A number of studies have shown that the capacity of an active muscle or muscle fiber to develop force is reduced following shortening. The force developed isometrically following an interval of shortening, or the force reached after shortening to a steady-state length in an isotonic contraction, is less than that obtained in a purely isometric contraction at the shortened length (Buchthal et al., 1951; Abbott and Aubert, 1952; Maréchal, 1955; Délèze, 1961; Aubert and Lebacq, 1971; Floyd and Smith, 1971; Dickinson and Woledge, 1973; Edman, 1975, 1980, 1981, 1996; Julian and Morgan, 1979b; Maréchal and Plaghki, 1979; Ekelund and Edman, 1982; Sugi and Tsuchiya, 1988; Granzier and Pollack, 1989; Edman et al., 1993). The reduced capacity to develop force is frequently referred to as ‘shortening deactivation’. In addition to reduced force output, there are other changes in muscle performance associated with shortening that may be attributable to deactivation. These are an increase in the rate at which force declines at the end of stimulation (Briden and Alpert, 1972; Edman, 1980), a depression of the rate of force redevelopment following shortening, the extent of which depends on the distance of the preceding shortening (Edman, 1981; Ekelund and Edman, 1982), a depression of shortening velocity in test periods following shortening (Granzier and Pollack, 1989), a
decline in the shortening velocity during the course of an isotonic contraction or of the force during isovelocity shortening (Aidley, 1966; Joyce et al., 1969; Floyd and Smith, 1971; Lännergren, 1978), a failure of force to rise during isovelocity shortening early in a tetanus when muscle activation is increasing (Colomo et al., 1986) and a failure of the force during isovelocity shortening to rise as rapidly as expected when the shortening occurs at long muscle lengths and hence shifts the length in the direction more favorable for force production (Granzier and Pollack, 1989).
Shortening deactivation is a widespread and perhaps universal feature of skeletal muscle. Most of the studies mentioned in the previous paragraph were performed using frog muscles but, in addition to frog muscle, evidence for deactivation with shortening has been found in cat and mouse limb muscles (Joyce and Rack, 1969; Joyce et al., 1969; Ekelund and Edman, 1982; Askew and Marsh, 1998), dogfish jaw muscle (Abbott and Aubert, 1952), fish swimming muscle (Altringham and Johnston, 1990; Rome and Swank, 1992), a crab respiratory muscle (Josephson and Stokes, 1989), scallop swimming muscle (Marsh and Olson,1994) and, as a somewhat special case, the asynchronous flight muscles of several insect orders (Pringle, 1978, 1981). Shortening deactivation in frog JEB2309
Active shortening of respiratory muscle L2B from the crab Carcinus maenas results in contractile deactivation, seen as (1) a decline of force during the course of isovelocity shortening, (2) a reduction in the rate of force redevelopment following shortening, (3) a depression of the level of isometric force reached following shortening, and (4) an accelerated relaxation at the end of stimulation. The degree of deactivation increases with increasing distance of shortening, decreases with increasing shortening velocity, and is approximately linearly related to the work done during shortening. Deactivation lasts many seconds if stimulation is maintained, but is largely although not completely removed if the stimulation is temporarily interrupted so that the force drops towards the resting level. Deactivation for a given distance and velocity of shortening increases with increasing muscle length above
the optimum length for force production. Stimulating muscle L2B at suboptimal frequencies gives tetanic contractions that are fully fused but of less than maximal amplitude. The depression of force following shortening, relative to the force during an isometric contraction, is independent of the stimulus frequency used to activate the muscle, indicating that deactivation is not a function of the background level of stimulus-controlled muscle activation upon which it occurs. Deactivation reduces the work required to restretch a muscle after it has shortened, but it also lowers the force and therefore the work done during shortening. The net effect of deactivation on work output over a full shortening/lengthening cycle is unknown.
Key words: muscle, contraction, shortening deactivation, work, deactivation, crab, Carcinus maenas.
Summary
Introduction
WORK-DEPENDENT DEACTIVATION OF A CRUSTACEAN MUSCLE
ROBERT K. JOSEPHSON1,* ANDDARRELL R. STOKES2
1School of Biological Science, University of California, Irvine, CA 92697, USA and 2Department of Biology, Emory
University, Atlanta, GA 30322, USA *e-mail: rkjoseph@uci.edu
muscle is a phenomenon without an obvious function, but in several of the other muscles in which it has been identified, shortening deactivation has been shown to be, or proposed to be, important in normal muscle operation. By decreasing the force at the end of shortening, and increasing the relaxation rate, shortening deactivation can reduce the work required to re-extend a muscle after shortening and thus possibly increase the net work done over a complete shortening–lengthening cycle. Shortening deactivation has been proposed to increase net power output from a crab respiratory muscle (Josephson and Stokes, 1989), fish trunk muscles (Altringham and Johnston, 1990; Rome and Swank, 1992) and a mouse limb muscle (Askew and Marsh, 1998) during operation at normal in vivo frequencies. In insect asynchronous muscles, shortening deactivation and its lengthening counterpart, stretch activation, result in positive power output during oscillatory contraction and are indispensable elements in the generation of the normal wingbeat cycle (Pringle, 1978, 1981; Josephson, 1997).
The following study examines deactivation with shortening in a muscle in which deactivation has been shown to be important functionally; respiratory muscle L2B of the green crab Carcinus maenas. Muscle L2B (so designated by Young, 1975) is a scaphognathite levator, the scaphognathite being the blade of a respiratory pump that moves water across the crab’s gills. This muscle was selected for study because there is already a substantial amount of information available about the organization and performance of the scaphognathite as a pump (see Mercier and Wilkens, 1984a,b, and references therein), on the basic contractile properties of the muscle, including force–velocity relationships during both shortening and lengthening (Josephson and Stokes, 1987, 1999), and on the power output of the isolated muscle (Stokes and Josephson, 1988; Josephson and Stokes, 1989). Characterization of the parameters determining the extent of deactivation with shortening is important in evaluating the effects of deactivation on net work output and for constructing realistic, theoretical models of work output from muscle, models whose predictions may be compared with the actual work output generated by living muscles, as has been done in several recent studies (Curtin et al., 1998; Williams et al., 1998; Askew and Marsh, 1998). One of the results that emerged from this study is that in the crab muscle, as in frog muscles (Maréchal and Plaghki, 1979; Granzier and Pollack, 1989), deactivation during shortening is more closely correlated with the work done during shortening than with the distance shortened. It is for this reason that we have termed the phenomenon ‘work-dependent deactivation’ rather than ‘shortening deactivation’ in the title of this paper.
Materials and methods
Green crabs (Carcinus maenas) were obtained from the Supply Department of the Marine Biological Laboratory, Woods Hole, MA, USA. The techniques used to isolate muscle L2B and its motor nerve and to mount the muscle for mechanical recordings were as described by Josephson and
Stokes (1987). The preparation was submerged in the crustacean saline of Govind and Lang (1981) (pH adjusted to 7.4 at 15 °C with NaOH before use). A cooling coil in the dish maintained the saline at 15 °C. Muscle length was controlled and force measured using either a Cambridge Technology (model 300H) ergometer or an ergometer constructed from a Ling 102A shaker with semiconductor strain gauges mounted on the shaker arm (Malamud and Josephson, 1991). The muscle was stimulated through a suction electrode on the motor nerve using 0.5 ms current pulses. In the experiments to be described, the muscle was always activated with bursts of stimuli at 100 Hz. Trials were paced regularly at 2 min intervals or, in experiments involving especially long or repeated bursts of stimulation, at 4 min intervals. Values of muscle length and force were digitized, and displayed and stored with a computer. At the end of an experiment, the muscle was fixed in situ with 70 % ethanol before being detached from the ergometer. The length of the fixed muscle was measured using an ocular micrometer. Muscles were stored in 70 % ethanol for several days to several weeks, after which they were rehydrated in saline overnight, dissected free from attached exoskeletal elements and weighed. Muscle masses were multiplied by 1.36 to compensate for the expected weight loss associated with ethanol fixation and rehydration (Josephson and Stokes, 1987). Muscle area was calculated as the ratio of muscle mass to length assuming a muscle density of 1 g cm−3.
