Nilfiris region in Tamilnadu. J Ethanopharmacol, 2007; 112: 189-191.
19. Kameswararao BK, Kesavulu MM, Giri R, Apparao C. Antidiabetic and Hypolipidemic
effects of Momordica cymbalaria Hook. fruit powder in alloxan-diabetic rats. J
Ethnopharmacol 1999; 67: 103-109.
20. Ewart RBL, Kornfeld S, Kipnis DM. Effect of lectins on hormone release from isolated
rat islets of langerhans. Diabetes 1975; 24: 705–714.
21. Cetto AA, Weidenfeld H, Revilla MC, Sergio IA. Hypoglycemic effect of Equisetum
mriochaetum aerial parts on STZ diabetic rats. J Ethnopharmacol 2000; 72: 129–133.
22. Abdel-Barry JA, Abdel-Hassan IA, Al-Hakiem MH. Hypoglycaemic and
antihyperglycaemic effects of Trigonella foenum-graecum leaf in normal and alloxan
www.wjpr.net 1500
23. Dunn JS, McLetchie NGB. Experimental alloxan diabetic in rats. Lancet 1943; 2: 384-
387.
24. Latha M, Pari L. Antihyperglycaemic effect of Cassia auriculata in experimental diabetes
and its effect on key metabolic enzymes involved in carbohydrate metabolism. Clin Exp
Pharmacol Physiol 2003; 30: 38-43.
25. Badole SL, Shah SN, Patel NN, Thakurdesai PA, Bodhankar SL. Hypoglycemic activity
of aqueous extract of Pleurotus pulmonarius (Fr.) Quel- Champ in alloxan induced
diabetic mice. Pharma Biol 2006a; 44(6): 421-425.
26. Badole SL, Bodhankar SL, Thakurdesai PA. Study of interaction of aqueous extract of
Pleurotus pulmonarius (Fr.) Quel- Champ with rosiglitazone in alloxan induced diabetic
mice. Pharmacologyonline 2006b; 3: 64-72.
27. Bailey CJ, Day C. Traditional treatment for diabetes. Diab care 1989; 12: 533 - 564.
28. Shah SN, Bodhankar SL, Badole SL, Kamble HV, Mohan VJ. Effect of trigonelline: an
active compound from Trigonella foenumgraecum Linn. in alloxan induced diabetes in
mice. J Cell Tissue Res 2006b; 6(1): 585-590.
29. Xie TT, Wang A, Mehendale S, Wu J, Aung HH, Dey L, Qiu S, Yuan CS. Antidiabetic
effect of Gymnema yannaense extract. Pharmacol Res 2003; 47: 323-329.
30. Catopano AL. Antioxidant effect of flavonoids. Angiol 1997; 48: 39-46.
31. Kameswararao B, Giri R, Kesavulu MM, Apparao C. Herbal medicines: In the
treatment of diabetes mellitus. Manphar Vaidya Patrika 1997; 1: 33-35.
32. Chakravarthy BK, Gupta S, Gambir SS, Gode KD. Pancreatic β cell generation- A novel
antidiabetic mechanism of Pterocarpus marsupium Rox. Ind J Pharmacol 1980; 12:
123-127.
33. Tiwari AK, Rao Madhusudana. Diabetes mellitus and multiple therapeutic approaches of