Work with each new preparation began with an evaluation of the stimulus strength needed to activate the muscle fully. The muscle was excited with 0.4 s bursts of tetanic stimuli, and the stimulus intensity was gradually increased from trial to trial until the muscle response reached a maximum. The stimulus intensity used in subsequent measurements was twice the minimum strength required to evoke a maximal muscle response. Next, the relationship between muscle length and tetanic force was characterized so that the subsequent experiments could be made on an appropriate part of the length–tension curve. The muscle was initially set at a length judged visually to be somewhat shorter than the optimal length for production of active force (active force = increase in force above the unstimulated level). The muscle was then stimulated tetanically (0.4 s burst) in a series of trials between each of which the muscle was lengthened by 0.4 mm (approximately 4 %). Trials were continued until active force clearly declined with increasing length. Determinations of an appropriate stimulus intensity and muscle length were followed by experimental trials exploring one or more aspects of deactivation following isovelocity shortening.
Results
Manifestations of deactivation
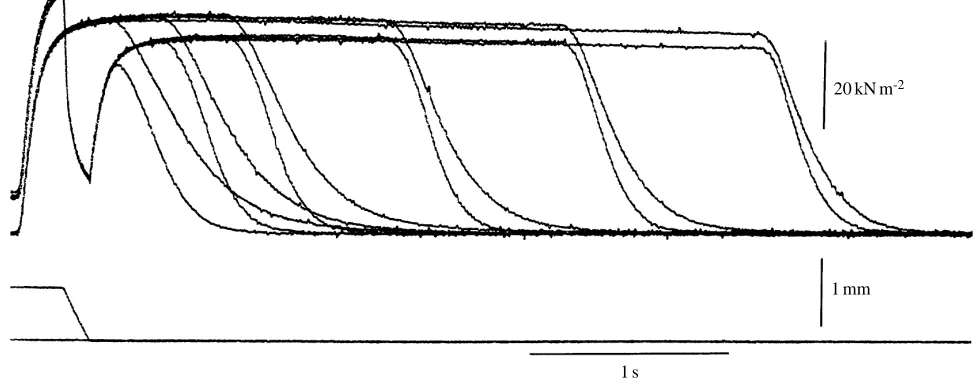
is demonstrated in three ways in trace 3, which is the force during the trial with shortening.
Depression of the isometric force redeveloped following shortening (Fig. 1Ai)
The initial and the final lengths in this experiment were selected such that the maximum tetanic tension was nearly identical at the two lengths. Therefore, were there no deactivation, one would have expected the force to drop during shortening and then to recover, at a rate similar to that of the initial tension development, to reach the same isometric force as before shortening. In fact, for some time after shortening, tension was substantially below that expected for an isometric contraction at the new length. The recorded force from the
stimulated muscle in Fig. 1A was the sum of the active force generated by the contractile elements and the passive force due to stretched parallel elastic elements. We assume that the passive force in a muscle is a function only of muscle length and was the same in the shortened muscle, after the shortening was complete, as in the muscle which was isometric throughout. Therefore, the reduction in isometric force following shortening was a reduction in the active force, that generated by the contractile elements.
Increased rate of relaxation (Fig. 1Aii)
Relaxation rate in the crab muscle, and in many other muscles as well, is a function of muscle length (Josephson and Stokes, 1989). Increasing length reduces the rate of relaxation. This is seen in Fig. 1, in which the rate of relaxation at the longer length (trace 2) was lower than that at the shorter length (traces 1, 5). The rate of relaxation at the shorter length was even faster in the trial in which the muscle was allowed to shorten (trace 3) than in those in which it was maintained at constant length. The more rapid loss of the ability to maintain tension at the end of a tetanus with shortening, compared with that of a muscle at the same length which contracted isometrically, is another reflection of shortening deactivation.
Declining force during isovelocity shortening (Fig. 1Aiii)
In some muscles, including fast muscles of frogs, the force during isovelocity shortening is reasonably constant, especially if the shortening is imposed during the plateau of a tetanic contraction and at lengths near the plateau of the length–tension curve (e.g. Cecchi et al., 1978; Westerblad and Lännergren, 1994). In the crab respiratory muscle, in contrast, force fell throughout the course of isovelocity shortening. During the latter part of the shortening, the force often declined at a rather constant rate. A similar decline in force during isovelocity shortening has been described for other muscles, including frog slow muscle (Floyd and Smith, 1971) and cat soleus muscle (Joyce et al., 1969), and has been ascribed to shortening deactivation.
A declining force during isovelocity shortening could reflect shortening deactivation, but it could also be due, in part, to decreasing passive force in parallel elastic elements as the muscle shortens and parallel elements become less stretched and to a delay in the progressive relaxation of stretched series elastic elements as these come into equilibrium with the reduced force level of the shortening myofibrils. The contributions of parallel elastic elements can be eliminated by subtracting from the force of an active muscle the passive force of the unstimulated muscle undergoing the same length changes. Doing this reduced the rate of force decline, especially early in the shortening when the muscle force and stiffness are highest, but it did not eliminate the decline in force in the crab muscle during isovelocity shortening (Fig. 1B). The releases in Fig. 1 were to a length at which there was little passive force, so subtracting the passive force from the active force had little effect on the slope of the force trace during the latter part of the shortening. Comparable results were obtained
10.0 mm
9.2 mm
20 kN m-2
0.5 s 1, 5
2
3 4
A
B
i
ii iii
1, 5 3, 4 2
[image:3.609.48.299.72.241.2]in six other preparations in which both passive and stimulated forces were measured during shortening with trajectories similar to that in Fig. 1. Contributions of stretched series elastic elements to the changing force during isovelocity shortening can be reduced or eliminated by imposing a small shortening step that appropriately unloads series elastic elements at the onset of the shortening ramp (e.g. Cecchi et al., 1978; Julian et al., 1986). Beginning the shortening ramp of the crab muscle with a step of appropriate size reduced the time taken to reach a steady rate of force decline but did not eliminate the decline (Fig. 2). The overall shapes of the force recordings during isovelocity shortening by the crab muscle were certainly influenced by decreasing force in parallel elastic elements and possibly by damped recoil of stretched series elastic elements, but the major decline in force, especially later in shortening, could not be explained by changing contributions of passive elastic elements. Most of the decline in force must have resulted from a decrease in the capacity of the myofibrils to generate tension, i.e. to deactivation.
Deactivation increases with increasing distance of shortening
[image:4.609.67.273.70.418.2]The approach used to examine and quantify the effects of shortening distance on the extent of deactivation is shown in Fig. 3. A tetanically stimulated muscle was allowed to shorten by varying amounts in different trials to a common final length. The shortening, which began during the plateau of the contraction, was at constant velocity (6 mm s−1), and the time of the release from isometric contraction to isovelocity shortening was adjusted such that, in each trial, the muscle reached the final target length at a fixed time following the onset of stimulation. Trials with shortening were alternated with control trials in which the muscle contracted isometrically at the final length reached during the shortening trials.
It was clear that increasing the distance of shortening resulted in greater deactivation (Fig. 3). How to quantify this relationship was not so clear. The force deficit between control contractions and trials with shortening decreased with increasing time after shortening, but the decrease was often a consequence of both growing force in the trials with shortening and decreasing force, usually at a rather slow rate, in the control trials. We have analyzed these recordings assuming that the recovery of force after shortening was a result of two processes: (1) an initial, rapid redevelopment of tension due to filament sliding and restretch of the series elastic elements which had become partially unloaded during the shortening, and (2) recovery from the deactivation engendered by the shortening. The initial recovery process (1) can be expected to be approximately as fast as the development of tension at the
0.1 s
Force
Length
A
B
20 kN m-2
0.5 mm
Fig. 2. Releases from isometric to isovelocity contraction as in Fig. 1, but with shortening steps of varying size imposed at the onset of the ramp shortening. A and B are from different preparations. The total distance of shortening was 7.2 % in A and 6.6 % in B. The slight oscillations in some of the force traces of B are probably a consequence of underdamping in the servosystem controlling length.
Fig. 3. (A) Increasing deactivation with increasing distance of shortening. During the plateau of an isometric contraction, the muscle was released to isovelocity shortening at 6 mm s−1. The distance of shortening allowed was varied from trial to trial. Trials with shortening alternated with control, isometric contractions at the length reached by the muscle at the end of shortening. (B) The method used to quantify deactivation. ∆F is taken as the force
depression at the end of the isovelocity shortening due to deactivation (see text for discussion).
20 kN m-2 Control
0.5 mm
0.2 s
∆F
A
B
[image:4.609.316.552.72.272.2]onset of stimulation and therefore to be complete in a few hundred milliseconds. Clearly, recovery from deactivation was substantially slower. We measured the deactivation by fitting a least-squares regression line to the last few hundred milliseconds of the contraction with shortening, a region of the force curve for which recovery is likely to be due nearly entirely to recovery from deactivation. The force predicted by extrapolating this line to the onset of force redevelopment was taken as the force that would have existed in the muscle if the redevelopment of force due to process 1 had been instantaneous. Similar regression lines were fitted to the isometric trials preceding and following the trial with shortening. The regression lines in the isometric trials were used to predict the force in an isometric contraction at the time corresponding to the onset of force redevelopment, using the same approach as that used to predict the extrapolated force in the shortening trials. The difference between the mean of the predicted forces in the two bracketing control trials and the extrapolated force in the trial with shortening (∆F in Fig. 3)
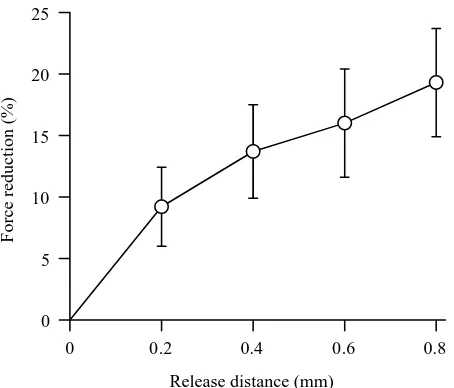
was taken as the depression of force due to deactivation as it existed at the end of the shortening. Deactivation measured in this way increased monotonically, but not linearly, with increasing shortening distance (Fig. 4).
Deactivation is inversely related to shortening velocity
Deactivation with shortening, seen as a reduction in the force redeveloped following shortening and as a speeding up of the relaxation at the end of stimulation, was greater following slow shortening than after rapid shortening over an
equivalent distance (Fig. 5). Deactivation following releases at different velocities was quantified using the approach illustrated in Fig. 3B. In five of the six preparations of this series, shortening deactivation increased with decreasing shortening velocity, from approximately 10 % when shortening for 0.8 mm at 24 mm s−1to 20 % when shortening for the same distance at 3 mm s−1(Fig. 6). For unknown reasons, one of the preparations of the six was quite different from the other five in that deactivation was large, approximately 30 %, and independent of the velocity of shortening.
Fig. 5 also illustrates an aspect of deactivation not previously considered for the crab muscle: a reduction in the rate of force redevelopment. The rate of rise of force at any point in the redevelopment of force following shortening depends on the instantaneous velocity of the contractile component of the muscle and the muscle stiffness. Both the shortening velocity of the contractile component and stiffness are functions of muscle force. If there were no deactivation of the contractile component, and the force–velocity properties of the muscle were therefore constant, the slope of the force rise should depend solely on the force level and should be the same at any force level irrespective of the extent of previous shortening. But, even at equivalent force levels, the slope of the force recovery was less step following slow shortening than following rapid shortening, which results in the crossing of the force traces following slow and fast shortening (inset, Fig. 5).
Deactivation is a function of work done during shortening
Deactivation varies with distance of shortening and with shortening velocity in the same way as does the work done during shortening. The work output during a contraction is the product of the distance of shortening and the average force during shortening. Because of the force–velocity properties of contracting muscle, the force during shortening varies
Release distance (mm)
0 0.2 0.4 0.6 0.8
Force reduction (%)
0 5 10 15 20 25
20 kN m-2 Control
0.5 mm
0.2 s
[image:5.609.61.288.71.265.2]C
Fig. 4. The relationship between release distance and deactivation measured as in Fig. 3. Data are shown as mean ± 2 S.E.M. (N=6). Because muscle lengths were not determined until the preparation was fixed at the end of the experiment, it was not convenient to adjust the shortening distance and velocity such that the strain and strain rate were identical in trials with different muscles. The muscle lengths in this set ranged from 9.4 to 11.6 mm, so the strain rate ranged from 0.52 to 0.64 lengths s−1in different preparations, and the strain in the largest release ranged from 6.9 to 8.5 %.
[image:5.609.318.566.71.194.2]inversely with velocity, as does the deactivation. We measured the work done during shortening in experiments such as those shown in Figs 3 and 5 in order to quantify the relationship between work and deactivation. The net work during shortening was obtained by summing the product F∆L over the
shortening period, where ∆L is the distance of shortening
between adjacent digitized points of the length channel and F is the force sample taken at the mid-point of ∆L. The magnitude
of the deactivation following shortening was found to be approximately linearly related to the work done during the shortening, and roughly the same relationship was found whether work was varied by changing shortening distance or shortening velocity (Fig. 7). The slope in four experiments such as those in Fig. 7 ranged from 3.0 to 6.2 % J−1kg (mean 4.1 % J−1kg). The deactivation following shortening is not solely a function of the amplitude of shortening, since it also varied with shortening velocity. Deactivation does seem to be a monotonic function of the work done during the contraction. For this reason, we have chosen to refer to the depression of contractile performance following shortening as work-dependent deactivation rather than shortening deactivation.
The linear regression between deactivation and work intersected the deactivation axis at a positive value in each of the four experiments such as those in Fig. 7. The mean value of deactivation at the intersection of the regression line with the y axis was 3.2 % (range 1.3–4.7 %). It was tempting to regard the deactivation at ‘zero work’ as reflecting internal
work done by the contractile component of the muscle in restretching series elastic elements that had shortened during
Velocity (mm s-1) 10
Force reduction (%)
0 5 10 15 20 25
2 5 20
N=6
[image:6.609.335.526.71.452.2]N=5
[image:6.609.46.289.73.295.2]Fig. 6. The relationship between shortening velocity and deactivation. Muscles shortened by 0.8 mm at the velocities indicated. Symbols are means; error bars, drawn in one direction only for clarity, indicate 2 S.E.M. One preparation was different from the other five in that deactivation was particularly large and independent of the velocity of shortening. The data have been plotted including (open symbols) and excluding (filled symbols) this odd muscle. The length of the muscles in this series ranged from 9.2 to 11.3 mm (mean 10.0 mm).
Fig. 7. The relationship between deactivation and work done during shortening. These results are from experiments such as that of Fig. 3, which examined the effects of distance of shortening on deactivation, and experiments such as that of Fig. 5, which quantified the relationship between velocity of shortening and deactivation. Results were obtained on the effects of both distance and velocity of shortening in four preparations. A and B illustrate two of these. A is the same preparation as is shown in Figs 3 and 5. In two of the four preparations providing data on both distance and velocity of shortening, one of which is shown in B, the relationships between work done and deactivation were quite similar in the length series and the velocity series. In the other two preparations, including that in A, there was some divergence between the results from the distance series and the velocity series. These experiments were not designed to test the possible equivalence of distance and velocity changes, and the measurements dealing with these parameters came at different times in a rather long series of measurements. The divergence between results from the distance series and the velocity series in A may reflect a change in the condition of the muscle in the interval between the distance measurements and those on velocity. The continuous line in each plot is the least squares regression:
r2=0.82, P<0.01 (A), r2=0.92, P<0.01 (B).
0 1 2 3 4 5
Deactivation (%)
0 5 10 15 20
Work (J kg-1) 0
5 10 15 20
Distance series Velocity series
A
B
0 1 2 3 4 5
the release. However, this is probably not the explanation for the non-zero intercept of the regression line with the deactivation axis. Imposing a small, fast shortening on the muscle can produce a large decrease in force (see Fig. 15). The recovery of resting force following such a release must require internal shortening of contractile elements and restretch of series elastic components. But small, fast releases, during which little external work is done, do not give measurable deactivation (see Fig. 15). Seemingly, the internal work associated with force redevelopment is too small, or in some other way inadequate, to produce deactivation. Given that deactivation is apparently zero when external work is zero, the plot of deactivation versus work must pass through the origin, and the initial slope of the relationship must be greater than that indicated by the regression line in Fig. 7.
Both increasing the distance of shortening at a given velocity and decreasing the velocity of shortening over a given distance increase the work done during a contraction and increase deactivation, but both also increase the duration of the shortening, which raises the possibility that it is the duration of the shortening event rather than the work done during the event that determines deactivation. In the four experiments such as those of Fig. 7, the correlation coefficient (r) for the linear regression between deactivation and work done (mean 0.93, range 0.90–0.96) was greater but not quite significantly so (P<0.06, two-tailed t-test) than the correlation coefficient between deactivation and duration of shortening (mean 0.86, range 0.79–0.92). The work done during contraction appears to be a better predictor of deactivation than is the duration of shortening.
[image:7.609.317.564.73.388.2]Deactivation increases with increasing muscle length above the optimal length for force production
Fig. 8A–D shows a set of recordings in which there was force redevelopment following isovelocity releases at a common velocity and over a common total distance but in which the releases began at different muscle lengths. As initial muscle length increased, the redeveloped force following shortening became a progressively smaller fraction of the isometric force at the shortened length. In Fig. 8A, the force at the onset of relaxation in the contraction with shortening was 92 % of the force at the onset of relaxation in the isometric contraction; in Fig. 8D, the equivalent ratio was 65 %.
The progressive reduction in the ratio of redeveloped force to isometric force with increasing muscle length suggests that deactivation increases with muscle length. However, quantifying deactivation using the approach illustrated in Fig. 3B gave a slightly different, and probably misleading, picture because it suggested that the relationship between muscle length and work-dependent deactivation had a minimum and was not a simple, monotonic increase (Fig. 9). At short muscle lengths, initial force development during tetanic stimulation and force redevelopment following shortening contraction are slower than at longer lengths. The approach used to quantify shortening deactivation (Fig. 3B) is predicated on the assumption that the recovery from
deactivation is much slower than the initial rate of force development and the rate of force redevelopment following shortening that would have pertained had there been no deactivation. When this assumption is true, the slope of the force recovery following shortening, measured several hundred milliseconds after the end of shortening, is due largely to recovery from deactivation. At short muscle lengths, when initial force development in a tetanic contraction is slow, the force redevelopment following shortening, even several hundred milliseconds after the end of shortening, is likely to include, as a significant component, the slow time course seen in the initial force development as well as recovery from
A
B
50 kN m-2
2 mm
C
D
-1 0 1 2 3
Force (%
Fmax,active
)
0 25 50 75 100
125
A
B
C
D
Active force Total force
0.5 s Muscle length (mm from optimum)
shortening deactivation. At short muscle lengths, therefore, the approach of Fig. 3B is likely to overestimate the extent of work-dependent deactivation. For this reason, the values at short muscle lengths in Fig. 9 are suspect. What is clear is that, at longer muscle lengths, deactivation does increase with increases in the length at which force redevelopment occurs.
Deactivation is long-lasting
The time course of shortening deactivation was examined
by comparing, during prolonged contractions, the force redeveloped following isovelocity shortening (0.8 mm at 6 mm s−1) with that produced isometrically at the shortened length. Stimulus durations were varied from 0.5 to 3.8 s in different trials. The release to isovelocity shortening came shortly before the plateau of the tetanus. The isometric trials preceded those with the same stimulus duration but with shortening. Work-dependent deactivation, as shown by depressed force production and faster tension relaxation in the trial with shortening, declined with increasing time after the end of shortening (Fig. 10). However, deactivation was still obvious at the end of the longest tetanic contractions tested, in which relaxation began 3.3 s after the end of shortening. Quite similar results were obtained in similar experiments with four other preparations.
Relaxation largely but not completely removes deactivation
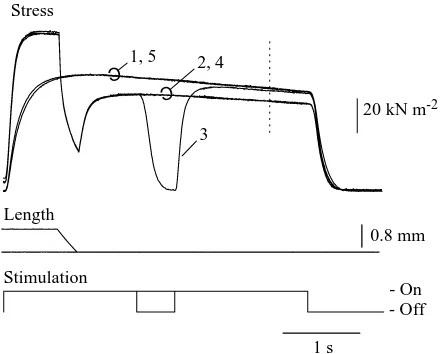
[image:8.609.52.276.74.266.2]The following protocol was used to determine whether deactivation persists beyond the end of a tetanic contraction. A muscle was held at a length somewhat longer than that optimal for tetanic force and stimulated tetanically (100 Hz) for 4 s. In some trials, the muscle was allowed to shorten by 0.8 mm at 6 mm s−1 and then to redevelop tension at the shortened length. The shortening began early in the plateau of the contraction. In one trial of the series, the stimulation was interrupted for 0.5 s beginning 0.75 s after the end of shortening (Fig. 11). Tension fell to near the resting level during the pause in stimulation. The sequence of trials in these experiments was: (1) isometric contraction at the short length, (2) contraction with release, (3) contraction with release and interrupted stimulation, (4) contraction with release, and (5) isometric contraction at the short length. Because of the long duration of the stimulation period, the intertrial interval in these experiments was 4 min. In quantifying the results, all forces were measured 3.5 s after the onset of stimulation.
Fig. 9. Deactivation following shortening as a function of muscle length. Muscles were released to isovelocity shortening (0.8 mm at 6 mm s−1) during the plateau of tetanic contractions. The abscissa is the muscle length reached at the end of shortening and at which force redevelopment occurred. Force reduction was quantified as in Fig. 3B. The filled circles are from the same set of trials as Fig. 8; the open symbols are from two other, similar experiments. Lopt, muscle length at optimal for work output.
Muscle length (%Lopt)
-10 0 10 20
Force reduction (%)
0 20 40 60 80 100
20 kN m-2
1 mm
1 s
[image:8.609.58.547.523.712.2]The final tension reached during the trial with shortening and interrupted stimulation (trial 3, Fig. 11) was greater than that in the contractions with shortening and continuous stimulation (trials 2 and 4) and similar to that in the isometric contractions at the short length (trials 1 and 5). This experiment was repeated with five preparations. In these, the force late in the contraction in the trial with interrupted stimulation averaged 100.3±1.9 % (mean ±S.E.M.) of that at the equivalent time in the isometric contractions at the short length (mean of trials 1 and 5). In contrast, the force in trials with release and continuous stimulation (mean of trials 2 and 4) was 89.6±1.5 % of that in the isometric contractions. From these experiments, it would appear that interrupting stimulation abolished deactivation. A different approach, however, indicates that some deactivation persists long after the end of stimulation.
In experiments such as that illustrated in Fig. 11, the force redeveloped following a break in stimulation by a muscle that had previously shortened was compared with the force in that muscle during continuous stimulation with and without shortening. It could be argued that interrupting stimulation allows the muscle to recover partially from accumulating fatigue. Or it could be proposed that, because of post-tetanic potentiation, stimulation following a break is particularly effective in activating the muscle. Either phenomenon – partial recovery from fatigue or post-tetanic potentiation – might increase the responsiveness of the muscle and mask accumulated shortening deactivation. The approach shown in Fig. 12 was used to examine the decay of shortening deactivation at the end of stimulation under conditions in which neither differential recovery from fatigue nor post-tetanic potentiation should affect the results. Each trial in these experiments began with a 1 s stimulation period during which
the muscle was isometric (trials 1 and 3 of each set) or allowed to shorten for 0.8 mm at 3 mm s−1 (trial 2). The initial contraction was followed, after a varied delay, by a 1 s test stimulation during which the muscle contracted isometrically. The presence or absence of deactivation was determined by comparing the force reached during the test contraction that followed shortening with the average of the force in the preceding and succeeding test contractions that followed isometric contractions. The durations of the quiescent intervals between the initial and the test stimulations ranged from 1 to 10 s and were presented in a mirror series. In three of the five preparations used, the intervals tested were, in order, 10, 5, 2, 1, 1, 2, 5 and 10 s. In the other two preparations, the first interval was 1 s and subsequent intervals were gradually increased to 10 s and then shortened. The results from the ascending part of the series and the descending part were averaged to obtain a single value for each tested interval for each preparation. The results from this experiment demonstrated that a small, but quite consistent, trace of deactivation persists for at least 10 s after the end of a contraction in which there was shortening (Figs 12, 13).
Deactivation does not depend on overall level of muscle activation
The activation level of muscle L2B can be manipulated by changing the stimulation frequency. Stimulating the muscle at 100 Hz (15 °C) produces a maximal or nearly maximal tetanic response. Stimulating at a lower frequency, down to approximately 20 Hz, initiates a tetanic contraction that is smoothly fused but one in which the force reached is less than
20 kN m-2
0.8 mm Stress
Length
Stimulation
- Off - On
1 s 1, 5 2, 4
3
[image:9.609.64.284.72.249.2]C C
Fig. 11. The effect on deactivation of a brief interruption in the stimulus train. Traces 2–4 included a period of isovelocity shortening (0.8 mm at 3 mm s−1); traces 1 and 5 were isometric at the length reached during shortening. In trace 3, the stimulus was interrupted for 0.5 s at the time indicated. The vertical broken line indicates the time at which measurements were made for comparing the force redeveloped following shortening with isometric force at the same length.
20 kN m-2
0.8 mm
1 s Stress
Length
[image:9.609.321.555.75.264.2]that with 100 Hz stimulation (Figs 14, 15; Josephson and Stokes, 1987). One component of deactivation with shortening in frog muscles is dependent on the level of muscle activation. For shortening under light loads, there is little deactivation if the shortening occurs during the plateau of a tetanus, while deactivation is pronounced when the shortening occurs during a twitch, a partially fused tetanus or the relaxation of a tetanic contraction (Edman, 1980). The following experiments, in which work-dependent deactivation was quantified in crab muscle during both partial and full activation, were performed to determine whether the extent of deactivation in the crab muscle varies with the state of activation.
A potential problem in evaluating shortening deactivation in a partially activated muscle is that the rate of rise of force as well as the final force reached are reduced compared with those of a fully activated muscle (Fig. 14). The finding that the muscle force, measured a fixed time after the end of shortening, is smaller relative to the isometric level in a partially activated
muscle than in a fully activated one might mean that shortening deactivation is more pronounced in the partially activated muscle, or it might reflect the fact that it takes the partially activated muscle longer to recover tension following release simply because its kinetic properties are intrinsically slower. A protocol was developed with which we could separate, from work-dependent deactivation, the potentially confounding effects of differences in the rate of force rise associated with differences in the steady-state level of muscle activation upon which the deactivation is imposed. We compared the force redeveloped following a small, fast shortening, which produced a large force change but little work-dependent deactivation, with the force redeveloped after a long, slow step of the sort that produces significant deactivation.
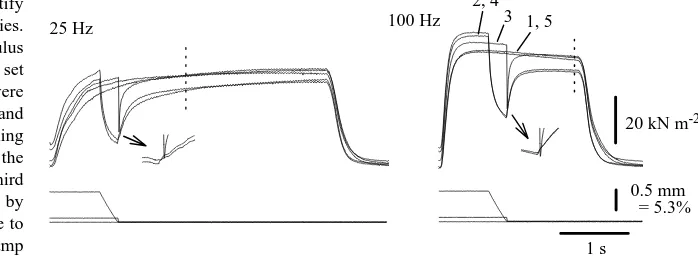
In the protocol used, the individual trials with a preparation were grouped into a series of sets (Fig. 15). The stimulus
Duration of pause (s) 2 5
1 10
Test force (% control)
[image:10.609.315.554.68.264.2]90 92 94 96 98 100
[image:10.609.68.272.73.268.2]Fig. 13. Isometric force in test trials following shortening relative to that following control trials without shortening (broken line). The data are from trials such as that of Fig. 11. Values shown are mean ± 2 S.E.M., N=5 preparations. See text for details.
Fig. 14. Peak isometric force and rate of force rise (dF/dt) as functions of stimulus frequency. Values (mean ± 2 S.E.M., N=10 preparations) are expressed relative to those at 100 Hz. The rate of force rise (N s−1) was measured as the average over the interval 20–40 % F0, where F0 is the peak isometric force reached in that trial.
Fig. 15. The approach used to quantify deactivation at different stimulus frequencies. Trials were given in sets at a common stimulus frequency. The first and last trial in each set (labeled 1 and 5 in the right panel) were isometric contractions. The second and penultimate trials (2, 4) included shortening (0.8 mm at 3 mm s−1) to the length at which the isometric contractions were recorded. The third trial was an isometric contraction interrupted by a small, step shortening that brought the force to approximately the same level as did the ramp shortening in the preceding and following trials.
Thus, the force redevelopment began at the same time, and from the same force levels, following the ramp and the step shortenings. The vertical dashed line is 1 s after the end of shortening and marks the point at which forces were measured to quantify deactivation. The inset, indicated by an arrow, is the force at the end of shortening on an expanded time scale. See text for further details.
Stimulus frequency (Hz) 0 20 40 60 80 100
Force (% maximum)
0 20 40 60 80 100
0 20 40 60 80 100
d
F
/d
t (% maximum)
Force
dF/dt
20 kN m-2 1, 5
3 2, 4
0.5 mm = 5.3%
1 s 100 Hz
[image:10.609.209.558.568.697.2]frequency was constant for all trials within a set and different between sets. The stimulus frequencies tested with each preparation were 12, 25, 50 and 100 Hz. In half the preparations (5/10), sets were presented in order of increasing stimulus frequency, in the other half in order of decreasing stimulus frequency. With 12 Hz stimulation, but not at the higher frequencies, there was usually incomplete fusion of tension. However, the ripple on the tension traces with 12 Hz stimulation was not great. The peak-to-peak height of the ripple ranged from 0 to 5.4 % of the maximum isometric force at 12 Hz (3.0±1.4 %, mean ±S.D., N=10).
The first and last trials in each set were isometric contractions and were included as a way of determining whether muscle performance had declined substantially during the set. The second and penultimate trials were responses in which an isometric tetanus was interrupted by a long, slow shortening ramp (0.8 mm at 3.0 mm s−1), which was expected to produce work-dependent deactivation. In the third trial, and sometimes the next few as well, an isometric tetanus was interrupted by a small, shortening step. The step occurred at the same time after the onset of stimulation as did the end of shortening in the trials with ramp shortening. The amplitude of the step was adjusted so that the minimal force at the end of the step was essentially the same as the minimal force reached following ramp shortening. If the force reached at the end of the step did not reasonably match that at the end of the preceding ramp, the step size was adjusted and the trial repeated. Force was measured 1 s after the end of the length change in the trials with ramp-and-step shortening. The extent of muscle deactivation during the ramp steps was determined by comparing the force redeveloped after shortening in the trial with step shortening with that at the equivalent time in the trials with ramp shortening that preceded and followed the step trial.
Specifically:
D =100{[S −(R1 + R2)/2]/S} ,
where S is the force 1 s after end of step shortening, D is deactivation (%) and R1and R2are the force 1 s after shortening in the trials with ramp shortening that preceded and followed, respectively, the trial with step shortening. Deactivation measured in this way varied from 12 to 18 % with no obvious trend over the frequency range tested (Fig. 16). Apparently, work-dependent deactivation in the crab muscle is not a function of the background level of muscle activation.
Discussion
Deactivation in the crab muscle is similar to that in frog muscle
Work-dependent deactivation in crab muscle L2B is quite like the deactivation seen in frog limb muscle following shortening at low to moderate speed. Deactivation following shortening has most often been measured as the deficit between the force redeveloped following shortening and that during an isometric contraction at the equivalent length. As is shown here for the crab muscle, the force deficit in frog muscle increases
with increasing distance of shortening, declines with increasing shortening velocity (or with decreasing load) and is approximately linearly related to the work done during the shortening (Maréchal and Plaghki, 1979; Sugi and Tsuchiya, 1988; Granzier and Pollack, 1989). The force deficit for a given distance and velocity of shortening increases with increasing muscle length in crab muscle and in frog muscle (Maréchal and Plaghki, 1979; Granzier and Pollack, 1989; Edman et al., 1993). Further, in both crab and frog muscle, the deficit is maintained throughout a tetanic burst; but in the crab muscle and in several studies with frog muscles the deactivation was found to be largely abolished if the stimulation was interrupted for long enough to allow force to drop to nearly zero (Julian and Morgan, 1979b; Edman et al., 1993). Granzier and Pollack (1989) report that interrupting stimulation removed the deactivation following isovelocity shortening (their Fig. 4C) but not that following isotonic shortening (their Fig. 9); why there might be a difference between isotonic and isovelocity shortening is not clear. The many similarities in the properties of work-dependent deactivation in frog muscle and in crab muscle suggest strongly that the underlying mechanisms of deactivation are also similar in crabs and frogs.
Sarcomere heterogeneity and deactivation
In frog muscle, much of the muscular deactivation following slow shortening, and perhaps all of it, may be related to differences in the contractile properties of sarcomeres in different regions along individual muscle fibers. Serial non-uniformity was first suggested as a mechanism for shortening deactivation by Abbott and Aubert (1952), and the concept was further developed by Julian and Morgan (1979b) and by Edman et al. (1993). The basic idea is that different segments along a fiber may differ in contractile strength. During an ‘isometric contraction’, there is an initial net shortening of the
Stimulus frequency (Hz)
0 20 40 60 80 100
% Deactivation
[image:11.609.330.553.70.289.2]0 10 20 30
contractile portion of a fiber as force rises and series elastic elements become stretched. But, throughout the contraction, strong segments shorten more relative to their length, i.e. have greater strain, than do weak ones. In fact, weak segments may actually lengthen during an isometric contraction (Sugi and Tsuchiya, 1988; Julian and Morgan, 1979a). At long muscle lengths, sarcomere heterogeneity can lead to slow, upward force creep during isometric contraction as stronger sarcomeres shorten and move to even more favorable lengths for force production (Gordon et al., 1966). The difference in the strain between segments is observed to be greater following loaded shortening than following unloaded shortening or during isometric contraction (Sugi and Tsuchiya, 1988; Edman et al., 1993). The greater heterogeneity in the segmental strain after loaded shortening is presumably a consequence of differences in the force–velocity properties, and hence the rate of shortening under load, which have been demonstrated to occur in different regions along a fiber (Edman et al., 1985). During an isometric phase following shortening, stronger segments continue to shorten, albeit at a low velocity, stretching weaker segments. If the weaker segments are longer than their optimal length, stretch will make them weaker still. The stronger segments, because they are shortening, develop less force than they would were they isometric. The greater the heterogeneity among segments, the greater the shortening velocity of the stronger segments and the lower the force in the fiber as a whole. The force redeveloped by a fiber following loaded shortening is proposed to be less than that following unloaded shortening, or that in an isometric contraction, because the heterogeneity of the strain among segments is greater following loaded shortening than following unloaded shortening or in an isometric contraction. The finding by Edman et al. (1993) that the degree of deactivation of a muscle fiber is linearly related to the variability in the strain measured from different segments along the length of the fiber supports the hypothesis that deactivation following loaded shortening is a result of serial non-uniformity in fiber strain. In addition, Edman et al. (1993) found that there was no force depression following isovelocity shortening of small segments of a fiber, within which sarcomere properties are likely to be nearly homogeneous. However, Granzier and Pollack (1989) report that there is deactivation even in small fiber segments following shortening under load-clamp, so the question as to whether serial non-uniformity is needed for there to be deactivation following slow shortening must remain open.
The many similarities between deactivation in the crab muscle and in frog muscles suggest that in the crab muscle, as in frog muscle, deactivation may be largely a consequence of an increase in sarcomere heterogeneity which develops during shortening. Of particular importance in implicating sarcomere heterogeneity as the mechanism of deactivation are the increase in deactivation for a given amount of shortening with increasing muscle length (Fig. 9), a finding that is consistent with model studies based on sarcomere heterogeneity (Edman et al., 1993), and the near elimination of deactivation when the stimulation is temporarily interrupted so that the force falls to
zero, a maneuver that should substantially reduce the heterogeneity in sarcomere length developed during loaded shortening. The correlation between work done and the extent of deactivation (Fig. 7) may be indirect. For example, decreasing the velocity of shortening increases muscle force during shortening, which increases the rate of work output but which may also be expected to increase the development of sarcomere heterogeneity during shortening (Edman et al., 1993). It may be the effect of muscle force on sarcomere heterogeneity rather than on work output that is the more important determinant of deactivation.
In the crab respiratory muscle, some deactivation following shortening persists for many seconds after the end of stimulation (Fig. 12). It seems likely that any heterogeneity in sarcomere lengths along the fibers accumulated during the loaded shortening would be dispersed when the muscle relaxed. It therefore appears that, in the crab muscle, at least some part of the deactivation following slow shortening results from factors other than developed non-uniformity of sarcomere lengths along the individual fibers, perhaps from the depletion of fuel or the build-up of metabolic products during contraction.
Categories of deactivation
Work-dependent deactivation is but one of several varieties of shortening deactivation that have been identified in muscle. Other kinds of deactivation, and some of their characteristic features, are summarized below. It has yet to be determined whether any of the other kinds of shortening deactivation in addition to work-dependent deactivation occur in crab muscle L2B.
Work-dependent deactivation (‘force depression after loaded shortening’; Edman et al., 1993)
Deactivation increases with increasing shortening distance, increasing load and, therefore, increasing work done during shortening. Deactivation lasts for many seconds following shortening, but is largely abolished by interrupting the stimulation for long enough for the force to drops to near zero. Deactivation for a given shortening distance and velocity increases with increasing muscle length. Judging by the results with crab muscle L2B (Fig. 16), the relative amount of deactivation is independent of the stimulus-induced level of activation at the time of shortening.
Activation-dependent deactivation (‘shortening-induced deactivation’ or ‘movement effect’; Edman et al., 1993)
shorter than the peak. The depression of the force-generating capacity apparently acts through the Ca2+control mechanisms of muscle, since the deactivation occurs under conditions in which muscle activation is less than maximal – during relaxation following twitch or tetanic contractions or, with skinned muscle fibers, when Ca2+ levels are less than saturating. The depression of the force-generating capacity lasts for 1–2 s after the end of shortening.
Delayed deactivation
The characteristics of delayed deactivation are rapid kinetics and a delay between length change and maximum deactivation (Pringle, 1978, 1981; Josephson, 1997). In a muscle showing delayed deactivation, force falls during a step shortening but then continues to fall to a minimum after the end of shortening, after which there may or may not be some recovery. The time from the end of shortening to the attainment of a stable force level is short, a few milliseconds to a few tens of milliseconds. The continuing decline in tension after the end of shortening results in a delay between the length change and maximum deactivation. This delay produces a phase shift between length and force during sinusoidal length change at an appropriate cycle frequency, the force lagging behind the length change. Delayed deactivation is prominent in insect asynchronous muscles and is the feature that allows oscillatory contraction in these muscles. Delayed deactivation also occurs in vertebrate limb and heart muscle, and in the heart muscle it is likely to be of functional significance (for a review, see Steiger, 1971).
Velocity-dependent deactivation
Colomo et al. (1986) report that, very early in the tetanic contraction of a frog muscle fiber, the force during isovelocity shortening is influenced by a preceding shortening. Pre-shortening at a velocity greater than that in the test period depresses the force at the test velocity, whereas pre-shortening at less than the test velocity results in a force during the test period that is greater than that which would have occurred had the shortening been continuously at the test velocity. Colomo
et al. (1986) conclude that shortening early in a tetanus deactivates muscle and that the extent of deactivation is a function of shortening velocity and not distance of shortening. This deactivation is similar to activation-dependent deactivation in that it only occurs during a time of incomplete muscle activation, but is apparently different in being dependent on the velocity and independent of the distance of shortening.
Does work-induced deactivation increase mechanical power output?
Increased respiratory demand in crabs is met by an increase in the stroke frequency and in the work per cycle of the scaphognathite pump (Mercier and Wilkens, 1984a). The increase in work per cycle can be expected to lead to increased deactivation during shortening by the scaphognathite muscles. How this increased deactivation affects the net work output of the muscles is not known. In a study of work output by crab muscle L2B, during which the muscle was subjected to sinusoidal strain, it was found that the work required to re-extend the muscle following shortening did not always increase with increasing amplitude of imposed strain and hence increasing distance of stretch during re-extension (Josephson and Stokes, 1989). At longer muscle lengths, and over part of the strain range, the work of re-extension (negative work) actually declined with increasing strain (Fig. 17). The decline in the work of re-extension was attributed to deactivation associated with the shortening that preceded the stretch. Because of the deactivation, the work required to re-extend the muscle was less following a moderately long shortening, during which much work was done, than following a small shortening with little work output. Judging by the reduction in the work of extension, the deactivation for a given amount of shortening increased with increasing muscle length (Fig. 17) as did, in the present study, the reduction in isometric force redeveloped following shortening (Fig. 9).
[image:13.609.215.566.69.197.2]During repetitive activity, deactivation apparently reduces the work absorbed by the muscle during lengthening, which in itself increases the net work done over a full shortening–lengthening
Fig. 17. Shortening work, lengthening work and net work from crab muscle L2B as functions of the amplitude of imposed, sinusoidal strain (data from Josephson and Stokes, 1989, which should be consulted for details). Measurements were made at the average muscle length optimal for work output (Lopt) and at muscle lengths 7.5 % shorter and 7.5 % longer than the optimum. The frequency of the sinusoidal oscillation was 2 Hz, the duration of stimulation was 100 ms, and the phase of stimulation in the
strain cycle was that determined to be optimal for work output at the optimum strain. Open symbols indicate the work done by the muscle during shortening (+ work), filled symbols show the work absorbed by the muscle during re-lengthening (−work) and the line without symbols is the net work done over one complete cycle. Note that at Loptand even more obviously at Lopt+7.5 %, there is a decline in the work required to re-extend the muscle (−work) with increasing strain over part of the strain range. This decrease in lengthening work is attributed to shortening deactivation.
0 10 20 30
Work (J kg
-1)
0 1 2 3
Strain (%)
0 10 20 30 0 10 20 30
+ Work
– Work
Net work
Lopt
cycle. But, judging by the decline in muscle force during the course of isovelocity shortening (Fig. 1), the onset of deactivation is rapid, and deactivation occurs throughout the course of shortening. Thus, deactivation, in addition to reducing lengthening work, presumably also reduces muscle force and the work done during shortening. Whether the reduction in negative work during lengthening attributable to deactivation is greater or less than the decrease in positive work because of deactivation during shortening is not known. The force trajectory of some muscles subjected to sinusoidal strain and phasic stimulation can be predicted reasonably well by models that do not include work-dependent deactivation, suggesting that the overall effect of deactivation may be quantitatively small (Curtin et al., 1998; Williams et al., 1998).
Force during muscle shortening and lengthening depends on a number of factors: the velocity of shortening and the force–velocity properties of the muscle; the length trajectory followed and the length–tension characteristics of the muscle; the time course of the activation initiated by stimulation, which itself may be influenced by muscle length; shortening deactivation; and stretch activation if it occurs. Disentangling the effects of deactivation from those of the other relevant parameters, so as to evaluate its overall influence on muscle force and work, is a formidable task. A clever approach by which the effects of deactivation might be analyzed is provided in a recent paper by Askew and Marsh (1998). In their study, Askew and Marsh calculated the expected force trajectory during a shortening–lengthening cycle on the basis of measured or assumed force–velocity and length–tension properties of the muscle. The difference between the calculated force trajectory and that actually measured was proposed to be a measure of the relative degree of muscle activation throughout the cycle; the difference between the time course of muscle activation measured in this way and that given by the rise and decay times of force during an isometric contraction was taken as being a measure of the effects of the shortening and lengthening experienced throughout the cycle on muscle activation and deactivation. There are some problems with the study of Askew and Marsh (1998). For example, a simple curve was used for the relationship between velocity and force in lengthening muscle, whereas in actuality the trajectory of muscle force during lengthening is time-dependent and rather complex and it is not predictable by a single force–velocity curve (Josephson and Stokes, 1999). Further, because of series elasticity and internal shortening, the time course of isometric force is at best a distorted measure of the time course of muscle activation. But these are problems that could be remedied by having more complete information about the mechanics of the muscle being studied, and they do not invalidate the approach. In the absence of more complete information, the most that can be said about the effect of work-induced deactivation on work output by crab muscle L2B is that it reduces the work required to re-lengthen a shortened muscle and it probably reduces to some extent the work done during shortening, but the net effect is uncertain.
This work was supported by NSF research grants DCB-9104170 and IBN-9603187.
References
Abbott, B. C. and Aubert, X. M. (1952). The force exerted by active
striated muscle during and after change of length. J. Physiol., Lond.
117, 77–86.
Aidley, D. J. (1966). Transient changes in isotonic shortening
velocity of frog rectus abdominis muscles in potassium contracture.
Proc. R. Soc. Lond. B 163, 215–223.
Altringham, J. D. and Johnston, I. A. (1990). Scaling effects on
muscle function: power output of isolated fish muscle fibres performing oscillatory work. J. Exp. Biol. 151, 453–467.
Askew, G. N. and Marsh, R. L. (1998). Optimal shortening velocity
(V/Vmax) of skeletal muscle during cyclical contractions: length–force effects and velocity dependent activation. J. Exp. Biol.
201, 1527–1540.
Aubert, X. and Lebacq, J. (1971). The heat of shortening during the
plateau of tetanic contraction and at the end of relaxation. J.
Physiol., Lond. 216, 181–200.
Briden, K. L. and Alpert, N. R. (1972). The effect of shortening
on the time-course of active state decay. J. Gen. Physiol. 60, 202–220.
Buchthal, F., Kaiser, E. and Rosenfalck, P. (1951). The rheology
of the cross striated muscle fibre. Dan. Biol. Medd. 21, 1–318.
Cecchi, G., Colomo, F. and Lombardi, V. (1978). Force–velocity
relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. J. Physiol., Lond. 285, 257–273.
Colomo, F., Lombardi, V. and Piazzesi, G. (1986). A
velocity-dependent shortening depression in the development of the force–velocity relation in frog muscle fibres. J. Physiol., Lond. 380, 227–238.
Curtin, N. A., Gardner-Medwin, A. R. and Woledge, R. C. (1998).
Predictions of the time course and power output by dogfish white muscle fibres during brief tetani. J. Exp. Biol. 201, 103–114.
Délèze, J. B. (1961). The mechanical properties of the semitendinosus
muscle at lengths greater than its length in the body. J. Physiol.,
Lond. 158, 154–164.
Dickinson, V. A. and Woledge, R. C. (1973). The thermal effects of
shortening in tetanic contractions of frog muscle. J. Physiol., Lond.
233, 659–671.
Edman, K. A. P. (1975). Mechanical deactivation induced by active
shortening in isolated muscle fibres of the frog. J. Physiol., Lond.
246, 255–275.
Edman, K. A. P. (1980). Depression of mechanical performance by
active shortening during twitch and tetanus of vertebrate muscle fibres. Acta Physiol. Scand. 109, 15–26.
Edman, K. A. P. (1981). Deactivation of the contractile system
induced by shortening of striated muscle. In The Regulation of
Muscle Contraction: Excitation–Contraction Coupling (ed. A. D.
Grinnell and M. A. B. Brazier), pp. 281–296. New York: Academic Press.
Edman, K. A. P. (1996). Fatigue vs. shortening-induced deactivation
in striated muscle. Acta Physiol. Scand. 156, 183–192.
Edman, K. A. P., Caputo, C. and Lou, F. (1993). Depression of
tetanic force induced by loaded shortening of frog muscle fibres. J.
Physiol., Lond. 466, 535–552.
Edman, K. A. P. and Kiessling, A. (1971). The time course of the
in single muscle fibres of the frog. Acta Physiol. Scand. 81, 182–196.
Edman, K. A. P., Reggiani, C. and te Kronnie, G. (1985).
Differences in maximum velocity of shortening along single muscle fibres of the frog. J. Physiol., Lond. 365, 147–163.
Ekelund, M. C. and Edman, K. A. P. (1982). Shortening induced
deactivation of skinned fibres of frog and mouse striated muscle.
Acta Physiol. Scand. 116, 189–199.
Floyd, K. and Smith, I. C. H. (1971). The mechanical and thermal
properties of frog slow muscle fibres. J. Physiol., Lond. 213, 617–631.
Gordon, A. M., Huxley, A. F. and Julian, F. J. (1966). Tension
development in highly stretched vertebrate muscle fibres. J.
Physiol., Lond. 184, 143–169.
Govind, C. K. and Lang, F. (1981). Physiological identification and
asymmetry of lobster claw closer motorneurones. J. Exp. Biol. 94, 329–339.
Granzier, H. L. M. and Pollack, G. H. (1989). Effect of active
pre-shortening on isometric and isotonic performance of single frog muscle fibres. J. Physiol., Lond. 415, 299–327.
Josephson, R. K. (1997). Power output from a flight muscle of the
bumblebee Bombus terrestris. II. Characterization of the parameters affecting power output. J. Exp. Biol. 200, 1227–1239.
Josephson, R. K. and Stokes, D. R. (1987). The contractile
properties of a crab respiratory muscle. J. Exp. Biol. 131, 265–287.
Josephson, R. K. and Stokes, D. R. (1989). Strain, muscle length
and work output in a crab muscle. J. Exp. Biol. 145, 45–61.
Josephson, R. K. and Stokes, D. R. (1999). The force–velocity
properties of a crustacean muscle during lengthening. J. Exp. Biol.
202, 593–607.
Joyce, G. C. and Rack, P. M. H. (1969). Isotonic lengthening and
shortening movements of cat soleus muscle. J. Physiol., Lond. 204, 475–491.
Joyce, G. C., Rack, P. M. H. and Westbury, D. R. (1969). The
mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J. Physiol., Lond. 204, 461–474.
Julian, F. J. and Morgan, D. L. (1979a). Intersarcomere dynamics
during fixed-end tetanic contractions of frog muscle fibres. J.
Physiol., Lond. 293, 365–378.
Julian, F. J. and Morgan, D. L. (1979b). The effect on tension of
non-uniform distribution of length changes applied to frog muscle fibres. J. Physiol., Lond. 293, 379–392.
Julian, F. J., Rome, L. C., Stephenson, D. G. and Striz, S. (1986).
The maximum speed of shortening in living and skinned frog muscle fibres. J. Physiol., Lond. 370, 181–199.
Lännergren, J. (1978). The force–velocity relation of isolated twitch
and slow muscle fibres of Xenopus laevis. J. Physiol., Lond. 283, 501–521.
Malamud, J. G. and Josephson, R. K. (1991). Force–velocity
relationships of a locust flight muscle at different times during a twitch contraction. J. Exp. Biol. 159, 65–87.
Maréchal, G. (1955). L’irréversibilité du diagrame tension–longueur
du Sartorius. Arch. Int. Physiol. 63, 128–129.
Maréchal, G. and Plaghki, L. (1979). The deficit of isometric tetanic
tension redevelopment after a release of frog muscle at a constant velocity. J. Gen. Physiol. 73, 453–467.
Marsh, R. L. and Olson, J. M. (1994). Power output of scallop
adductor muscle during contractions replicating the in vivo mechanical cycle. J. Exp. Biol. 193, 139–156.
Mercier, A. J. and Wilkens, J. L. (1984a). Analysis of the
scaphognathite ventilatory pump in the shore crab Carcinus
maenas. I. Work and power. J. Exp. Biol. 113, 55–68.
Mercier, A. J. and Wilkens, J. L. (1984b). Analysis of the
scaphognathite ventilatory pump in the shore crab Carcinus
maenas. III. Neuromuscular mechanisms. J. Exp. Biol. 113,
83–99.
Pringle, J. W. S. (1978). Stretch activation of muscle: function and
mechanism. Proc. R. Soc. Lond. B 201, 107–130.
Pringle, J. W. S. (1981). The evolution of fibrillar muscle in insects.
J. Exp. Biol. 94, 1–14.
Rome, L. C. and Swank, D. (1992). The influence of temperature on
power output of scup red muscle during cyclical length changes. J.
Exp. Biol. 171, 261–281.
Steiger, G. J. (1971). Stretch activation and myogenic oscillation of
isolated cardiac, skeletal and insect flight muscle. In Insect Flight
Muscle (ed. R. T. Tregear), pp. 221–268. Amsterdam: North
Holland.
Stokes, D. R. and Josephson, R. K. (1988). The mechanical power
output of a crab respiratory muscle. J. Exp. Biol. 140, 287–299.
Sugi, H. and Tsuchiya, T. (1988). Stiffness changes during
enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J. Physiol., Lond. 407, 215–229.
Westerblad, H. and Lännergren, J. (1994). Changes of the
force–velocity relation, isometric tension and relaxation rate during fatigue in intact, single fibres of Xenopus skeletal muscle. J. Muscle
Res. Cell Motil. 15, 287–298.
Williams, T. L., Bowtell, G. and Curtin, N. A. (1998). Predicting
force generation by lamprey muscle during applied sinusoidal movement using a simple dynamic model. J. Exp. Biol. 201, 869–875.
Young, R. E. (1975). Neuromuscular control of ventilation in the crab